Abstract
Lectins have the potential to act as anti-nematode proteins in strategies to control both sedentary and migratory endoparasitic nematodes. It has been shown in the past that lectins bind to the surface of sedentary nematodes. In this report, results are presented for the binding of three types of lectins, concanavalin A of Canavalia ensiformis, wheat germ agglutinin of Triticum aestivum and Helix pomatia lectin, to the surface of the migratory endoparasite Radopholus similis, which is the most important and damaging nematode parasite of banana. Binding occurred in the head region, at the excretion pore, the pores of the reproduction system and those of the phasmids. These results indicate an interaction between lectins and R. similis that could have toxic effects or inhibit chemoreception of host root signals. It was also shown that the secretions of R. similis can be stained and visualised with the protein-specific dye Coomassie Brilliant Blue R. Secretions appear at the amphids, the excretion pore, the vulva, the spicules and the phasmids. This technique can be applied in experiments to test the effect of lectins on the production of secretions by R. similis and other migratory and sedentary nematodes.
1. INTRODUCTION
1.1. Molecular strategies for nematode resistance
In general, three basic strategies to create nematode resistance have been formulated [1,2]: (a) the use or reinforcement of natural plant defence mechanisms, (b) the direct killing of nematodes, and (c) interference with the plant-nematode interaction. The first strategy includes cloning of natural resistance genes and reintroduction of these genes into susceptible plants. This has been accomplished for the HsIpro-1 gene of Beta procumbens, the wild relative of B. vulgaris, which is resistant to Heterodera schachtii [3]. Transgenic plants had the same level of resistance as their wild-type counterparts [4].
In the second type of strategy, external and internal nematode structures are the target of lytic enzymes, such as chitinases and collagenases, and inhibitory or toxic proteins, such as protease-inhibitors or Bacillus thuringiensis endotoxins. The aim of using chitinases is to increase the permeability of the nematode eggshell, which could be lethal to embryos. However, the chitin layer is not exposed directly to the environment, but is protected by a vitellin layer [5,6]. The same problem arises in the use of collagenases to degrade the cuticle of nematodes, as the cuticle is protected by the glycocalyx or surface layer [6]. Protease inhibitors are part of the natural defence response of plants and have already proved useful to control insect pests. Their use as anti-nematode proteins is based on the assumption that nematodes digest food with proteases. Results so far, for sedentary nematodes only, are encouraging [7-11]. The application of B. thuringiensis toxins is hampered by the fact that they are highly species-specific [1,6,12].
Interference in the plant-nematode interaction can be accomplished by the expression of a repelling protein, an inhibition of the induction or maintenance of feeding structures by sedentary nematodes, or by the expression of a protein that hampers host recognition [1,2]. No research has been done so far on the use of antisense RNA to inhibit the transcription products of nematode-induced genes, while negative effects on host plant roots have been observed after the local expression of the RNase barnase to kill feeding structures [13]. Antibodies, specifically targeted against nematode secretions responsible for feeding structure induction and maintenance, have not been successful in controlling M. incognita in transgenic tobacco [14]. An alternative is to direct antibodies towards the structures and secretions of nematodes, responsible for chemoreception of host signals. However, clear indications about such an effect are not available [15,16].
