The examples utilized in this section demonstrate the varying levels of complexity, especially with respect to disease control, that are adhered to in carrying out introductions. Details could well vary from country to country for particular species or species groups.
The following are the minimum measures considered necessary for preventing the introduction of specified disease agents with introductions and transfers of salmonid ova and fish. Genetic and ecological considerations would also have to be dealt with:
1.1 The importing country must list which diseases or disease agents are proscribed. Fish shall not originate from any farm site in which the disease agents have occurred in the last two years or from waters where there is cause to consider the agent is present, unless ova only are involved. Where the proscribed pathogens may be carried intra-ovum, e.g., all viruses and Renibacterium, the ova are treated as for fish. Other proscribed pathogens may be discounted if disinfection of ova against bacterial pathogens is practised and ova are incubated in parasite-free waters.
The list of proscribed agents will depend on what diseases are present in the importing country and what precautions its experts consider are necessary against importing exotic strains of pathogens already present. An example of a typical list would be:
Any filterable replicating agent capable of causing cytopathic effects in acceptable cell lines including but not limited to:
- Viral Haemorrhagic Septicaemia Virus (VHS)
- Infectious Haematopoietic Necrosis Virus (IHN)
- Infectious Pancreatic Necrosis Virus (IPN)
Renibacterium salmoninarum (Bacterial kidney disease, BKD)
Aeromonas salmonicida (Furunculosis)
Yersinia ruckeri (Enteric Red Mouth, ERM)
Myxosoma cerebralis (Whirling Disease, WD)
Proliferative kidney disease (PKD)
1.2 Site of origin sampling procedures for (a) to (f) must be carried out twice a year, the first test being in the early summer (May/June) and the second in early winter (November/December), including the spawning period for broodfish.
Each lot of fish on site will be tested. A lot is defined according to species, but specimens must be taken from each tank, pond, raceway or cage, to ensure that strain, water supply, origin and age are sampled in each lot. Prior to testing, each fish lot must be examined for overt signs of disease and then divided up to provide fish for the various tests. Apparently healthy fish should be randomly distributed.
Testing of lots is required during the two years prior to export of live salmonids. The minimum number of samples of fish from each lot should comprise:
Early summer - 90, 0+ yr fish for virus examination, 30, 1+ yr and 30, 2+ yr fish for virus, bacteria and whirling disease examination.
Early winter - 150, 0+ yr fish for virus, bacteria and whirling disease examination.
Note At least five specimens must be taken from each tank, pond, raceway or cage containing fish of a specific lot and this may result in processing more than the recommended minimum of specimens. All fish in the early winter test must be at least five months old.
In addition, serum for testing anti -R. salmoninarum agglutinins must be collected from broodstock at spawning time. Serum extraction is non-lethal and must be carried out on 10, 3+ yr fish; 10, 4+ yr fish and 10, 5+ yr fish.
If any fish in any group have a titre of 1/32 or more, the test must be repeated using 30 fish from the failed group(s). If titres of 1/32 or more are again recorded, lethal sampling of 60 fish in failed group(s) must be carried out using gram stains, fluorescent-antibody tests (FAT) and culture methods. Only if these fish are negative will the site be permitted to proceed to testing as listed in (b).
Testing of lots required for a site after the initial 2-year testing period detailed in (a). The samples of fish from each lot should comprise:
Early summer - 90, 0+ yr fish for virus examination, 30, 1+ yr and 30, 2+ yr fish for virus, bacteria and whirling disease examination.
Early winter (November/December) - 150, 0+ yr fish for virus, bacteria and whirling disease examination.
1.3 Laboratory Procedures for the Examination of Fish for Notifiable Diseases:
Virological tests
Sampling: In fish under 8 cm, transverse body sections are sampled to include the main visceral organs. With larger fish; samples of liver, kidney, spleen and pyloric caecae are taken, except in the case of salmon older than one sea winter, when kidney samples alone are taken. The samples should be processed within 48 hours, during which time they must be stored at 4°C. On no account must samples be frozen before testing since this greatly reduces the sensitivity of the test.
