M. G. SAROGLIA
The main items of expenditure that breeders running fish farms have to meet are : the purchase (or production) of fry, the purchase of feed, and the electric power required to operate the pumps and aeration equipment.
In order to recover the money invested and to secure his profit margin, the breeder has to aim to reduce all kinds of consumption by raising a marketable product in as short a time as possible, by minimizing fish mortality, by optimizing his feed conversion factor, and by cutting energy costs to the irreducible minimum.
All these aims have to be pursued by controlling all the environmental - physical, chemical, and biological factors that may directly or indirectly exert stress on the breeding stock.
Of the environmental factors, particular importance is attached to the quality of the water, management techniques, feed quality, and hygienic breeding conditions.
Breeders have to perform various operations (such as cleaning the ponds, sorting out the various sizes, moving tanks and stock, etc…) that , as is well known, imposes a strain on the fish.
Apart from these normal husbandry operations, fish may suffer stress through poor-quality water, the presence of infestation or infection (e.g. from parasites, fungi, batteria, and viruses), as well as the presence of predators, etc…
From the breeder's angle, stress should be seen as a state caused by a number of procedures, environmental factors, or events what interfere with the ability of the fish to grow to marketable size in accordance with the time schedule.
Bred fish show a wide range of responses to stress stimuli, including such visible ones as loss of appetite, changes in behaviour, or death. Hidden responses include inefficient feed conversion and deterioration in physiological parameters. Here we intend to review, by way of an example, the stresses caused by fluctuations in catabolites and dissolved gases, attempting to pinpoint parameters that indicate a state of suffering, and suggesting possible courses of action to check such stress.
Most species of fish used in aquaculture can tolerate fluctuations in a large number of environmental chemical and physical parameters, provided these reamin within clearly-defined limits. As long as these limits are not exceeded, the fish may be said to remain in good health and homeostasis is maintained (see fig. 1). As regards fluctuations exceeding the aforesaid limits and coming within the risk threshold, the result is that the fish undergo stress, even though there is still some homeostatic compensation.
Beyond the risk threshold there occurs an imbalance in the homeostasis, and the fish begin to show marked deterioration of their physiological parameters. Should these conditions persist, the “break” point is passed , and the fish will die, or have their health seriously undermined, even if environmental conditions return to normal.
For fluctuations above the normal range, however, although general physiological conditions remain acceptable, there is a considerable rise in energy consumption due to the fact that compensation and defence mechanisms get in.
The result of all this may be a reduction in the amount of energy available foe the process of growth.
If we feed the fish a daily quantity of commercially-available dry feed equal to 2 % of their weight, this means they have about 100 calories (419 joules) Per head per day. Of these, the net energy available will amount to only 59 calories, which will be expended on general metabolism or, to be more precise, on maintenance, basic motory activity when resting, calorie-loss, activation of disintoxication processes and stress protection, as well as flight reacitons, and active swimming.
The energy left for growth will thus only be what remains and, if breeding conditions are optimized, this may at most amount to 45 % of the initial energy intake.
On the other hand, in non-optimal conditions, the percentage of energy available for growth will diminish until, at the worst, there is none at all, with a consequent loss of biomass.
Of the environmental factors of a chemical nature that, by stimulating defence mechanisms, may result in the expenditure of energy, let us now take the example of nitrogen compounds on catabolites. The most important of these is ammonia, which is produced by catabolism of the proteins and amino-acids.
The amount of ammonia produced by a fish in 24 hours depends on the temperature, individual size and type of feed.
For example, as regards the sea-bass (Dicentrarchus labrax), at a temperature of18–24° C for medium size fish, the figure is about 400 mg of ammonia nitrogen per kg of fish per day (fig. 2). This quantity will have to be removed by suitable changing of the water.
However, ammonia production is not constant throughout the day, increasing up to 3 – 4 times the basic values after feeding (Fig. 3). These daily fluctuations are set up in the ammonia concentration inside ponds, and this will have to be taken into account since: even if the chemical measurements carried out (for example, in the morning) show an acceptable concentration of ammonia-nitrogen, the concentration may still exceed the tolerance threshold at certain hours of the day.
In such an event, the fish will respond by reducing the quantity of ammoniac excrements they produce, with consequent increase in the ammonium ions in the blood (ammoniemia) following the build-up of the products of excretion (Fig.4). Exposure to ammonia will, in addition, reduce the functional area of the gills, reduce respiration, and slow down metabolism.
Analysis, both microscopic and otherwise, of freshly-obtained gills is a reliable aid to the diagnosis of suffering caused by high concentration of ammonia, even if the latter are intermittent.
Other environmental factors, such a temperature, salinity, and PH interfere with overall ammonia toxicity.
The undissociated ammonia components, NH3may be considered the toxic form of ammonia-nitrogen.
From the graphs in Fig. 5 and 6, we see that this component is influenced by the pH and temperatures values.