1.2. Alternative strategies to control migratory nematodes
1.2.1. Need for novel strategies
Research has generally been focused on resistance against sedentary endoparasitic nematodes, such as the root-knot nematode Meloidogyne spp., and the cyst nematodes Globodera spp. and Heterodera spp. The reasons are twofold. On the one hand, there is the agronomic impact: root-knot nematodes are an important threat to agricultural production in the tropics and sub-tropics, and cyst nematodes cause severe yield losses in the temperate regions. The sedentary nematodes have, on the other hand, an intriguing biology: they are obligate parasites that spend the greater part of their life cycle inside the plant root, during which they engage in a long-term interaction with their host. They do not kill it, but induce genes and initiate a redifferentiation process. The reactions of the hosts are extensively characterised and often include a hypersensitive reaction. Moreover, Arabidopsis thaliana is used as a model host [2,17], as both Meloidogyne spp. and Heterodera spp. can complete their life cycle in its roots. This is an advantage for the molecular genetical analysis of plant-nematode interactions, because the genome of A. thaliana is small, with a low number of repetitive DNA sequences, it has been sequenced, and genetic transformation has been proved to be efficient. The interaction between migratory endoparasitic nematodes and plants is far less studied, as appears from the existing strategies for the induction of resistance against nematodes. Migratory nematodes do not interact in a sophisticated manner with their host tissue; they do not induce local, permanent feeding structures. Instead, they move themselves continuously through root tissue while feeding, which makes a localised, directed resistance mechanism, such as the inhibition of feeding structures, impossible. Moreover, all developmental stages of migratory endoparasitic nematodes, from secondary juveniles to adults, can move through root tissue as well as through the soil, in which they spend a great deal of their life cycle.
The most important nematode species that infect banana are migratory endoparasites, including Radopholus similis, Pratylenchus spp. and Helicotylenchus multicinctus. The development of valuable strategies against migratory nematodes is thus essential. Some of the aforementioned anti-nematode proteins are unsuccessful, while others are aimed at the feeding structure of sedentary nematodes. In this paper, lectins are presented as candidate anti-nematode proteins in an alternative strategy to control migratory endoparasitic nematodes.
1.2.2. Lectins as candidate anti-nematode proteins
Plant lectins are proteins that possess at least one non-catalytic domain that binds reversibly to a specific mono- or oligosaccharide. In plants, seven different classes of lectins are defined according to their structure and evolution. They function mainly in the storage of nitrogen and in recognition processes. Some plant lectins are involved in defence mechanisms against insects [18]. In nematology, lectins have been used, in the first place, to characterize sugar moieties on nematode surfaces or in secretions via binding experiments. They have been shown to bind to several plant (mainly sedentary) parasitic nematodes at the excretion pore, the cuticle, the amphids or the head region in general, as well as at the spicules and the vulva [19-25].
A novel application in nematology is for testing the capacity of lectins to control nematodes. In contrast to the previously mentioned anti-nematode proteins, lectins are readily available and relatively easily applicable on a laboratory scale. They can be used in control strategies at two levels: expression inside the host root, or expression and secretion into the plant's rhizosphere. It is assumed that lectins, by analogy with their effect in insects, have an intestine-associated activity in nematodes: the permeability of the digestive tract increases after ingestion of lectins. This has not previously been tested in vitro because of the lack of artificial diets for phytophagous nematodes. However, Burrows and co-workers [26] reported on the expression of Galanthus nivalis lectin (GNA) in transgenic potatoes at a level of 0.1-0.5% of total root proteins. One out of four of these transgenic lines reduced female G. pallida numbers by 50%. Recently, tests have been set up in the Laboratory of Tropical Crop Improvement of K.U.Leuven (Belgium), to screen transgenic A. thaliana, expressing different types of lectins, for resistance against migratory and sedentary endoparasitic nematodes of banana (K. Carlens, personal communication).
Marban-Mendoza and co-workers [27] have done research on the control of root-knot nematodes of tomato by lectins. They found that an application of 12 µg of Canavalia ensiformis lectin (Con A) around the base of tomato plants, every week, during a period of 4 weeks, reduced root-knot formation by 75% compared with control plants. An explanation might be that lectins bind to the nematode surface and as a consequence have a toxic effect or inhibit the host-finding mechanism of nematodes. It has been substantiated that nematodes are attracted to host plants by chemotaxis [28]. The amphids, located in the head region, are considered to be the primary sensory organs for the perception of root signals. These chemical substances are captured by the amphidial secretions, which fill the amphidial channel, and are brought to the amphidial membranes by diffusion [29]. Knowing this, Zuckerman [30] formulated a strategy to create resistance against nematodes; blocking the host's signals or the signal perception should disturb the localisation of host plants. Not being able to detect a host plant, nematodes will wander about in the soil, with little chance of finding a food source. A reduction of chemotaxis was accomplished in Caenorhabditis elegans by binding of lectins to the amphidial secretions [31]. However, negative results were obtained by Forrest and co-workers [32]: Con A had no effect on the capacity of G. rostochiensis juveniles to detect and reach pieces of agar soaked with potato root diffusates.
