Abstract
Banana and plantains are important fruit crops in tropical and subtropical countries, and provide employment, nutrition, and food security. Tremendous progress has been made in the genetic improvement of Musa in recent years, and new varieties are now becoming available from breeding programmes. There is still further scope for improvement of banana against disease resistance, abiotic resistance, and other agronomical traits. Since breeding is time-consuming, induced mutations and biotechnology would be an ideal approach to improve banana and plantains in an environmentally friendly way. International collaboration is very much needed for germplasm exchange, e.g. the Global Programme for Musa Improvement (PROMUSA). It is a broadly based programme and was developed as a means to link the work carried out to address the problems of export banana producers, with those initiatives directed towards improving banana and plantain production at the subsistence and smallholder level. There are several working groups of PROMUSA for banana genetic improvement under two major programmes: (a) genetic improvement and (b) pest and disease research. It is highly desirable that the farmers accept newly developed banana varieties and minimise the use of chemical pesticides. In the future, banana research will need more attention for the improvement of nutrition, genomics, genetic mapping, low cost micropropagation, and somatic embryogenesis.
1. INTRODUCTION
Banana and plantains are a most important food crop, which rank fourth in the world. They are grown in more than 100 countries, with an annual production around 88 million metric tones; are cheap to produce, and can grow in a range of environments and produce fruit year-round; and are used both as a staple food (cooking banana) and dietary supplements (dessert banana). Banana fruit production is currently threatened by several diseases and pests, including bunchy top virus, burrowing nematodes (Radopholus similes), Moko disease (Ralstonia solanacaearum), black Sigatoka or black leaf streak (Mycospharella fijiensis), and Fusarium wilt (Fusarium oxysporum f sp. cubense) [1]. The breeding programme for bananas, especially edible bananas, is hampered by high sterility, triploidy and seedlessness. Few diploid banana clones produce viable pollen, and the germplasm of commercial banana clones is both male- and female-sterile [2]. However, in spite of this undesirable situation, important progress has been made in the genetic improvement of Musa in recent years, and new varieties are now becoming available from breeding programmes. There is great potential to improve sustainable banana yield by applying biotechnology [3], which could become an additional useful tool for plant breeders to ensure food security by stabilizing sustainable crop production and improving the socio-economic status of the growers. Large-scale multiplication of banana plants has been done by direct organogenesis or micropropagation, and is used by commercial companies for multiplication and transport of elite banana germplasm. The recovery of transgenic banana plants has been reported by several groups [4-7], although a commercial transgenic banana variety has yet to be released.
1.1. Breeding programmes
Major breeding programmes that use conventional breeding methodologies are located at the Fundacion Hondurena de Investigacion Agricola (FHIA) in Honduras, the Centre de Coopération Internationale en Recherche Agronomique pour le Développement (CIRAD-FLHOR) in France and Guadeloupe, the International Institute of Tropical Agriculture (IITA) in Nigeria and Uganda, the Centre de Recherches Régionales sur Bananier et Plantain (CARBAP) in Cameroon, and the Empresa Brasiliera de Pesquisa Agropecuaria (EMBRAPA) in Brazil.
The first breeding strategy developed by FHIA, and now adopted by other programs, relies on the production of improved diploids. The diploids possess useful resistance characteristics introduced from wild sources in an improved genetic background, and are then used as male parents in crosses with the desired triploid as female parent. The improved diploids developed by FHIA are now being used for the improvement of many different types of bananas, including dessert varieties, plantains, cooking bananas, and East African highland bananas. The main constraints of this breeding strategy are: (a) the low fertility rate of the desired female parents, and (b) the possibility of residual fertility in the tetraploid progeny could lead to seed-bearing fruit under certain circumstances. To overcome this latter constraint, efforts are now being focused on the production of secondary triploids.