Extraction procedure: The pooled visceral samples should be processed by one of the following methods:
- ground using a mortar and pestle with sufficient sterile sand until a thick paste is formed. Tissue culture maintenance medium (MM) containing antibiotics (see (a) (iii)) should be added in the ratio 1:1 w/v to the original visceral sample. The visceral extracts are then centrifuged at 1,500 g for 15 minutes and the supernatant collected. This supernatant should be diluted a further 1:25 in MM.
- diluted 1:10 in MM and transferred to a Seward stomacher bag and homogenised in a Seward stomacher “80” for 2 min. The homogenised tissue is then centrifuged at 1,500 g for 15 minutes and the supernatant collected. This supernatant should be diluted a further 1:5 in MM.
- subjected to speed rotary blending or ultransonification using
the methods described by Hedrick et al. (1986) Prog. Fish-Cult.,
48, 47–51.
The sampled material should be kept as cool as possible during
the whole procedure.
Elimination of bacterial and fungal contamination: Normally, broad-spectrum antibiotics are included with nystatin or fungizone incorporated to combat fungal contamination.
Inoculation of cell cultures: Culture wells or flasks of BF or CHSE 214 and/or FHM cells or other acceptable cell line(s)* should be inoculated with each extract at a rate of 1/10th the normal volume of MM should be added. The cultures should then be incubated at 15°C (duplicates at 15° and 20°C in cases of suspect SVC) and examined daily for signs of cytopathic effect (CPE). If no CPE developes after 7 days the cultures should be harvested by freezing and thawing and passed using 1:10 dilution into MM, and then onto fresh cell cultures for a second incubation period of 7 days at 15°C (duplicates at 15° and 20°C in cases of suspect SVC).
If CPE develops, the cultures should be harvested by freezing and thawing, diluted 1:100 and inoculated onto fresh tissue cultures. If no CPE develops during the second incubation the test can be declared negative. If viral CPE develops during the second incubation period the virus must be identified.
Cell cultures*: Only young, actively growing cultures, ie., 1–3 days old and 75–95% confluent, should be used for the isolation tests. BF and CHSE 214 cells should be grown at 20–25°C and FHM cells at 25°–30°C. When testing specifically for one disease a single suitable type of cell culture is used, but for general virological examinations CHSE 214 or BF and FHM cell cultures are used in parallel. Currently, tests for the viruses of viral haemorrhagic septicaemia (VHS), infectious haematopoietic necrosis (IHN) and spring viraemia of carp (SVC) are carried out on FHM cell cultures, but BF or CHSE 214 cell cultures are used for 1PN virus tests.
Positive controls: It has been reported from several laboratories that some fish cell lines appear periodically to lose their receptivity to some fish viruses. Therefore, the susceptibility of the cell cultures to the virus(es) under test should be confirmed. This may be done at the time of testing by inoculating replicate cultures of the cells with known low infective doses of the viruses.
Identification of viruses: The cause of any viral-type CPE must always be identified. Where specific antisera against a suspected virus is available, serum neutralization of virus infectivity is the test of choice using either the plaque reduction method or the constant serum (1:100) varying virus method.
Bacteriological Tests
Bacterial Kidney Disease, Renibacterium salmoninarum (BKD)
Presumptive Tests
Smears: duplicate kidney imprints should be taken for Gram stain and FAT tests.
Serum agglutinins. Serum sampling is non-lethal and may be used for valuable brood fish, e.g., 3 years and older. If any fish in any group have a titre of 1/32 or more, the test must be repeated using 30 fish from the failed group(s). If titres of 1/32 or more are again recorded, lethal sampling of 60 fish in failed groups must be carried out using Gram stains, FAT and culture methods. Only if these fish are negative will the site be viewed as free of BKD.
Culture: swabs must be taken from the mid-to-anterior kidney of all fish in the sample group and must be plated without delay onto selective kidney disease medium (SKDM); Austin, Embley & Goodfellow (1963), FEMS Microbiol, Letters, 17, 111–114), incubated at 15°C and examined weekly for 6 weeks for the presence of Renibacterium salmoninarum.
