J.C. Waterlow and P.R. Payne
It has been accepted for many years that at any fixed level of N intake a change in the level of energy-yielding nutrients often brings about a marked change in N retention. There are numerous physiological and dietary factors which may modify the extent of this effect, for example the level of intake of both N and energy considered in relation to the age of the animal, i.e. its potential for growth, the state of nutrition or adaptation of the animal to its previous diet, and also to the relative proportions of the nutrients supplied. The literature abounds with data described in qualitative terms, and has been extensively reviewed by Munro (1,2).
We have made an attempt to fit at least some of the data into a unified scheme. In doing so it has been necessary to be selective, often rejecting experiments in which there was no clear demonstration of the identity of the physiological or dietary factor limiting N retention. The picture thus presented may be to some extent an oversimplification, but will perhaps lead to some general conclusions which will help in assessing practical nutritional problems.
Even when an adequate intake of protein is provided, energy intakes at or below the level equivalent to BMR usually result in negative N balance. Commencing from such low intakes, successive increments of energy give rise to proportional reductions in the rate of N loss, until a minimal calorie intake (CM in the diagram) is reached which will just support N equilibrium. If the animal is capable of growth the response line can be extended further into the region of positive balance, until a limit is reached beyond which the response to additional energy is quantitatively much smaller, as indicated by the reduced slope of the line (I1) on the diagram. At a higher level of N intake (I2) the line of steep response to calories is extended to a greater positive balance before the change of slope occurs. There are thus two types of N balance response to energy changes. One in which energy intake is the sole limiting factor, and in which the response is maximal, and another in which changes in N intake (e.g. I1 - I2) are effective, and in which the influence of small changes in energy is much reduced.
It is possible to select data from a number of experiments on growing and adult rats, and on adult men, to give values both for the slope of the calorie limited response line, and also for CM, the calorie intake which will just provide for maintenance of N equilibrium. Table 1 gives a number of values for the amount of N retained for each extra calorie provided. These range from 6 to 7 mg N/K calorie (i.e. 22 - 26 K cals/g protein) and are higher than many of those quoted by Munro (1) probably because only values were used in the calculations which were considered representative of the region of energy limited response.
Estimates such as these, of the N sparing effect of calories under energy limited conditions may be regarded as measures of the energy cost of growth, since the demands of all processes involved in growth will be included. Kielanowski (7) has measured separately the energy cost of deposition of protein and fat in growing pigs and gives values of 15.9 12.9 kcals/g respectively. If we assume that the minimum lipid content of tissues formed is between 0.4 to 1.0 g per g protein; then each gram of protein deposited would require between 21 and 29 kcals, so that Kielanowski's independent estimate is in good agreement with the N balance data. The need for 15.9 kcals/g of protein deposited (which includes the heat content of the protein itself) implies an energy cost of synthesis of between 10 and 11 kcals per gram. This figure while surprisingly high, is in accord with the calorie conversion figures actually achieved with fast growing animals. Moreover, Waterlow (8) in reviewing estimates of the rates of whole body turnover in animals of different sizes, commented that they were related to body weight in the same way as are fasting metabolic rates. His estimates of the ratio basal calories/g of protein turnover, range from 8 - 12 K cals/g. Thus an energy cost of synthesis of this order of magnitude will account for the greater part of the observed BMR. It is however difficult to account for more than about 10 to 20% of this amount of energy in terms of the requirement for formation of the ATP and GTP involved in peptide synthesis, and it must be admitted that we are a long way from an understanding in biochemical terms of the energy needs of the growing animal the only certainty being that a major part is associated with protein deposition.
The BMR can thus be regarded as the energy needed to sustain the re-utilization of amino N at a rate characteristic of a fasting subject. However, it is found experimentally that a somewhat greater amount of energy (CM on the diagram) is required to maintain N equilibrium. This is almost certainly due to the fact that at maintenance, protein must be synthesised sufficient to replace obligatory losses. Thus for example for an adult man having a BMR equivalent to 20 kcals/kg/day, the endogenous losses in urine and faeces amount to about 50 mg/day/kg. At between 6 and 7 mg N retained for calorie, this will require about 8 kcals, i.e. about a 50% increase above the basal level. Table 2 shows values for CM obtained for a number of species of animal. When expressed as calories per day per kg of metabolic body size, these are very similar, and are close to 1.5 times the interspecies average for basal metabolic rate (70 Kcals/kg3/4). The table also shows that the minimal amounts of energy found to be necessary for constant body energy content are also very similar.
These levels of intake apply to animals and to human subjects under very sedentary conditions and in a thermoneutral environment, and should therefore be regarded as the lower limit of adaptation to reduced intakes of energy.