This strategy requires the presence of lectins in the surroundings of the rhizosphere of nematode host plants. Expression inside the plant cell is thus not sufficient and signal peptides are necessary for the transport of the proteins to the plant's apoplast and rhizosphere. Secretion of transgenic proteins has already been accomplished successfully in N. tabacum by Herbers and co-workers [33] and Borisjuk and co-workers [34] and in A. thaliana by Ziegler and co-workers [35]. Preliminary experiments have been set up in the Laboratory of Tropical Crop Improvement of K.U.Leuven (Belgium) to test the efficiency of signal peptides in the transport of expressed transgenes in banana [36]. So in the lectin secretion strategy, localised expression in root tissue is not necessary, and nematode infection is hampered before or at the moment of contact with the root. Moreover, this defence mechanism could also be effective for controlling the infective stage of sedentary nematodes, namely the juveniles, which are trying to locate suitable root cells to settle in and become sedentary.
1.3. Test of the hypothesis
What needs to be tested is the hypothesis that the binding of lectins to the sensory organs of nematodes, the amphids, or their secretions can inhibit chemoreception of host signals, and in this way disturb the localisation of hosts by nematodes. Moreover, the binding of lectins to the surface of nematodes could limit their movement or could be toxic. In this paper, preliminary results of lectin binding to the banana-parasitic nematode R. similis are presented, to confirm that there is indeed an interaction between lectins and this nematode species. Additionally, results are shown of attempts to stain and visualise secretions of R. similis. The technique can then, in a later phase, be applied to test the effect of lectin binding on the production of secretions by the nematode.
2. MATERIALS AND METHODS
2.1. Nematodes
A Radopholus similis population isolated from banana roots in Uganda was obtained from monoxenic alfalfa callus cultures [37] for the lectin-binding experiments and the staining of nematode secretions. Under sterile conditions, nematodes were extracted from callus medium by a modified Baermann funnel [38] and collected in siliconised Eppendorf tubes.
2.2. Lectin-binding assay
The fluorescein isothiocyanate (FITC)-conjugated lectin of Canavalia ensiformis (Con A) was obtained from Sigma, as well as the colloidal gold-conjugated lectins of Triticum aestivum (WGA) (10 nm gold particles) and Helix pomatia (HPA) (5 nm gold particles). Nematodes were washed twice in Tris-buffer, pH 8 (15 mM Tris-HCl, 50 mM NaCl, 1 mM CaCl2 and 1 mM MnCl2), concentrated to 100 µl and incubated in 200 µl of Con A-FITC, dissolved in the same Tris-buffer at 500 µg/ml. After 2 h in the dark at 4°C, nematodes were held at 26°C for 0.5 h, washed extensively with Tris-buffer on a 25 µm-pore sieve and collected for analysis. Treated nematodes were mounted on glass microscope slides and observed under a Nikon fluorescence microscope with an excitation wavelength of 450-490 nm and a barrier filter of 515 nm. For the binding experiment with gold-conjugated WGA and HPA, nematodes were washed twice in phosphate-buffered saline (PBS), pH 6.8, with 0.1 mM CaCl2, 0.1 mM MnCl2, 0.05% Tween-20 and 0.5% albumin (PBSAT). WGA and HPA were added to the nematode suspension at a dilution of 1:20 and incubated in the dark at room temperature for 4 h. Nematodes were washed with PBSAT on a 5 µm pore sieve, followed by an extensive rinse with distilled water. The surface of the sieve was dried and silver enhancement of the gold particles was applied (BB International). Nematodes were then collected with distilled water and mounted on glass microscope slides for observation under a Nikon light microscope. The control treatment for both experiments consisted of an incubation of R. similis in the appropriate buffer without lectins but with otherwise identical handling of the samples.