The effects on growth of fish begin to be evident for values exceeding 0.05 mg/l of N - NH3 which occur about 6 mg/l of total ammonia at pH 7,2 and at 1,0 mg/l for a pH value of 8,2.
Oxidation of the ammonia into nitrites and nitrates is caused by bacteria of the Nitrosomonos and Nitrobacter genera with consumption of oxygen.
When the concentration of O2 is low, or ammonia is present in too high concentrations, the oxidation may stop at the intermediate stage of nitrite and therefore accumulate in the water.
This nitrogen form also has a high level of toxicity, since it can interfere with the oxygen, transport mechanisms by means of irreversible oxidation of the haemoglobin into metahaemoglobin, a pigment that can no longer convey oxygen.
Due to exposure to a lethal concentration of nitrites, we find , from the very first hours, the appearance of high percentages of metahaemoglobinemia (see Fig. 7).
After 1–2 days, a serious form of haemolythic anemia, with a marked reduction in total haemoglobin, begin to appear.
Measurement of the haematic concentration of metahaemoglobin and total haemoglobin are good indications for diagnosing excessive levels of nitrose-nitrogen in water. Histochemical analysis can also be used to pinpoint suffering caused by nitrites. Indeed, if coloration of spleen tissue with prussian blue is carried out, haemolytic anemia will be shown up by high deposits of ferric iron in the spleen, which will develop a deep blue colour.
The beginning of mortality among the population will correspond to the drop in total haemoglobin concentration.
The effect of the stress caused by nitrites may continue even when exposure to toxic substances has ended. Thus, a few hours after exposure to a lethal concentration of nitrite, even though metahaemoglobinemia is rapidly disappearing, the process leading to haemoloythic anemia will continue irreversibly (see Fig. 8).
In the case that we give, there is no return to physiological values of haemoglobinemia until more than 24 days have passed since the end of the event causing stress.
It is therefore obvious that speedy diagnosis of nitrite intoxication may make it possible to avoid considerable damage to production such as might become strongly apparent even may days after the severe exposure concerned.
Of the environmental factors, salinity is the one that has the greatest effect on the toxicity of nitrites.
In particular, the chloride ions, by subjecting the branchial membrane to the effect of nitrite, may even reduce toxicity to one third, changing from fresh water to sea water with 37 % salinity (Fig. 9).
This would appear to show that, in the case of stock raised in fresh water, especially if the water is partially recycled, anti-nitrite prophylaxis might be achieved by adding limited quantities of chloride ions (for example, by adding sea water to the recirculation systems).
To recapitulate : in the case of water with a limited concentration of catabolites the fish are likely to grow well, with a good feed conversion factor and limited losses through mortality. High concentrations of catabolites, on the other hand, may lead to high energy losses, with a deterioration in the feed conversion factor, the risk of outbreaks of infection, and loss of stock through both acute and chronic mortality.
The parameter that we now consider, which is no less important than that of the nitrogenized catabolites, is dissolved oxygen.
The capacity of fish to consume oxygen changes with the partial pressure of that gas in water, and therefore with its concentration (see fig. 10).
Below the incipient lethal concentration, fish resist for a limited period of time, and then die.
In the area of respiratory dependence, fish activate their compensatory and regulatory mechanisms, which call for the expenditure of energy, and reduce energy availability for other vital processes as swimming, food utilization, and growth, The best protection for fish is obtained at oxygen tension values in the area of respiratory regulation.
Thus oxygen, when not present in sufficient concentration, can also act as a brake on growth and on efficient feed conversion.
Hence, farmers usually take action by installing mechanical systems such as rotating-blade oxygenators, pumps, etc…, or by the insufflation of liquid oxygen. All these systems, and even the more frequent changing of the water, represent an increase in electric power or fuel costs. Their use has therefore to be programmed and confined to those points in ponds where oxygen is definitely needed.
While a limited excess of oxygen may merely mean a waste of electric power, values beyond a certain supersaturation threshold can produce a syndrome known as gas bubble disease (GBD).
GBD can kill fish under breeding conditions as soon as the risk threshold is exceeded.
Certain factors, such as the species and level of ontogenetic development, as well as temperature and the nature of the dissolved gases, can influence the risk threshold (see Fig. 11).
Gaseous supersaturation may occur for may different reasons, such as, for example, insolation, the malfunctioning of pumps, and excessive oxygenation of the water in pressurized tanks and may follow a cyclic pattern.
To avoid the death of fish, signs of gas bubble disease, which can lead to embolism, emphysema, and haemorrhage, must be spotted early.
To conclude, as has already been emphasized, stress factors factors that can interfere with the life of fish at a breeding station are of many different kinds, and very often several such agents are present at the same time.
Stresses that exceed physiological tolerances will sooner or later prove lethal. Less severe stresses that are, even so, more frequent will reduce growth, make fish susceptible to infection, and reduce their ability to stand up to additional stress.
