

 |
 |
| Agenda Item 4.5 | Conference Room Document 28 |
second fao/who global forum of food safety regulators
Bangkok, Thailand, 12-14 October 2004
(Paper prepared by the departments of the European Commission)1
The European Community is a unique entity which is one of the main importers of foodstuffs. Its originality lies in its structure: it is referred to by the FAO and the Codex Alimentarius as a Regional Economic Integration Organization. It is not a federal State, like the USA or Brazil, but an association of sovereign States which, by means of a treaty, have decided to share some of their national prerogatives by integrating them into a supranational legislative body. This process of integration has not been completed and history shows that, since the European Community was established in 1956, it has been continually expanding to include new Members while progressively extending and strengthening its areas of responsibility. This process resulted in the enlargement of the EU to include ten new Member States on 1 May 2004.
In the field of food safety, national legislation has largely been incorporated into Community legislation, although there are still a few areas in which national law continues to apply. However, the purpose of European integration is not to ensure that everything is systematically harmonized. Under the subsidiarity principle, which is enshrined in the Treaty, action can be taken by the European Union only if the EU is really capable of taking more effective action than the Member States acting individually.
The European Community joined the FAO in 1991, the WTO in 1995 and the Codex Alimentarius in 2003, and became an official observer of the OIE in 2003.
The Union Treaty stipulates that a high level of human health protection will be ensured in the definition and implementation of all Community policies and activities. The WTO Agreement on the Application of Sanitary and Phytosanitary Measures allows the Members to establish the level of protection which they consider appropriate in accordance with the provisions of the Agreement. In the context of European integration, the European Community has laid down joint conditions for imports of foodstuffs of animal or plant origin, taking account of the need not only to protect consumer health but also to protect the territory of the Union from the introduction of devastating animal or plant diseases. One practical consequence of European integration is the freedom to market imported foodstuffs throughout Community territory provided that they comply with the requirements laid down by the Community and its Member States.
The general principles on which the rules on food imports are based are no more restrictive than the health rules applied by the Member States on European Community territory. These principles, which recognize the fact that it is impossible to check all products at the border or at the place of destination, imply that the monitoring and checking of the ability of foodstuffs to meet health requirements takes place at as early a stage as possible in the production chain. The competent authorities in the countries in which foodstuffs are produced are therefore responsible for carrying out inspections and certifying that the health rules on production and the health safety standards laid down in Community legislation are correctly applied and checked. These specific rules for each category of animal products or products of animal origin can be found on the European Commission's website at the following address: http://europa.eu.int/comm/food/fvo/pdf/ guidethirdcountries_en.pdf.
Other Community rules have also been put in place for the other food products, particularly plant products.
Animal products and foodstuffs of animal origin may be exported to the European Community only by the countries included on the list of countries authorized for the product in question. The Community takes three criteria into account when drawing up the list.
As the principle is to delegate responsibility for checks at the point of origin to the national competent authority, this authority must be evaluated by the Food and Veterinary Office of the European Commission and recognized by the Commission as being equivalent, for the product under consideration, to the national authorities of the Member States.
This evaluation is based on Community legislation (Council Directive 2002/99/EC) and complies with the Guidelines of the Codex Alimentarius, in particular the Principles for Food Import and Export Certification (CAC/GL 20-1995) and the Guidelines for the Design, Operation, Assessment and Accreditation of Food Import and Export Inspection and Certification Systems (CAC/GL 26-1997). It also takes account of the relevant chapters of the Animal Health Code of the OIE (World Organization for Animal Health), in particular for the evaluation of veterinary authorities.
The evaluation starts with a documentary study of national legislation and the organization of the competent authority, its legal powers, independence, resources, human and material resources, particularly the number of inspectors, their level of training and the availability of laboratories, and procedures for inspecting and certifying exported products. This information, which is requested in a detailed questionnaire, is analyzed and a discussion takes place between the competent authority in the exporting country and the Food and Veterinary Office of the European Commission (FVO).
When the information available is judged to be satisfactory, a team of experts from the FVO is sent on an inspection visit to carry out on-the-spot checks to establish the veracity of the information. The inspection visit, which can last between one and two weeks in the exporting country, is prepared in close collaboration with the national competent authorities. The cost of the visit is borne entirely by the FVO (i.e. it is covered by the Commission budget) and the exporting country has nothing to pay.