Confirmation of Renibacterium salmoninarum: slowly-developing colonies on SKDM plates should show Gram-positive diplococco bacilli in Gram-stained smears, give positive fluorescent antibody test results using specific antiserum prepared in rabbits and show characteristic biochemical profiles (Austin et al. (1983); Sanders & Fryer (1980), Int. J. Sys. Bact., 30, 496–502).
Furunculosis (Aeromonas salmonicida)
Culture: swabs from the kidney and any furuncles must be plated onto tryptone soya agar (TSA), incubated at 22°C and examined daily for 7 days for the presence of furunculosis.
Confirmation of Aeromonas salmonicida: colonies grown on TSA plates often produce dark-brown pigment. The gram-negative rods are non-motile and fail to grow at 37°C. Colonies should give a specific agglutination in the A. salmonicida latex test (McCarthy (1977), Fish Health News, 6(3), 146–147), or meet the confirmatory criteria of Popoff (1984), Bergey's Manual of Systematic Bacteriology.
Enteric redmouth (ERM), (Yersinia ruckeri)
Culture: swabs from the faeces and kidney must be plated onto TSA, incubated at 22°C and examined daily for 7 days for the presence of ERM.
Confirmation of Yersinia ruckeri: colonies grown on TSA plates should show Gram-negative rods on Gram smears, ferment glucose, produce catalase but not cytochrome oxidase, and exhibit a typical biochemical profile (Green & Austin (1983), Aquaculture, 34, 185–192; Ewing et al. (1987), Int. J. Sys. Bact., 28, 37–44).
Parasitological Tests
Whirling disease (WD) (Myxosoma cerebralis)
Fish less than 5 months old showing clinical signs of disease: A transverse section is taken through the head cartilage posterior to the eyes and anterior to the opercula and fixed in 10% formal saline. Following fixation, the tissues are processed histologically and subsequently 5 um sections stained with Giemsa, examined microscopically for the trophozoite or spore stages of Myxosoma cerebralis and accompanying pathology in the cartilage.
Fish over 5 months old: Fish should be examined by either:
- the histological method outlined in (i) or
- whole heads are removed from the fish and processed within 72
hrs. With large salmon older than one sea winter gill arches may
be used instead of whole heads. Samples must not be frozen.
Samples are soaked in warm water until the skin and muscle can be
easily stripped from the cranial skeleton, or filaments from the
gill arches. The material is macerated in a blender and filtered
through muslin to remove any large fragments. The sample is then
concentrated using the plankton centrifuge method of O'Grodnick
(1975), J. Wild). Dis., 11, 54–57. A drop of the final
suspension is examined using phase contrast microscopy at x 250
magnification. At least 50 microscope fields are examined for
each suspension. Confirmatory identification of spores is based
on the criteria of Lom & Hoffman (1971), J. Parasit., 57,
1302–1307.
Proliferative Kidney Disease (PKD). The following are adopted from the Fish Health Blue Book of the American Fisheries Society:
Diagnostic Procedures for Disease Situations, Differential Diagnosis: Clinically, the following diseases have similar manifestations: IHN, bacterial kidney disease (BKD), sanguinicollasis (Sanquincola klamathensis), nephrocalcinosis, and low-grade copper toxicity.
Presumptive Diagnosis: The presence of lightly staining extra- and intramacrophage protozoa containing 1–7 “daught cells” in stained imprints of posterior kidney and spleen. The “parasitized” macrophages are often surrounded by small lymphocytes, the reported “satellite” condition. Confirmatory Diagnosis: With transmitted electron microscopy (TEM), the primary cell contains multivesicular bodies, limpid bodies, mitochondria, and electro-dense bodies (“haplosporosomes”) which contain an electron-lucent bar. With light microscopy, the organism is PAS-positive. In the kidney, particularly the posterior kidney, there is marked lymphocytic hyperplasia to the point that the nephrons are often compressed. Organisms are often seen in the renal tubules and blood vessels. In the spleen, there is a marked diminution of the erythrocytic elements due to the lymphocytic hyperplasia.
Procedures for Detecting Asymptomatic Infections: Sample the suspect population in accordance with the method to provide a 5% prevalence detection level.