A different breeding strategy for the production of improved triploids is being developed by CIRAD-FLHOR and CARBAP. This is based on colchicine treatment for doubling the chromosome number of desired diploids, which are then used in tetraploid × diploid crosses for the production of triploids. However, despite natural difficulties in breeding bananas, the main constraint in the distribution of improved varieties is the Banana Streak Virus (BSV). Certain hybrids (particularly plantain hybrids) from FHIA, IITA, CARBAP and CIRAD have already been found positive for this virus, and are therefore not available for distribution. There is growing evidence that integrated BSV sequences could be the source of episomal infectious BSV particles in a wide range of Musa genotypes, including most hybrids that have been bred for disease resistance, improved yield, or fruit quality. It was therefore proposed that episomal viral genomes could arise from integrated BSV sequences through a complex recombination pattern [14]. So far, all screened interspecific hybrids harbouring the B genome contained integrated BSV sequences which are readily activated. Therefore, high priority has been given to all research activities related to deeper understanding of the mechanisms involved in BSV integration and expression.
There is growing interest in Musa improvement not only in Cavendish but also in other major groups of bananas and plantains. However, considering the global importance of the crop, the number of existing Musa breeding programmes still remains small. Despite this, research is progressing well both in conventional breeding and biotechnology. Banana breeders could increase breeding efficiency by exploiting technologies such as induced mutations, tissue culture, somatic embryogenesis, organogenesis, somaclonal variation, and embryo rescue. Furthermore, research is advancing rapidly in the use of molecular markers as a breeding tool, and the molecular characterisation of germplasm.
1.2. Biotechnology programmes
Biotechnology has taken great strides in crop improvement. Plant cell and tissue culture has contributed to plant regeneration of banana through somatic embryogenesis [8], micropropagation [9-11], and recently from protoplasts [12]. Mass propagation of banana has been achieved using different explants, such as meristems, rhizomes, inflorescences, zygotic embryos, etc. [3]. The cost of production of micropropagated banana plants has become high due to factors such as sugar, agar, electricity, and labour [11]. Consequently, small farmers in the developing countries are unable to purchase costly micropropagated banana plants. Plants have been regenerated from banana somatic embryos using explants such as meristems, rhizome tissues, leaf bases, immature zygotic embryos, and young male flowers of diploid and triploid banana genotypes [3].
Genetic transformation of banana has been accomplished by using techniques such as particle bombardment, Agrobacterium-mediated transformation, and electroporation [3]. May et al [13] transformed banana by Agrobacterium-mediated transformation and obtained transgenic plants, identified phenotypically and with molecular tools. They succeeded in the recovery of putative banana (var. Grande Naine) transformants within 4 weeks of cocultivation of explants with Agrobacterium by using kanamycin as the selective agent for the npt-II gene. Recently, Ganapathi et al. [4] used the Agrobacterium-mediated transformation system for embryogenic cell suspension cultures from in vitro-grown thin-shoot sections of the banana cultivar Rasthali (AAB). Two hundred putative transformants were recovered, and 16 of them expressed the GUS gene, as determined histochemically. Two of them were grown to maturity under greenhouse conditions, and their fruit tested positive for GUS expression. Sagi et al. [5] transformed somatic embryogenic cell suspension cultures of banana using the particle bombardment method; the cells showed high transient expression of the b-glucuronidase (GUS) reporter gene both in banana and plantain. It is now therefore possible to transform several banana tissues for transient GUS gene expression, including somatic embryos (originating from embryogenic cell suspension, ECS), ECS (originating from male flowers, scalps) and protoplasts (ECS) [3].
1.3. Future prospects
New generations of products are expected to contribute to an increased production of bananas in an environmentally friendly way. Both breeding programmes and recombinant DNA strategies require detailed knowledge of the genetics and genomics of the bananas. Therefore the quality of the products and their availability to farmers will depend on both the improvement strategy and the progress made on genetics and genomics.
Different types of products can be distinguished: tetraploid and triploid hybrids with resistance to pests and diseases; and released varieties in which resistance or tolerance to pests and diseases have been enhanced using foreign genes or banana-derived genes. However, hybrids are only another step waiting for the expansion of knowledge required to reconstruct the actual triploid varieties. The development and implementation of different biotechnology tools could allow the cultivars to be reconstructed with new resistance to the main pests and diseases.
Micropropagation is a highly labour-intensive technology for large-scale plant production. Beside high labour costs, other parameters such as costly chemicals, electricity, etc. raise the cost of in vitro multiplied plantlets. Efforts should be made to develop a reliable low cost technology without affecting the rate of plant multiplication.