For certain types of animal products or products of animal origin, the health status of the exporting country as regards animal diseases which are transmissible through meat or animal products is an important criterion. The Community is free of certain epizootic diseases and must seek to minimize the risks of introducing these diseases onto its territory. The recent experience with foot-and-mouth disease has shown that the methods used to protect the Community need to be strengthened. When evaluating the health status of an exporting country, the FVO takes account of the information provided by the national veterinary services and of the OIE's recommendations. The inclusion of an exporting country in the list of authorized countries also depends on the types of manufacturing processes used for foodstuffs. Countries in which certain animal diseases are endemic may be included in the lists of countries authorized to export to the Community only with respect to food products which have undergone treatment sufficient to destroy the pathogens of these diseases.
In accordance with the rules established by the OIE and Article 6 of the SPS Agreement, the Community makes provision in its legislation for a regionalized arrangement which takes account of the fact that different areas of the same country can have a different health status. The requirements for the importation of animal products from these areas are established while taking account of the specific health status of the region in question and the traceability of products.
Specific provisions have also been established for beef and beef-based products, depending on the classification of the exporting country in terms of BSE risk and the obligation to remove risk material in accordance with the requirements of the OIE.
The third criterion relates to the monitoring by the national competent authorities of residues of veterinary medicines and contaminants in food products of animal origin. Certain veterinary medicines are not authorized because of their toxicity and no residue, even traces of it, may be present in food. Other medicines are authorized but limits have been set for their residues in food in order to protect consumer health. Carrying out systematic checks of all residues in all products exported to the Community is too unwieldy and costly a task to be considered as a routine option. The Community has therefore decided that monitoring plans should be established which target particular veterinary medicines and animal species. The Member States have the task of drawing up the monitoring plans and submitting them to the Commission. Similarly, the exporting countries are required to submit to the Commission for approval an annual monitoring plan in which the products and residues checked are clearly identified. All products of animal origin which are produced through animal husbandry are affected (including aquaculture products and honey), as well as wild game. However, fish and fisheries products from the natural environment are not subject to monitoring of this kind.
When these three criteria are met, the exporting country can be included in the list of authorized countries for the product concerned. (N.B. There is a separate list for residue monitoring plans approved by the Commission.)
The hygiene rules relating to the production of food in the Community have been harmonized under Community legislation. These rules cover general structural requirements relating to the design, installation and equipment of food production establishments and more specific rules depending on the production type, particularly for products of animal origin. They also cover functional requirements for cleaning and disinfecting the establishments, which are included in a quality assurance programme based on HACCP principles.
These requirements are equivalent to the Recommended International Code of Practice — General Principles of Food Hygiene — of the Codex Alimentarius (CAC/RCP 1-1969, Rev. 4 (2003)). They apply to all food production establishments in the Community and, in the same way, to establishments in non-Community countries which want to export food products to the Community.
More specifically for animal products and products of animal origin, the establishments in the exporting countries must appear on a list of authorized establishments which correspond to the category of products which are prepared and exported. There are currently 14 categories of lists of establishments which correspond to different products, such as fresh meat, meat-based products, poultry, fish and fisheries products, dairy products, game meat, etc., depending on the interests of the exporting country (see Annex).
The competent authorities in the exporting countries are responsible for inspecting and authorizing establishments which want to export products to the Community. The inspectors must have a sound knowledge of Community requirements as regards the hygiene of the establishments and must assess whether the establishments meet equivalent requirements before proposing that they be included in the list of authorized establishments. The competent authorities send the Commission the list of authorized establishments, with the guarantee that they have been inspected and deemed to comply with the specific hygiene rules which correspond to the category of animal product or product of animal origin in question. The lists are then adopted by the Commission after an opinion of the Member States and sent to the border inspection posts in the Community.
The lists are periodically updated and are published on the Commission's website at the following address: http://forum.europa.eu.int/irc/sanco/vets/info/data/listes/table0.html
In the specific case of bivalve molluscs (oysters, mussels, clams, etc.), Community legislation stipulates that these products must come from particular harvesting areas where sources of pollution are under control and in which a plan for monitoring toxic planktonic organisms has been established. The levels of microbiological contamination and of dangerous toxins must be monitored continually by the competent authority which issues the authorization for harvesting. The list of authorized harvesting areas is also adopted by the Commission, after the Member States have given their opinion, and is published on the same website. A traceability system must ensure that bivalve molluscs which are exported to the Community do in fact come from the authorized harvesting areas.
Food products which are exported to the Community must be accompanied by a health certificate if Community legislation so stipulates. The certificates are unique documents which are legally binding on the signatory. The format and content of the certificate are laid down in the legislation and must be respected. The certificates are issued and signed by the competent authority in the exporting country, and are evaluated and approved by the European Commission (see Directive 96/93/EC on the principles of certification) in accordance with a procedure which complies with the Guidelines on certification systems established by the Codex Alimentarius as regards fraud prevention, for example. The certification procedures are audited by the FVO inspection team in the exporting country and must not be amended without the agreement of the Commission, which will decide whether the inspection visit should be repeated. The Commission is examining the possibility of introducing secure electronic certification procedures.