Collect acetone-fixed imprints of posterior kidney and 10% neutral-buffered formal in-fixed samples of posterior and mid-kidney, spleen and gastrointestinal tract. Early in the “PKD season”; i.e., mid-March to mid-May, acetone-fixed smears of pyloric caecal and large intestinal mucosa scrapings should be examined. The acetone-fixed imprints and smears may be stained using either the methylene blue technique or the Leishman-Giemsa method. The formalin-fixed material, after sectioning, may be stained using the PSA and/or the H&E techniques.
Procedures for Determining Prior Exposure to the Etiological Agent; No methods are currently available to detect previous infections with the PKD-causing organisms.
Procedures for Transportation and Storage of Samples to Ensure Maximum Viability and Survivability of the Etiological Agent. All samples must be fixed on site in accordance with the procedure described. The organism will deteriorate very rapidly in iced samples - often to the point that it becomes unrecognizable.
1.4 As the range of disease agents that may be carried by eyed ova (especially after disinfection) is very much less than for fish, conditions for imports of ova may be less stringent. However, all ova must be disinfected with iodophors before leaving the exporting country.
Salmonid eggs are safely disinfected as green eggs following ferilization and water hardening, or as early eyed eggs. Iodophors for disinfection are usually providone or polyalcoholic complexes of iodine, in which the soluble iodine confers its broad-spectrum germicidal activity but is not as corrosive or irritating as in its elemental form. A number of typical disinfectants* of this type are available commercially in North America; among these are OvadinR, BridineR, Betadine R, Actomar K30R, WescodyneR and ArgentyneR. Most contain a 1%–2% active iodine concentration.
Preparation of the disinfectant
Dilute the stock iodine-based disinfectant to give a solution containing 100 parts per million (ppm) of active iodine. The disinfectant must be prepared in water with a low organic content to minimize loss of the free iodine. Use a plastic, glass, stainless steel or fibreglass tank for preparing and holding the solution.
Check the pH of the diluted disinfectant and, if necessary, adjust to 6.5–7.5 using 8% aqueous sodium bicarbonate (baking soda).
Disinfection procedure
Use a fresh solution of diluted disinfectant.
To avoid temperature shock, adjust the disinfectant solution to the same temperature as the subsequent egg incubation temperature.
In the case of freshly fertilized eggs, allow eggs to water harden one hour before disinfection.
Immerse water-hardened green eggs or early eyed eggs in the disinfectant for ten minutes.
Treat approximately 2,000 eggs per litre before discarding the disinfectant.
Rinse eggs thoroughly in uncontaminated water after disinfection.
Arrange the egg handling programme to ensure that disinfected eggs do not have subsequent contact with contaminated equipment, water or personnel.
Diluted iodophors can also be used to disinfect work surfaces, utensils, nets and other equipment used during the egg-taking process, but they must be rinsed thoroughly in clean, uncontaminated water following the disinfection.
1.5 The exporting country should have significant experience in testing for the proscribed disease agents, i.e., an officially recognized authority. This authority must have records for two years of testing by approved methods for any fish farm source of ova or fish before consideration can be given for direct introductions without quarantine.
Where the exporting country has little or no relevant experience or no history of testing of relevant stocks, the import must be considered a risk and be placed in quarantine on arrival in the importing country. In quarantine, appropriate tests will be conducted. Quarantine should last until the F1 generation is three months into first feeding for imports of ova, and until the F2 generation is three months into first feeding for fish imports.
1.6 Fish and ova for import should be considered from one of four categories of source:
Fish or ova are from specified pathogen-free (SPF) farms, (see 1.2 and 1.3 for sampling and laboratory test requirements) which conform to certain physical requirements, that is:
The site must be entirely supplied by water from spring, bore-hole or well which, from source to inlet, is under the control of the site owner.
The water supply must be free of feral salmonids and the site should be screened to prevent their ingress.
The site water supply must be free from risk of pollution from ground water or flooding by other water courses.
All piscivorous birds or other animals must be excluded from the site.
Food stores should be sited so that transporter vehicles do not traverse the farm but unload at some peripheral point, thus minimizing any risk of introducing infection from another farm.