There is a recognition of a need to introduce a wider genetic base to breeding programmes, as well as a need for a better understanding of the Musa genome both at the molecular and karyological levels. Efforts are ongoing to address these issues, particularly in the framework of PROMUSA. In this respect, a Musa genomics consortium has been formed to enhance collaboration in genomics research. Genetic mapping is already in progress, using molecular techniques such as restriction fragment length polymorphisms (RFLPs), amplified fragment length polymorphisms (AFLPs), and microsatellite markers. Complementary research is being carried out to establish a physical map of the Musa genome. Several segregating populations are being established in support of this work. These efforts will allow the identification of specific genes of interest (pest and disease resistance). This, together with improved techniques that are now available for classical breeding and genetic transformation, will increase the production of improved varieties much more quickly.
A mechanism for collaboration and information exchange between researchers involved in Musa genetic improvement has been put in place in the form of PROMUSA, the Global Programme for Musa Improvement. This has allowed the global prioritisation of research needs, and the acceleration of progress through the formation of synergistic partnerships.
2. THE GLOBAL PROGRAMME FOR MUSA IMPROVEMENT (PROMUSA)
The Global Programme for Musa Improvement (PROMUSA) is a broad-based programme, aiming to involve all major players in Musa improvement, and act as a resource to link the groups addressing the problems of export banana producers with initiatives directed towards improving banana and plantain production at the subsistence and smallholder level.
The global programme builds upon existing achievements and ongoing research initiatives, and pays attention especially to genetic improvement and supportive research, and priority is given to research which has a global or regional significance. PROMUSA is therefore a mechanism to maximise the outputs and accelerate the impact of the overall Musa improvement efforts. The program is an innovative mechanism to bring together research carried out both within and outside the Consultative Groups on International Agricultural Research (CGIAR), creating new partnerships between National Agricultural Research Systems (NARS) and research institutes in both developing and developed countries. Such partnerships also strengthen the capacity of NARS for conducting Musa-related research.
PROMUSA operates as a consortium, and relies on various funding mechanisms. Decisions are made on the basis of scientific priorities identified by program participants and on users' needs. The global and regional Musa evaluation programme plays a major role in this regard, providing a mechanism for the two-way exchange of information between NARS and research and breeding programmes. The provision of feedback information regarding farmers' needs is of particular importance in setting research priorities. The existing regional banana research networks also provide a useful channel through which information from national programs is fed back to the global programme. The major thrust of PROMUSA is to develop a wide range of new banana hybrids suitable for production by banana growers worldwide. The programme brings together conventional breeding based on hybridisation techniques, and genetic engineering and biotechnological breeding approaches. This broad-based genetic improvement effort is supported by research that is carried out on specific pests and diseases within the various PROMUSA working groups. An efficient mechanism for evaluating new varieties produced within the framework of PROMUSA is also an essential component of the programme.
2.1. Program structure
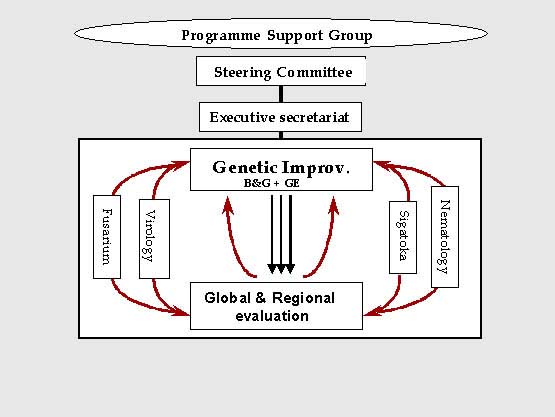
PROMUSA's organisational structure is simple and efficient to ensure the maintenance of maximum support for research activities (Figure 1). The Programme Support Group provides visibility, guidance and support to the programme. It endorses the overall direction and strategy of the program and contributes to identifying and providing additional funding and other resources as necessary. The Steering Committee is responsible for proposing direction and providing oversight to the programme. It sets priorities based on technical advice from the working groups compiled by the Executive Secretariat, and advises donors on the allocation of resources to the programme. The Steering Committee also approves the programme strategy, medium term plan, and annual work plan. It commissions reviews of the programme, advocates on behalf of the programme, and seeks external technical advice as appropriate. The Executive Secretariat is provided by INIBAP. It serves as the programme coordinator and is responsible for ensuring the smooth running of the programme as well as providing a programme secretariat. It also facilitates the organisation of technical meetings, both thematic and interdisciplinary, and disseminates information to programme partners. It prepares reports and compiles lists of priorities, based on technical advice provided by the thematic working groups. Internal communication is a particularly important aspect of the programme, and the Executive Secretariat plays a critical role in stimulating contacts between groups. Regular inter-group information exchange is ensured through a programme newsletter, which is either published separately or included as a 'ProMusa' section in an existing newsletter. The Executive Secretariat also has an important role in providing feedback to the programme and ensuring a link with the end-users. The Executive Secretariat can also, if required, play the role of executing agency for funding provided to the programme.