In the region of the diagram to the right of CM, an increase of nitrogen intake for example from I1 to I2 at a fixed calorie intake gives rise to an increase in N balance, and a similar rise would result from a change in protein quality from a low to a higher value. However, for any fixed level of N intake changes in the energy level still exert some effect on N balance, so that the lines on the diagram corresponding to I1 and I2 are not horizontal. The N sparing effect in this region which is considerably less in magnitude than 6.8 mg/N/ calorie, has been described by Munro as the effect of surfeit calories. The magnitude of this depends upon both the quality of the protein and upon its proportion in the diet, increasing as both of these are raised. Under the dietary conditions likely to be found in infant feeding, values between 1 and 3 mg N per K calorie are theoretically possible, and it is interesting to note that Ashworth et al (12) showed that infants recovering from malnutrition on high intakes of protein gained weight in amounts equivalent to 2.1 mg N for each extra K calorie consumed even up to very high levels of energy intake.
Since it is the result of a change in the ratio of N to total calories fed, this may best be regarded as an indication of the adaptation of metabolism to an alteration of nutrient ratios in the diet, and is probably connected with the adaptive changes which have been observed in the levels of a number of liver enzymes known to be involved in the oxidative deamination of amino acids; for example Muramatsu and Ashida (13) and Harper (14) have shown that in many cases these are related to the level of protein in the diet previously fed. Thus the organism adapts in such a way that the proportion of energy derived from different substrates, is related to their relative proportions in the diet. The existence of the protein sparing effect suggests that even the amino acid which is most limiting in the diet is not immune from oxidation and is a further indication of the very high priority placed upon the satisfaction of energy demands. Another aspect of the same phenomenon has been described by many authors as an inverse relation between efficiency of N retention and protein level in the diet. For example with rats (15) - (20). With chicks (21,22) and in infants (23,24).
Hegsted and his associates (25) (26) have interpreted their studies of N retention in rats as indicating that the efficiency of utilization of dietary N is independent of the ratio of protein to energy in the diet, and hence by implication have denied the existence of a protein sparing effect of excess calories. However, the interpretation of their experiments is complicated by the fact that increases in ad libitum food consumption of young growing rats, tend to compensate for falling efficiency as the level of protein in test diets is increased thus resulting in an approximately linear dose response curve.
The existence of energy limited situations, in which the attainment of N equilibrium or of any degree of positive retention may be decided by the level of calorie intake rather than the amount or quality of protein fed, means that calorie intake must be taken into account in assessing the adequacy of diets to meet protein requirements. In other words it is necessary not merely to compare intakes of protein and of calories with respective requirement levels but also to establish which of the two is the limiting factor. For this we need estimates of minimum requirements, for both protein and calories; only then is it possible to assess the degree to which protein deficiency in a population is due primarily to a shortage of protein, and to what extent it is the result of caloric insufficiency.
As an example, at one year of age the calorie intake needed for maintenance of N would be 1.5 × BMR = 1.5 × 500 or 750 Kcals. If the growth component of the protein requirement is 2 g/day then the total calories needed will be 750 + (2 × 24) = 800 K cals/day. Similarly for the reference adult male, the minimum calorie intake for N maintenance would be 1.5 × 1500 = 2250 K cals/day.
In both cases, provision of adequate intakes of protein but with calorie supplies at less than these levels, would be ineffective. To the extent that these estimates of minimal energy needs are derived from animals and human subjects under controlled, and probably very sedentary conditions, they must be regarded as probably underestimating the effects of calorie restriction on protein utilization. It is known for example that the energy cost of maintenance of N is increased by exposure to cold environments, Payne and Jacob (27), as would also be the case for more than minimal amounts of physical activity.
Sukhatme (28) has emphasized the importance of comparing both protein and calorie intakes with minimal requirements in assessing the nature of a nutritional situation. In India and probably in many other parts of the world, such an analysis shows that supplementing the diet with protein, or with an amino acid is likely to be futile since the limiting factor for most of the population is the level of calorie intake. In many such instances, provision of more food of the type already being consumed is the best practical solution.
The existence of a marked effect on N balance of increasing energy intake even above the level at which it ceases to be the limiting factor, is probably of most significance in relation to the feeding of infants. The importance of an adequate supply of energy in the recovery of malnourished children has been stressed by Ashworth et al (12) and by Graham et al (29), as has the need to consider not only protein quality, but also the ratio of protein to calories in assessing the protein values of diets, Platt and Miller (30).