All introductions of live fish or ova must be agreed to by the state or local government certifying authority as being from an establishment of similar health status.
It is essential that vehicles carrying live fish to or from the farm are thoroughly cleaned and disinfected before accepting their cargo. Such farms, called SPF Category I, must have had SPF status for two years. Additionally, no fish or ova other than from a farm with SPF Category I health shall be or have been introduced in the past two years to the site. Fish or ova from SPF Category I farms may be allowed direct entry without quarantine if accompanied by the appropriate certificates (Appendix IV).
Fish or ova are from farms which have been in the SPF category for two years and to which no fish or ova other than from a farm of similar or better health status have been introduced in the past two years to the farm shall be called SPF Category II farms. These farms do not meet the physical site requirements of SPF, Category I farms.
Fish from such SPF Category II sites may be allowed direct entry only in certain situations, i.e., when pathogens on the proscribed list are common to both countries or absent in the exporting country, but assuming the shipment is certified free of all pathogens on the proscribed list. Otherwise, such fish must be placed in quarantine for life and the Fl progeny not released until testing for the proscribed pathogens is completed to the satisfaction of the “official authority” in the importing country.
Ova are from wild sources. The sampling of fertilized eggs or the sex products of fish cannot be relied upon to detect all disease agents. The threat of introducing disease agents with such ova comes from parent fish; however, even here there is cause to doubt if current methods are sufficient to detect low levels of carrier infection of some disease agents in such parent fish. It is for this reason that four tests are conducted before fish farms are accorded SPF status, during which time it has been found that if low levels of infection are present, they almost always express themselves as higher levels of infection in the confines of culture environments. For these reasons, ova of wild fish must be quarantined preferrably for a whole life cycle until the next generation (Fl) is three months post feeding and certainly for not less than the 2-year period equivalent to the SPF testing period. Ova for quarantine should come from parent wild fish tested individually and found free of all viral and other agents which may be transmitted via ova.
Fish are from wild sources. A high risk is associated with such fish which should be quarantined for life. Fish should come from groups where samples are tested and found free of proscribed diseases. The safest procedure would be to allow only the F2 generation from quarantine to be introduced but certainly the minimum period must be two years for the Fl generation.
When an importer seeks to introduce fish or ova, whether directly or via quarantine, it is obligatory that a health certificate from a recognized authority be provided for each shipment. Each approved shipment must be accompanied by an appropriate import permit from the authorities in the importing country. Such import permits should clearly indicate if entry is conditional, e.g., is quarantine to be imposed, and if so, the regulatory authority in the importing country must supervise the conditions.
Because of the relatively high commercial value of a number of molluscan species, they have been subject to a great deal of movement within or between countries. This movement continues, both as part of ongoing commercial operations or when a new non-indigenous species is brought in. The introduction or transfer of these molluscan species create the same range of inherent genetic, ecological and pathological problems as would the import of other aquatic organisms. However, because the majority of the molluscs introducted or transfered are sessile or capable of only localized movement, it is possible that one could more effectively deal with an analysis of genetic or ecological risks than might be the case with a fish species which exhibited broader distribution characteristics.
A centre for the study of molluscan introductions (usually as adults for broodstock) could be established in a location well removed from contact with related indigenous species which might be impacted on either ecologically or genetically. With proper containment (barriers, removal before spawning, etc.), indigenous and introduced species could be brought together to permit assessment of genetic and ecological interaction. By limiting the size of the initial importations, it would be easier to eradicate the group should something go wrong.
Pathological considerations could be dealt with at the same time as the genetic and ecological considerations.
2.1 Control of On-going Commercial Operations:
2.1.1 General recommendations:
A list of known infectious undesirable pathogenic diseases must be established by the importing country. An example of a list would be:
- Iridovirosis of adults and larvae
- Marteiliosis
- Bonamiosis
- Haplosporidiosis
- Perkinsus type parasite.
The exported molluscs can not be provided from a site where one of these diseases has been identified during the previous four years.
Disease inspection by the exporting country must be carried out four times per year in the different production zones as well as in the hatcheries.
Each exporting country must have accredited laboratories which agree to carry out the disease control regulations.