Checks on animal products or products of animal origin imported into the Community must be carried out before entering Community territory at a border inspection post (BIP) which has been authorized by the Commission. The main purpose of this requirement is to minimize the risk of introducing animal diseases into the Community and to protect consumer health. (Plants are subject to other types of checks relating to the protection of plant health.) There are around 300 authorized BIPs, at ports, airports and land borders.
In order to be authorized, the BIP must meet a number of requirements — staff, facilities, storage premises, cold stores, testing laboratory, etc. — which can differ depending on the type of products imported. There are therefore BIPs specializing in certain animal products or products of animal origin which cannot receive all products. The Commission gives its approval after the BIP has been inspected and given a positive assessment by the FVO.
It should also be borne in mind that checks on luggage belonging to passengers from non-Community countries are carried out on arrival in the Community in order to prevent, as far as possible, the introduction of food which could contain bacteria which are pathogenic to animals and likely to lead to epidemics.
There are also specific rules relating to products of animal origin which are in transit within the Community: only products which comply with animal health requirements may be stored and circulated on Community territory.
All consignments of animal products or products of animal origin are subject to three checks: a documentary check, an identity check and a physical check.
If an unfavourable result is obtained, a more stringent check is carried out on the next ten consignments from the same origin. In order to improve and speed up the flow of information among the BIPs, the Commission has put in place an electronic communication system called TRACES, which records all the results of the checks carried out in all the Member States.
Where a product is found repeatedly to contravene the rules and pose a risk to health, temporary safeguard measures can be introduced to ensure that a specific product undergoes systematic checks for a particular risk.
The Member States can also carry out random checks on food products when they reach their destination and at the marketing stage in order to verify whether they comply with the regulations.
The conditions governing imports of food products which are not of animal origin are less detailed than for animal products. However, specific plant-health rules apply to plants or plant products which could introduce harmful organisms onto Community territory.
Community legislation currently does not contain any requirements concerning the lists of countries and/or establishments which are authorized to export foodstuffs of non-animal origin to the Community. However, the new Regulation (EC) No 852/2004 on the hygiene of foodstuffs, which will come into force on 1 January 2006, stipulates that foodstuffs imported into the Community must comply with the relevant provisions of food legislation or the requirements which the Community deems to be at least equivalent. The general hygiene requirements laid down in the Regulation are consistent with the General Guidelines of the Codex Alimentarius, particularly as regards food hygiene and the application of HACCP principles. The new Regulation (EC) No 882/2004 on official controls on foodstuffs, which will also come into force on 1 January 2006, stipulates that the Commission may, if necessary, introduce conditions and detailed procedures to be respected when importing products of non-animal origin; these conditions and procedures may include a list of non-Community countries from which specific products may be imported into the Community.
There is no requirement to submit to the Commission an annual residue monitoring plan and its results, as is the case for veterinary medicines in animal products. This does not mean that the Community does not have any requirements concerning natural contaminants or pesticide residues. It is for the food industries to take account of these requirements in their quality assurance programme (HACCP) when they earmark their products for the Community market. In the event of repeated non-compliance, the Commission can introduce safeguard clauses which involve the competent national authorities in a system of compulsory inspection and certification which is in line with the relevant Guidelines of the Codex Alimentarius.
Imports of foodstuffs of non-animal origin are not yet subject to compulsory checks at a Border Inspection Post accredited by the Commission. These checks can be carried out on Community territory at all stages of the importation and marketing processes unless specific plant-health rules apply. However, in the event of repeated non-compliance, the Commission can decide, on the basis of a risk assessment, that checks be carried out at a designated BIP for a foodstuff of a particular origin. The checks can focus, in particular, on the presence of contaminants, pesticide residues, unauthorized food additives, labelling, etc.
The new Regulation (EC) No 882/2004 on official controls will come into force on 1 January 2006. The official controls on imported foodstuffs will comprise a systematic documentary check, a random identity check and, where appropriate, a physical check based on risk. They must be carried out at entry points designated by the Member States and the importers must provide prior notification of the arrival of a consignment and of its contents.
Community legislation does not lay down any requirements for the general certification of products of non-animal origin imported into the Community. However, the Commission can introduce certificates under the safeguard clause in the event of repeated non-conformity.
The new Regulation (EC) No 882/2004 on official controls on foodstuffs, which will come into force on 1 January 2006, stipulates that the Commission may, where necessary, decide on the establishment of models of certificates accompanying consignments of food imported into the Community.