2.3. Staining of nematode secretions
Secretions of R. similis juveniles, females and males were stained according to the method of Premachandran and co-workers [39]. Briefly, nematodes were concentrated to 100 µl in a siliconised Eppendorf tube and 200 µl of a staining solution containing Coomassie Brilliant Blue R (CBB-R) (ACROS) was added. A drop of the suspension was put on a glass microscope slide under a coverslip and then sealed with clear nail polish. Samples were incubated in the dark at room temperature for 24 h, followed by observation and analysis under a Nikon light microscope.
3. RESULTS
3.1. Binding experiment
Radopholus similis juveniles, females and males showed autofluorescence (FITC-filter) of the muscles in the head region, the muscles of the spicules, the oesophageal glands, and to some extent in the anterior part of the body. The pale yellow-green colour was, however, clearly distinguishable from the bright green colour of FITC-Con A. This lectin was found to bind to R. similis in the head region, but the principal lectin-binding sites were the pores or the secretions of the vulva and the spicules. Labelling of the excretion pore and the phasmids or their secretions was less frequent (Table 1).
Table 1 Binding of labelled lectins Con A, WGA and HPA to the head, excretion pore, vulva, spicules and phasmids of R. similis
|
Binding sitea |
FITC-Con A |
WGA-gold |
HPA-gold |
|
Excretion pore |
+/- |
+/- |
+/- |
|
Vulva |
++ |
++ |
++ |
|
Spicules |
++ |
- |
+ |
|
Phasmids |
+/- |
+/- |
+/- |
++, strong binding; +, intermediate binding; +/-, less frequent binding; -, no binding
a includes secretions
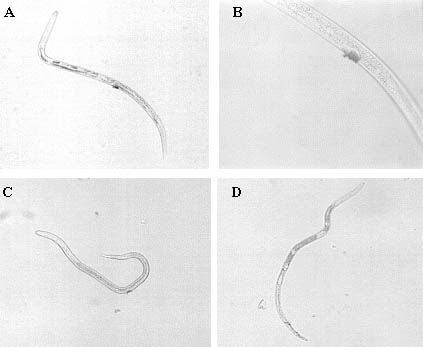
WGA and HPA, conjugated to colloidal gold particles, showed most extensive binding at the vulva of R. similis females (Figure 1A-C). The spicules or their secretions were also stained when treated with HPA (Figure 1D). Less frequent was the binding of WGA and HPA at the excretion pore, the phasmids and the pores of the amphids (Table 1). In general, the resolution at 800 × magnification was not sufficient to make more detailed observations for Con A, WGA and HPA. Binding of these lectins to the pores or to the secretions of certain organs could not be distinguished.
Figure 1 Binding of gold-conjugated lectins, WGA (A and B) and HPA (C and D), visible after silver enhancement, to R. similis at the vulva (A, B and C) and the spicules (D). (Scale bar = 25 µm)
3.2. Staining of secretions
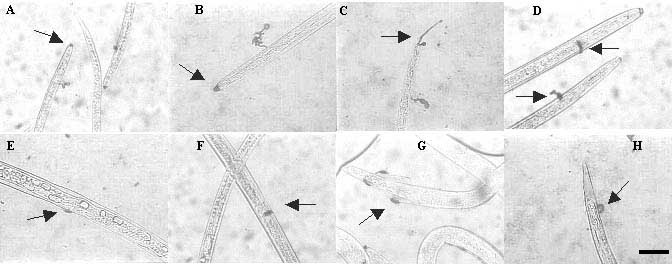
In preliminary tests different solutions of CBB-R were tested: (a) 1% CBB-R dissolved in water, (b) 0.2% CBB-R dissolved in 20% methanol, (c) 0.2% CBB-R dissolved in 40% methanol and 10% acetic acid, and (d) 0.1% CBB-R dissolved in 40% ethanol and 10% acetic acid. Solutions (c) and (d) gave clear and reproducible staining of the secretions of R. similis. It was decided to proceed with solution (c). The secretions in the head region of R. similis juveniles, females and males were stained as one big spot or as two distinct rays. These represent the secretions of the amphids, as was shown with the same staining solution for H. schachtii [40]. The secretions of the excretory pore were also visible as one big spot or as a thin ray. Additionally, secretions were stained at the vulva, the spicules and the phasmids (Figure 2).