Each shipment must be accompanied by a health certificate attesting to the absence of listed diseases and which indicates the presence or absence of any abnormal mortalities.
The mollusc for export must originate from production areas in which predator populations (Urosalpinx, Turbellariums, etc) and competitors (crapidula, algae) are not prevalent. In all cases the export molluscs will be sorted and cleaned to remove surface predators and other potential competitors.
2.1.2 Preparation and analysis of samples: For contagious diseases described in the literature all the tests can be made by microscopy, except for Bonamia which now can be identified by simple serological diagnosis. This can be done because of the advanced state of diagnostic techniques now applicable to molluscs.
By utilizing a sample size of 100 for analysis of all the disease organisms results will be sufficiently precise to indicate their presence even at lower levels than (1% at a 95% confidence limit).
After carefully opening the shell, in order not to damage the different organs of the animal, each individual is inspected in vivo. During this inspection the external quality of the flesh will be noted as well as all other abnormalities such as abscess, lesions etc. on different organs.
Other samples are cut sagittally, and fixed in Carson's liquid fixer which offers the advantage of allowing one to use these samples for subsequent treatment and observation under the electron microscope.
One is advised to search for Bonamia with the help of the diagnostic kit ELISA which has proven slightly more sensitive and quicker to use.
Identification of abnormalities or pathogenic agents would lead to the speciman being reexamined using the electron microscope.
2.2 Controls and operations to carry out for other introduction:
In addition to the analyses carried out by the exporting and importing countries as described above, operators concerned only with smaller lots of spawner should carry out the following procedures:
Destroy all shipping materials and carefully brush the shells of each spawner to remove attached living organisms.
Place in a quarantine station where all effluent will be treated by sterilizing techniques such as ultraviolet; ultrafiltration, chorination, bromation, etc.
Maintain strict health controls on site.
After spawning and successful production of an F1 generation, the broodstock should be destroyed or utilized to study interactions with indigenous species at special isolated sites.
The F1 generation can be released if further pathological testing of larvae and juveniles is satisfactory.
Problems encountered as a result of F1 pathological, genetic or ecological testing could necessitate holding oysters to the F2 or F3 generation prior to release from quarantine.
Hatcheries could be established to provide disease-free stock to commercial enterprises, rather than continually importing new animals.
Eel culture is mainly dependent on the supply of elvers, the migrating young stage, which are caught at sea or in rivers and transported to aquaculture installations where they are cultivated. Transportation may also occur for restocking in natural waters. In many cases this implies an import from one country to another. These movements introduce considerable risk of transfer of infectious agents. For example in 1976 Sano & al. reported that a Rhabdovirus had been isolated in Japan from elvers imported from France. Castric and Chastel (1980) presented studies on viruses recovered from elvers on the French Atlantic coast in 1977 and 1978 which stated an IPN virus (type Sp) and two different types of Rhabdovirus (B12 and C30) had been found in eels from the Loire area.
Proscribed diseases and disease agents for eels are:
Dactylogyrus sp.
The following procedures for handling the transfers of eels are aimed at minimizing the chance of introducing disease agents into new areas:
3.1 Each country should compile an inventory of current practices in eel transfers, including the following data:
History of outbreaks of fish disease in introduction areas associated with import of elvers or of other species.
Imports of eels (at any stage) should come from certain areas i.e., areas known to be free from disease and disease agents according to lists previously developed.
3.2 Before export of local elver populations to another country, these populations must be sampled for pathogens or pests (virus, bacteria, etc.) to avoid introduction of any new disease to importing country.
3.3 A six week quarantine upon arrival in the importing country should be mandatory and should follow all the elements of quarantine outlined under universal protocols in section (3.4), including the distruction or sterilization of all material in contact with the eels during import. Transport to importing country should take place without exchange of transport water. A schemetic diagram, Figure 1, based on Swedish practice is included for consideration.
Water leaving the quarantine installation must either be filtered through soil of an appropriate particle size or treated with calcium or sodium hydroxide to raise the pH to 10 or higher.