The importation into the Community of plants or plant products is governed by Council Directive 2000/29/EC concerning the protection of the Community from harmful organisms. Certain types of unprocessed fruit and vegetables intended for human consumption are subject to the provisions of this Directive.
The general principles of this Directive are based on the provisions of the International Plant Protection Convention (IPPC) of the FAO. These include:
The most important plant health provisions concerning the importation of plants or plant products into the Community can be summarized as follows:
These import requirements are summarized in the flow chart below, which gives non-Community countries wishing to export edible plants or plant products to the Community an overview of the steps and various options involved in complying with the Community rules on plant health.
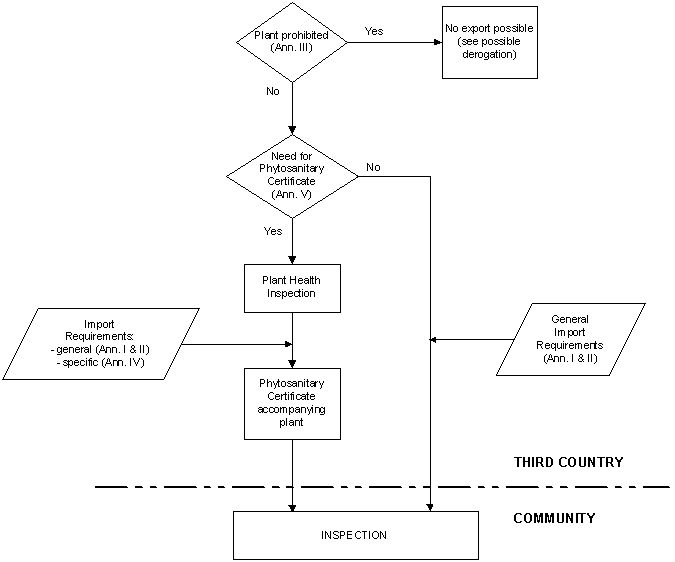
Compulsory plant health checks are carried out on certain plants or plant products which are imported into the Community. As with animal products, these checks comprise a documentary check (plant health certificate), an identity check and a physical check in order to verify whether the imported products comply with Community requirements on plant health.
If these checks reveal the presence of a harmful organism, a procedure for the notification of interception of the product is applied in accordance with Community legislation and plant health measures are taken to prevent the introduction and spread of these organisms.
The requirements concerning the importation of foodstuffs into the European Community are being progressively harmonized, although national standards still apply in some cases. The harmonization process will take an important step forward on 1 January 2006, when the new Regulations on hygiene rules and official controls will come into force. Before that date, the European Commission plans to adopt as many decisions as possible which harmonies national hygiene rules, such as microbiological criteria or maximum limits for residues and contaminants, although these efforts will certainly continue for several years.
The purpose of the Community requirements on food imports is not only to protect consumers but also to limit the risk of introducing onto Community territory animal or plant diseases which could have disastrous economic or social consequences.
The Community rules on food imports comply with the Guidelines of the Codex Alimentarius, the OIE and the IPPC in that they give the country of origin the responsibility for inspecting, checking and certifying consignments which are exported. The European Community is aware that its requirements are sometimes difficult for developing countries to meet. Under the new Regulation on official controls, the Commission is therefore able, in the context of the policy on development cooperation, to provide developing countries with specific assistance as regards food safety. The Commission will therefore be able to decide that the requirements be applied gradually with respect to developing countries, while assisting the competent authorities with the presentation of information, drawing up guidelines to help the competent authorities organize official checks on exported products and sending out experts from the Community to help them, and enabling inspection staff from developing countries to participate in training programmes organized by the FVO.
CODE |
PRODUCT OF ANIMAL ORIGIN |
Directive |
01-RM |
Fresh Meat |
72/462 |
02-PM |
Fresh Poultry Meat |
71/118 |
03-GM |
Farmed Game Meat |
91/495 |
04-WM |
Wild Game Meat |
92/45 |
05-MM |
Minced Meat and Meat Preparations |
94/65 |
06-RPM |
Meat Products |
72/462 |
07-PMP |
Poultry Meat Products |
92/118 |
08-GMP |
Farmed Game Meat Products |
92/118 |
09-WMP |
Wild Game Meat Products |
92/118 |
10-MMP |
Milk and Milk-based Products |
92/46 |
11-FFP |
Fish and Fishery Products |
91/493 |
12-LBM |
Live Bivalve Molluscs |
91/492 |
15-CAS |
Animal Casings |
92/118 |
17-GEL |
Gelatine |
92/118 |
Source: http://forum.europa.eu.int/irc/sanco/vets/info/data/listes/table0.html
1 Does not necessarily represent the official position of the Commission.