Figure 2 Staining of the secretions of R. similis with 0.2% CBB-R in 40% methanol and 10% acetic acid, at the head (A and B) and more specifically the amphids (C), the excretory pore (D), the vulva (E and F), the spicules (G), and the phasmids (H). (Scale bar = 20 µm for all pictures)
4. DISCUSSION
Lectins are generally used in nematology to identify sugar moieties on the surface or in secretions of nematodes. For the past twenty years, research has been focused on the free-living nematode C. elegans and the sedentary endoparasitic nematodes of plants, Meloidogyne spp., Globodera spp. and Heterodera spp. Only Robertson and co-workers [24] included migratory nematodes, such as R. similis, in lectin-binding experiments. They observed no binding of Con A to R. similis, in contrast to the results presented in this paper. WGA binds to the amphids or the head region in general, and to the excretion pore. Robertson and co-workers [24] do not mention the vulva and the spicules, which were clearly stained in the present experiments. The reason for lectin binding in the region of the vulva is not clear. On the other hand, Aumann and Wyss [21] mentioned the binding of Con A, WGA and HPA to the spicule pores of H. schachtii. Coomans and De Grisse [41] described the spicules as chemoreceptors that produce secretions that can be labelled by lectins. These chemoreceptors are, however, only used to test the surroundings of the vagina. In long-distance migration towards a female nematode, sex pheromones are detected by the amphids.
According to Robertson and co-workers [24], the binding of WGA to the amphids, the amphidial secretions and the excretion pore of R. similis is specific. This means that the binding is inhibited by N-acetylglucosamine oligomers. In their experiments, non-specific binding of Con A and WGA at the vulva and anus of Longidorus spp. was observed. Davis and co-workers [22] also report that the binding of Con A and WGA to the amphidial secretions of secondary juveniles of Meloidogyne is not inhibited by the specific sugars of either lectin. Con A and WGA have a broader sugar specificity, or their affinity for sugars in nematode secretions is too strong to be inhibited. A third explanation is the affinity of certain lectins for other ligands than sugars. Lectins from the Leguminosae, like Con A, contain a conserved hydrophobic binding site, and WGA and HPA can also bind hydrophobic ligands [42]. So non-specific interactions between lectins, cell surfaces and secretions can occur. The binding of Con A, WGA and HPA to the vulva, spicules and other body pores of R. similis that was observed in the present experiments is possibly non-specific, and needs to be examined by including the appropriate inhibitory sugars in the treatments, as described by Jansson and co-workers [20]. A hydrophobic ligand could also be included: all three lectins show affinity for 1,8-anilinonaphthalene sulphonic acid [42].
Secretions of plant parasitic nematodes have many functions. In sedentary species they take part in the infection of roots and the induction and maintenance of feeding structures [43]. Amphidial secretions wash and protect the dendrites of the sensillae and are believed to be responsible for the capture of chemotactic compounds and their transport to the membranes of the sensillae [30,31]. The secretory system is often thought to be involved in the production of the glycocalyx. This surface layer is a lubricant when nematodes move through the soil, and supplies the nematode body with recognition domains, important in the interactions between nematodes and parasitic micro-organisms and between nematodes and host plants. Moreover, the glycocalyx is regularly renewed to avoid recognition and the induction of resistance in the host plant [44]. In the experiments with CBB-R, only localised staining at the different body pores was observed, instead of all over the body surface. These findings confirm the doubts of Spiegel and McClure [45] and Spiegel and co-workers [46] about the involvement of the secretory system in the secretion of the glycocalyx.