The quarantine station and its operation must be inspected and approved by qualified government inspectors before use. When in use the facility should be supervised by a local veterinarian, authorized by the government to ensure equipment maintenance and hygienic conditions in the installation are maintained.
Sampling of elvers (at least 20–30 animals per sample) for disease agents must be carried out within 1–3 weeks after arrival of the elvers at the quarantine facility. Standard virological examination of pooled 5 and 5 elvers should be carried out using RTG-2 cells. If results of testing are positive for virus or other disease agents, elvers must not be released for farming or other stocking purposes.
3.4 The quarantine must be supplied with a test-fish system consisting of two test tanks (Fig 1).
- The test fish must be juvenile (5 g–15 g) salmonids, e.g. rainbow trout or salmon. Juvenile fish at this stage are considered to be most sensitive to viral diseases.
- The test-fish tanks (100 litres water/tank) must be stocked with at least 100 fish.
- In one of the test tanks the test fish should be exposed to effluent water from the eel tanks, diluted and adjusted to 10°C. In the other test tank the same number fish should be kept in water which is not contaminated by effluent from the eel tanks.
- After exposure to eel tank effluent (minimum 14 days) the test fish (and reference fish) are examined virologically. Until testing is completed no eels will be released from quarantine.
- If the examination results are positive elvers (eels) must not be released for farming or stocking purposes. If the results are negative the eels can be released immediately (about 5–6 weeks from the start of the quarantine).
- Infected fish must be destroyed.
- The quarantine period should be prolonged, if necessary, to thoroughly investigate suspected disease or infectious agent carrier conditions.
A number of factors (species, age, condition of fish, water temperature, virus concentration or infection dose, and the virulence of the pathogen) apparently influence the eventual outcome of a test-fish exposure in situations as those described above. Absence of clinical symptoms in virologically IPN-positive salmonids, as well as negative results in experimental Rhabdovirus infection, could be explained in a number of different ways. As we are unable to control some of the important factors for the efficiency of a test system we must admit that negative results can not necessarily be taken as absolute evidence for absence of virus carriers in a tested population.
A French study of viral infections among elvers taken from a certain area of the eastern Atlantic coast in 1984 recovered viruses in 10 of 23 samples examined. Against this background it is realistic to consider the risk of introducing infectious agents as very high in elvers collected and sold for fish culture or stocking purposes from such areas, even if viral examinations of samples from the lot in question are negative.
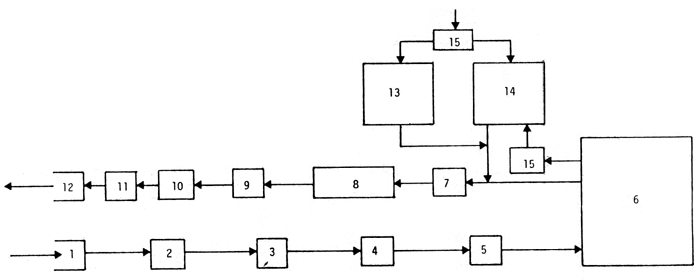
Fig 1. Principle for a quarantine installation for elvers (Modified after Ackefors et al., in press).
Water is pumped through (1) and passes through a screen (2) where particles bigger than 2 mm (e.g., mussels, coarse sand) are removed. The water then passes through a heat exchanger (3), where the temperature is adjusted to the desired level, and through one or two glass-fiber filters (4) or triangle filters, where particles down to 10 u are removed. After aeration (5) the water then enters the elver tanks (6). After leaving these tanks, faeces and food waste are removed from the water swirl separator (7) or similar arrangement and collected in a reservoir (8). The water is then pumped (9) to an installation (10) where the pH is increased to 10. The pH adjusted water is normally held for one or two hours (11) before releasing it(12).
There are two test-fish tanks (13 and 14). One of them (14) is partly fed with water from the elver tanks and partly with fresh incoming water; the other is fed with only fresh incoming water (13). All the ingoing water is temperature adjusted to 10°C (15). The outgoing water from the fish-test tanks is treated as is the outgoing water from the elver tanks.
Note: UV-light may be inserted between (4) and (5), but because of several technical drawbacks this treatment is generally not recommended.