2.1.1. Thematic working groups
Programme activities are carried out through a series of thematic working groups, which allow continual interaction between group members. Interdisciplinary contact also occurs at regular intervals through meetings at the program level, and on a continuing basis through the program secretariat. The working groups are open to all interested parties to encourage new ideas and interests within the working groups. Therefore, they have two levels of participation: (a) the discussion group, which comprises all the members of the group who exchange views and information; and (b) the core group which consists of members specifically working on the priority research needs which they have identified, and who should be able to participate in collaborative projects and to develop new proposals; it includes the maximum number of institutes both from the north and the south; and ensures good representation of all participants, including National Agricultural Research Systems (NARS), Advanced Research Institutes (ARIs), International Agricultural Research Centres (IARCs), and Private Sector.
PROMUSA partners benefit from the global prioritisation of research needs, and participation improves opportunities for funding because of the recognition of the program by donor agencies. Participants may develop close interactions with other research groups within their area of specialisation, and increase opportunities for interdependent research projects.
2.2. Research strategy and priorities
The research strategy of PROMUSA is to produce improved farmer-accepted Musa varieties through the development and application of conventional and biotechnological breeding approaches, incorporating resistance to pests and diseases to increase productivity and minimise pesticide use, and close collaborative partnership.
The major objectives of PROMUSA are to:
(a) increase the sustainable banana and plantain production for both local and export markets,
(b) foster the development of improved Musa varieties with a wide genetic base and consumer acceptability, and disseminate these varieties to farmers through participating National Agricultural Research Systems (NARS),
(c) facilitate and stimulate partnerships among NARS, ARIs, and IARCs to increase the efficiency and cost-effectiveness of global Musa improvement.
Research activities are developed in collaboration with different working groups and their thematic research can be changed according to the priorities defined by the core group called the 'Genetic Improvement Working Group'. Research activities within PROMUSA are currently distributed among five different working groups: 'Genetic Improvement' as the core group, and 'Sigatoka WG', 'Nematode WG', 'Fusarium WG' and Virology WG' as additional working groups. A new initiative on weevils is now under consideration.
Figure 1 Structure of PROMUSA.
2.2.1. Genetic improvement
The PROMUSA genetic improvement working group (GIWG) defines the different needs and orientations of research needed to accelerate the development of new varieties resistant to pests and diseases; and collaborates with the other different working groups to refine their strategy.
Geneticists agree that, to enhance the utilisation of plant genetic resources by breeding programmes, it is important to have better access to existing Musa collections to obtain increased availability of natural germplasm. More efforts are needed for adequate characterisation of the germplasm, including the development of molecular tools. They should also express the importance of facilitating the exchange of breeding materials. Research into efficient breeding methodologies should also be developed to strengthen overall breeding efforts, in order to address national and regional needs better (genetically important traits, marker-assisted selection, multilocational evaluation of germplasm).