The binding experiments show that different types of lectins interact with the surface of the migratory nematode species R. similis. Potentially, these lectins or others could have a toxic effect or inhibit chemotaxis. However, an important problem with transgenic plants is the effect of the expressed proteins, lectins in this case, on non-target organisms that feed on or live in the surroundings of the plants. The lectin of Phaseolus vulgaris, for example, is toxic to human and animal consumers of raw beans [47]. The insecticidal properties of lectins have long been acknowledged and are being tested through the use of transgenic plants. Galanthus nivalis lectin (GNA) is moderately active against chewing insects and highly toxic for sucking insects, like aphids, in tests with artificial diets, but also in experiments with transgenic plants. The effect of these plants might not be limited to the targeted insect or nematode pest; the consequences might spread over the food chain. It has been shown that the fecundity and the survival of eggs of ladybirds decrease if they feed on aphids which themselves have fed on transgenic potatoes expressing GNA [48,49]. Another important aspect is the effect of lectins on non-target soil organisms and the processes in which they are involved, like nitrification and decomposition of organic matter. Griffiths and co-workers [49] have done tests with transgenic potatoes expressing GNA and Con A. Both in pot and field experiments, no changes in the decomposition of organic matter or negative consequences for plants in the following growing season were observed. As for bacteria and fungi, the cell wall hampers any interaction of lectins with glycoconjugates on the cell membrane as well as the entrance of lectins into the cytoplasm. Through an indirect mechanism, such as an interaction with sugar moieties of the cell wall or extracellular glycans, lectins might have an influence on the mobility of bacteria or cell-wall synthesis in fungi in the case of chitin-binding plant lectins [18]. Lectins are thus potential anti-nematode proteins whose impact on the environment should nevertheless be thoroughly studied.
REFERENCES
[1] DE WAELE, D., "Potential of gene transfer for engineering resistance against nematode attack", Proceedings of the workshop on biotechnology applications for banana and plantain improvement, San José, Costa Rica, 27-31 January 1992, INIBAP, Montpellier (1993) 116-124.
[2] SIJMONS, P.C., Plant-nematode interactions, Plant Mol. Biol. 23 (1993) 917-931.
[3] CAI, D., et al., Positional cloning of a gene for nematode resistance in sugar beet, Science 275 (1997) 832-834.
[4] JUNG, C., WYSS, U., New approaches to control plant parasitic nematodes, Appl. Microbiol. Biotechnol. 31 (1999) 439-446.
[5] BONANTS, P.J.M., et al., A basic serine protease from Paecilomyces lilacinus with biological activity against Meloidogyne hapla eggs, Microbiology 141 (1995) 775-784.
[6] BURROWS, P.R., DE WAELE, D., "Engineered resistance against plant parasitic nematodes using anti-nematode genes", Cellular and Molecular Aspects of Plant-Nematode Interactions, (FENOLL, C., et al., Eds), Kluwer Academic Publishers, Dordrecht (1997) 217-236.
[7] ATKINSON, H.J., et al., "Novel plant defences against nematodes", Advances in Molecular Plant Nematology, (LAMBERTI, F., et al., Eds), Plenum Press, London (1994) 197-210.
[8] URWIN, P.E., et al., Engineered oryzacystatin-I expressed in transgenic hairy roots confers resistance to Globodera pallida, Plant J. 8 (1995) 121-131.
[9] ATKINSON, H.J., et al., Image analysis of the growth of Globodera pallida and Meloidogyne incognita on transgenic tomato roots expressing cystatins, J. Nematol. 28 (1996) 209-215.