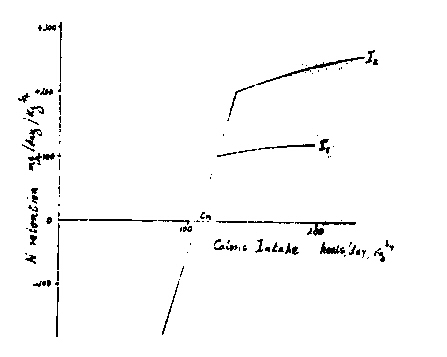
Table 1
The effect of energy intake on N balance
| Subjects | mgN balance/calorie |
| Adult man (3) | 6.7 |
| Young rats (4) | 6.8 |
| Adult rats (5) | 5.4 |
| Adult rats (6) | 6.3 |
Table 2
Minimum energy intakes necessary for maintenance
of body N or energy content
| kcals/kg | Metabolizable energy per day kcals/kg3/4 | ||
| Adult men (3) | 34 | 97 | body N maintenance |
| Young rats (31) | 250 | 118 | |
| Young rats (4) | 220 | 107 | |
| Lambs (9) | 70 | 105 | body energy maintenance |
| Calves (10) | 42 | 107 | |
| Pigs (7) | 35 | 103 | |
| Cows (11) | 22 | 104 | |
1. Munro, H.N. Physiol. Rev. 31, 449 1951
2. Munro, H.N. In Mammalian Protein Metabolism. H.N. Munro and J.B. Allison Eds. 1963 Academic Press London
3. Calloway, D.H. and H. Spector. Am. J. Clin. Nutr. 2, 405 1954
4. Miller, D.S. and P.R. Payne. J. Theoret. Biol. 5, 1398 1963
5. Benditt, E.P., E.M. Humphreys, R.W. Wissler, C.H. Steffee, L.E. Frazier and P.R. Cannon. 1948 J. Lab. Clin. Med. 33, 257
6. Calloway, D.H. and H. Spector. J. Nutrition 56, 533 1955
7. Kielanowski, J. and M. Kotarbinska. Proc. VIII Intnl. Cong. Nutrition, Prague 1969
8. Waterlow, J.C. The Lancet II, 1091 1968
9. Walker, D.M. and K.T. Jagusch. In Energy Metabolism of Farm Animals. Eds. K.L. Bloxter, 1969 J. Kielanowski and G. Thorbek, Oriole Press London. P. 187
10. Van Es, A.J.H. H.G. Nijkamp, E.J. Van Weerden and K.K. Van Hellemond. Ibid p. 197
11. Van Es, A.J.H. and H.J. Nijkamp. Ibid p. 209
12. Ashworth, A., R. Bell, W.P.T. James and J.C. Waterlow. The Lancet II, 600 1968
13. Muramatsu, K. and K. Ashida. J. Nutrition 76, 143 1962
14. Harper, A.E. Am. J. Clin. Nutr. 21, 358 1968
15. Barnes, R.H., M.T. Bates and V.E. Maack. J. Nutrition 32, 538 1946
16. Forbes, K.M., L. Vaughan and M. Yohe. J. Nutrition 64, 291 1958
17. Miller, D.S. and P.R. Payne. Brit. J. Nutr. 15, 11 1961
18. Morrison, H.B., Z.I. Sabry, N.T. Gredgeman and J.A. Campbell. Canad. J. Biochem. 41, 275 1963
19. Rafalski, H. and E. Nogal. Proc. VII Intnl. Congr. Nutrition 4, 307
20. Tagle, M.A. and G. Donoso. J. Nutrition 93, 579 1967
21. Summers, T.D. and H.H. Fisher. J. Nutrition 75, 435 1961
22. Brown, W.O. and E. Squance. In Protein Utilization by Poultry. Morton and Amoroso Eds. Oliver and Boyd London
23. DeMaeyer, E.M. and H.L. Vonderborght. NRC pubn. No. 843 1961
24. Snyderman, S.E., A. Boyer and E.L. Holt. Ibid.
25. Hegsted, D.M. and Yet Oy Chang. J. Nutrition 87, 19 1965
26. Hegsted, D.M. and R. Neff. J. Nutrition 100, 1175 1970
27. Payne, P.R. and M. Jacob. J. Nutrition 87, 221 1965
28. Sukhatme, P.V. Brit. J. Nutr. 24, 477 1970
29. Graham, G.G., A. Cordano and J.M. Baertl. J. Nutrition 84, 71 1964
30. Platt, B.S. and D.S. Miller. Proc. Nutr. Soc. 18, Vii 1959
31. Narayana Rao, M. and A.B. Morrison. Canad. J. Biochem. 44, 1365 1966