The genetic engineering subgroup also defines some of the research priorities that should be developed as a complementary approach to classical breeding. Significant progress has been made in the development of efficient transformation systems for Musa, and the technology is being transferred to various laboratories. However, different research activities are still very much needed to improve the development of genetically transformed bananas. This research should include the development of efficient tissue-culture systems that do not cause somaclonal variation. The focus should be on the development of somatic embryogenic cell suspension cultures for inducing mutations as well as for developing somatic embryos. A single cell origin of somatic embryogenic cultures would avoid chimerism in regenerated plants derived from material treated with physical and chemical mutagens, as well as plants obtained from genetic transformation work. Reliable constructs for transformation with strong promoters for transgene expression, including promoters from banana plants, are very necessary, as well as a molecular toolbox to control gene expression.
The phenomena of gene silencing and genome interaction may influence the characteristics of newly obtained material. Alternation of gene expression could be important to generate genetic variability in vitro, while gene silencing may alter the expression of transgenes.
The development of molecular tools such as genetic mapping, construction of Bi-BAC libraries, cloning genes of important traits, etc., are important problems to be to addressed through the genomics consortium on Musa, which was created in the framework of PROMUSA. It now comprises more than 26 different institutions. Research in Musa genomics has been identified as the main priority for the GIWG. It is expected that the information derived from Musa genomics will facilitate the development of new cultivars in different biological, ecological and cultural environments. Three main priority areas of Musa genomics have been identified:
(a) Development of Musa genetic maps. Already existing Musa genetic maps need to be saturated by international collaboration, by focusing on the development of Sequence Tagged Microsatellite Site (STMS) markers which are locus-specific, co-dominant and highly polymorphic.
(b) Development of physical maps and integrated genetics for sequencing. Different resources are required for physical mapping, including Bacterial Artificial Chromosomes (BAC) and complementary DNA (cDNA) libraries and Expressed Sequence Tags (ESTs).
(c) Identification and isolation of genes. Different approaches should be considered, such as map-based gene cloning, comparative genomics, differential screening, and gene tagging.
Chemical and insertional mutagenesis has also been designated as a useful technique that could be developed by the GIWG. Mutagenesis is considered an attractive tool to obtain plants with traits that are not available in nature. It is expected that mutagenesis will play an increasingly important role in Musa genome mapping projects, where it will be used to generate deletion stocks and knockout mutants.
2.2.2. Pest and disease research activities related to genetic improvement
The other PROMUSA working groups, such as the Sigatoka, Nematodes, Fusarium and Virology working groups have collaborated with breeders, geneticists and biotechnologists to orientate and define the different research activities required to create new varieties resistant to pests and diseases, as well as their distribution.
2.2.2.1. Sigatoka
The Sigatoka working group is mainly focused on the distribution and relative incidence of the different species involved in the different diseases grouped under the broader terminology of Sigatoka: Mycosphaerella fijiensis (black Sigatoka), Mycosphaerella musicola (yellow Sigatoka) and Mycosphaerella eumusae (Septoria). It is important to develop appropriate diagnostic tools to differentiate the pathogens responsible for Sigatoka. The development and implementation of molecular tools are already in progress, using restriction assay of PCR-amplified ITS regions of rDNA. They have prioritised (a) the development of methods to follow the changes in pathogen populations in response to selection pressure from the new resistant banana genotypes; (b) the mechanisms of resistance, especially partial resistance; and (c) improving the screening methods for evaluation of disease resistance in germplasm.
2.2.2.2. Fusarium wilt
The Fusarium working group (FWWG) has defined key issues in the management of Fusarium wilt. Examples of some current priorities that have been developed are pathogen diversity, disease management strategies, and epidemiology. The development of a standard screening test to evaluate the resistance to Fusarium wilt has been identified as the main priority to assist the different genetic improvement requirements. The availability of DNA-based identification of all races and strains of Fusarium oxysporum f.sp. cubense (FOC) directly from plant and soil is also a priority that needs to be addressed. Despite the development of the different tools mentioned above, the group agreed upon the importance of identifying additional resistance in landraces and hybrids. It would also be interesting to collate existing data for resistance of wild banana types, and to obtain a better understanding of the mechanisms of resistance to Fusarium wilt.