[10] URWIN, P.E., et al., Resistance to both cyst and root-knot nematodes conferred by transgenic Arabidopsis expressing a modified plant cystatin, Plant J. 12 (1997) 455-461.
[11] URWIN, P.E., et al., Transgenic resistance to the nematode Rotylenchulus reniformis conferred by Arabidopsis thaliana plants expressing proteinase inhibitors, Mol. Breed. 6 (2000) 257-264.
[12] BURROWS, P.R., JONES, M.G.K., "Cellular and molecular approaches to the control of plant parasitic nematodes", Plant Parasitic Nematodes in Temperate Agriculture, (EVANS, K., et al., Eds), CAB International, Wallingford, UK (1993) 609-630.
[13] OHL, S.A., et al., "Anti-feeding structure approaches to nematode resistance", Cellular and Molecular Aspects of Plant-Nematode Interactions, (FENOLL, C., et al., Eds), Kluwer Academic Publishers, Dordrecht (1997) 250-261.
[14] BAUM, T.J., et al., Expression in tobacco of a functional monoclonal antibody specific to stylet secretions of the root-knot nematode, MPMI 9 (1996) 382-387.
[15] STEWART, G.R., et al., A glycoprotein specific to the amphids of Meloidogyne species, Parasitology 106 (1993) 405-412.
[16] STEWART, G.R., et al., Studies on the amphid specific glycoprotein gp32 in different life-cycle stages of Meloidogyne species, Parasitology 107 (1993) 573-578.
[17] SIJMONS, P.C., et al., Arabidopsis thaliana as a new model host for plant-parasitic nematodes, Plant J. 1 (1991) 245-254.
[18] PEUMANS, W.J., VAN DAMME, E.J.M., Lectins as plant defense proteins, Plant Physiol. 109 (1995) 347-352.
[19] McCLURE, M.A., ZUCKERMAN, B.M., Localization of cuticular binding sites of concanavalin A on Caenorhabditis elegans and Meloidogyne incognita, J. Nematol. 14 (1982) 39-44.
[20] JANSSON, H.B., et al., Fluorescent and ferritin labelling of cuticle surface carbohydrates of Caenorhabditis elegans and Panagrellus redivivus, J. Nematol. 18 (1986) 570-574.
[21] AUMANN, J., WYSS, U., Lectin binding sites on mobile stages of Heterodera schachtii Schmidt (Nematoda: Heteroderidae), Nematologica 33 (1987) 410-418.
[22] DAVIS, E.L., et al., Characterization of carbohydrates on the surface of second-stage juveniles of Meloidogyne spp., J. Nematol. 20 (1988) 609-619.
[23] FORREST, J.M.S., et al., Changes in the structure of amphidial exudates and the nature of lectin labelling on freshly hatched, invaded and emigrant second stage juveniles of Globodera rostochiensis, Nematologica 34 (1988) 422-431.
[24] ROBERTSON, M.W., et al., Surface carbohydrates of plant parasitic nematodes, Nematologica 35 (1989) 180-186.
[25] AUMANN, J., et al., Lectin binding to cuticle exudates of sedentary Heterodera schachtii (Nematoda: Heteroderidae) second stage juveniles, Revue Nématol. 14 (1991) 113-118.
[26] BURROWS, P.R., et al., Plant-derived enzyme inhibitors and lectins for resistance against plant-parasitic nematodes in transgenic crops, Pest. Sci. 52 (1998) 176-183.
[27] MARBAN-MENDOZA, N., et al., Control of root-knot nematodes on tomato by lectins, J. Nematol. 19 (1987) 331-335.
[28] PERRY, R.N., "Plant signals in nematode hatching and attraction". Cellular and Molecular Aspects of Plant-Nematode Interactions, (FENOLL, C., et al., Eds), Kluwer Academic Publishers, Dordrecht (1997) 38-50.
[29] WRIGHT, K.A., "Nematode sense organs", Nematodes as Biological Models. Vol. 2, Aging and other Model Systems, (ZUCKERMAN, B.M., Ed.), Academic Press, London (1980) 237-295.