2.2.2.3. Nematodes
The nematode working group (NWG) arranged the different research priorities into three main lines: nematode communities, including biodiversity; damage and yield loss potential of populations; and resistance screening including methods, sources and mechanisms. Several aspects were identified and linked with genetic improvement and activities that could contribute towards genetic improvement. There are not enough resources of resistant material, and it has been suggested that the centre of origin of Musa in Southeast Asia should be examined for new sources of resistance. The development of molecular markers and any study on the genetic basis of the inheritability would first require the availability of segregating populations.
2.2.2.4. Virology
The PROMUSA virology working group has set up certain priorities for research involving genetic improvement. They stressed the importance of developing reliable diagnosis of BSV, using appropriate diagnostics by developing a better understanding of BSV diversity, and through education on the significance of the A and B genomes. The production of a PROMUSA pamphlet on current procedures for virus diagnosis has also been suggested.
2.2.2.5. Genomics
Research is needed to understand better the heterogeneity of the B genome, and the contribution of the A genome in activating virus integrants in advanced breeding lines. It is also crucial to identify the mechanism of silencing of BSV integrants in the genome. Research is also required to determine the geographical diversity of BSV vis-à-vis movement of germplasm, particularly with respect to epidemiological information and risk assessment. Virologists have also demonstrated the importance of developing resistance-screening methods, and also securing the supply of diagnostics.
REFERENCES
[1] JAIN, S.M., A review of induction of mutations in fruits of tropical and subtropical regions, Acta Hort. 575 (2002) 295-302.
[2] NOVAK, F.J., et al., Somatic embryogenesis and plant regeneration in suspension cultures of dessert (AA and AAA) and cooking (AAB) bananas (Musa spp.), BioTechnology 7 (1989) 147-158.
[3] ROUT, G.R. et al., Biotechnology of the banana: a review of recent progress, Plant Biol. 2 (2000) 512-524.
[4] GANAPATHI, T.R. et al., Agrobacterium-mediated transformation of embryogenic cell suspensions of the banana cultivar Rasthali (AAB), Plant Cell Rep. 20 (2001) 157-162.
[5] SAGI, L., et al., Genetic transformation of banana and plantain (Musa spp.) via particle bombardment, BioTechnology 13 (1995) 481-485.
[6] REMY, S., et al., Co-transformation as a potential tool to create multiple and durable resistance in banana (Musa spp.), Acta Hort. 461 (1998) 361-365.
[7] BECKER, D.K., et al., Genetic transformation of Cavendish banana (Musa spp. AAA group) cv. Grand Nain via microprojectile bombardment, Plant Cell Rep. 19 (2000) 229-234.
[8] GEORGET, F., et al., Morphohistological study of the different constituents of a banana (Musa AAA, cv. Grande naine) embryogenic cell suspension, Plant Cell Rep. 19 (2000) 748-754.
[9] MENDES, B.M.J., et al., A statistical approach to study the dynamics of micro-propagation rates, using banana (Musa spp.) as an example, Plant Cell Rep. 18 (1999) 967-971.
[10] ROUX, N., et al., Effectiveness of three micropropagation techniques to dissociate cytochimeras in Musa spp., Plant Cell Tiss. Org. Cult. 66 (2001) 189-197.
[11] KODYAM, A., et al., Low-cost alternatives for the micropropagation of banana, Plant Cell Tiss. Org. Cult. 66 (2001) 67-71.
[12] ASSANI, A. et al., Plant regeneration from protoplasts of dessert banana cv. Grande Naine (Musa spp., Cavendish sub-group AAA) via somatic embryogenesis, Plant Cell Rep. 20 (2001) 482-488.
[13] MAY, G.D. et al., Generation of transgenic banana (Musa acuminata) plants via Agrobacterium-mediated transformation, BioTechnology 13 (1995) 486-492.
[14] NDOWORA, T.C.R., LOCKHART, B.E.L., Development of a serological assay for detecting serologically diverse banana streak virus isolates, (First International Conference on banana and plantain for Africa, Kampala, Uganda. 14-18 October 1996), (CRAENEN, K., et al., Eds), Acta Hort. 540 (2000) 377-388.
| [50] INIBAP Parc Scientifique Agropolis II 34397 Montpellier cédex 5 France [51] International Atomic Energy Agency |