[30] ZUCKERMAN, B.M., Hypotheses and possibilities of intervention in nematode chemoresponses, J. Nematol. 15 (1983) 173-182.
[31] ZUCKERMAN, B.M., JANSSON, H.-B., Nematode chemotaxis and possible mechanisms of host/prey recognition, Annu. Rev. Phytopathol. 22 (1984) 95-113.
[32] FORREST, J.M.S., et al., A possible role for the amphids of potato cyst nematode Globodera rostochiensis in host finding, Nematologica 34 (1988) 173-181.
[33] HERBERS, K., et al., A thermostable xylanase from Clostridium thermocellum expressed at high levels in the apoplast of transgenic tobacco has no detrimental effects and is easily purified, BioTechnology 13 (1995) 63-66.
[34] BORISJUK, N.V., et al., Production of recombinant proteins in plant root exudates, Nature Biotechnol. 17 (1999) 466-469.
[35] ZIEGLER, M.T., et al., Accumulation of a thermostable endo-1,4-b-D-glucanase in the apoplast of Arabidopsis thaliana leaves, Mol. Breed. 6 (2000) 37-46.
[36] WUYTS, N., Components of a novel strategy to control migratory nematodes with transgenic plants, MSc Thesis, Catholic University of Leuven (2001).
[37] ELSEN, A., et al., Aseptic culture systems of Radopholus similis for in vitro assays on Musa spp. and Arabidopsis thaliana, J. Nematol. 33 (2001) 147-151.
[38] HOOPER, D.J., "Extraction and processing of plant and soil nematodes", Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, (LUC, M., et al., Eds), CAB International, Wallingford, Oxon (1990) 45-68.
[39] PREMACHANDRAN, D., et al., A method for staining nematode secretions and structures, J. Nematol. 20 (1988) 70-78.
[40] AUMANN, J., WYSS, U., Histochemical studies on exudates of Heterodera schachtii (Nematoda: Heteroderidae) males, Revue Nématol. 12 (1989) 309-315.
[41] COOMANS, A., DE GRISSE, A., "Sensory structures", Plant Parasitic Nematodes, vol. 3, (ZUCKERMAN, B.M., ROHDE, R.A., Eds), Academic Press, London (1981) 127-174.
[42] ROBERTS, D.D., GOLDSTEIN, I.J., Binding of hydrophobic ligands to plant lectins: titration with arylaminonaphtalenesulfonates, Arch. Biochem. Biophys., 224 (1983) 479-484.
[43] BIRD, A.F., Plant response to root-knot nematode, Annu. Rev. Phytopathol. 12 (1975) 69-85.
[44] BIRD, A.F., et al., A role for the 'excretory' system in secernentean nematodes, J. Nematol. 20 (1988) 493-496.
[45] SPIEGEL, Y., McCLURE, M.A., The surface coat of plant-parasitic nematodes: chemical composition, origin and biological role - a review, J. Nematol. 27 (1995) 127-134.
[46] SPIEGEL, Y., et al., Meloidogyne javanica surface proteins: characterisation and lability, Parasitology 115 (1997) 513-519.
[47] PUSZTAI, A., et al., The relationship between survival and binding of plant lectins during small intestine passage and their effectiveness as growth factors, Digestion 46 (1990) 308-316.
[48] BIRCH, A.N.E., et al., Ecological impact on predatory 2-spot ladybirds of transgenic potatoes expressing snowdrop lectin for aphid resistance, Mol. Breed. 5 (1999) 75-83.
[49] GRIFFITHS, B. S., et al., Testing genetically engineered potato, producing the lectins GNA and Con A, on non-target soil organisms and processes, J. Appl. Ecol. 37 (2000) 159-170.
| [37] Laboratory of Tropical
Crop Improvement and Laboratory of Phytopathology and Plant Protection Catholic University of Leuven Kasteelpark Arenberg 13 B-3001 Leuven Belgium |