Reduced feed conversion efficiency and the incidence of infectious diseases may also be stress indicators.
Unfortunately, however, by the time such indicators are perceived, it is too late to do anything about the financial damage incurred.
It is therefore necessary to know about and have available stress protection factors that will raise the risk threshold, as well as parameters that will give timely warning beyond any shadow of doubt.
Suitable feed additives can provide defence for the organism, as in the case of ascorbic acid, a vitamine that has been proved to provide protection against stresses caused by heavy metals and pesticides, as well as stresses due to the simultaneous presence of several environmental factors at maximum tolerance level.
For example, high temperature and salinity with oxygen concentrations that are not optimal have, in the post, been responsible for a serious syndrome in sea-bass. The disease presented a clinical picture characterized by disturbances in calcium metabolism, bone fractures, haemorrhagic rashes, detachment of the splanchnocranium from the trunk, and death of the fish.
The addition of strong doses of ascorbic acid to the diet has been found sufficient to reduce the incidence of this syndrome. Even when the illness has not been noticed, an increase in vitamine C in the diet has been found to produce an improvement in the general condition of fish, as well as a better feed conversion factor.
Moreover, the reaction of the tissues to acute stresses includes the depletion of muscular and liver glycogen and of vitamine C in the kidney and liver.
Systematic periodic analyses, including checking on these parameters, carried out on samples at random, might well make possible early diagnosis of a general state of stress in fish being raised at breeding stations. These atypical stress indicators could then be combined with specific investigations and analyses such as those mentioned here. Research is progressing along these lines, with periodic random tests aimed at establishing valid methods of early diagnosis.
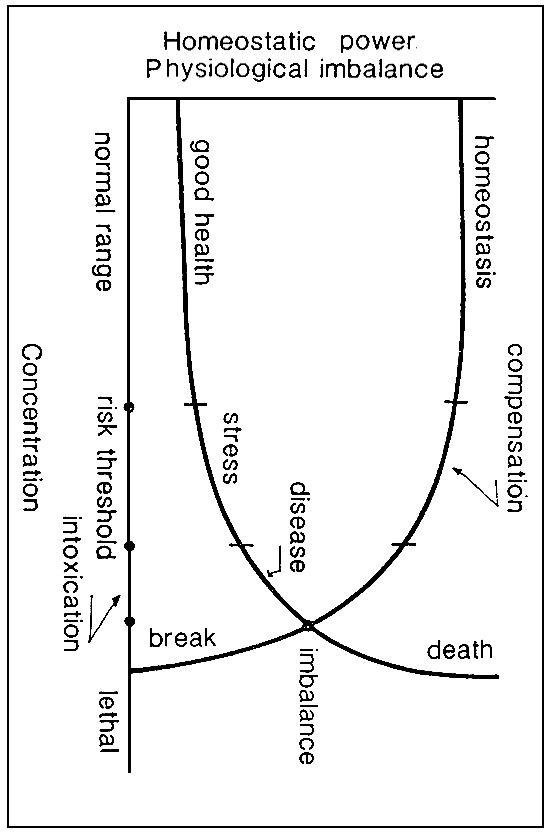
Fig. 1. Stress agent concentration versus physiological conditions (Soruce: D. Calamari, 1976).
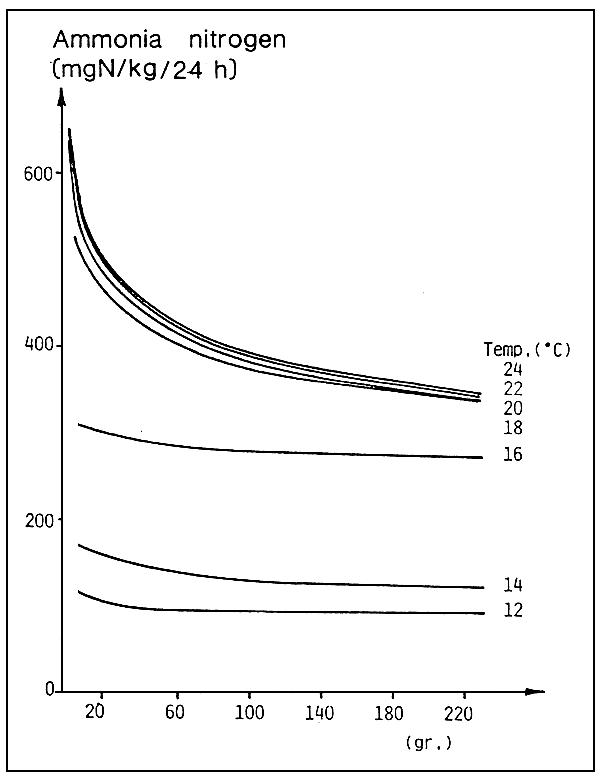
Fig. 2. Daily excretion of ammonia-nitrogen versus individual weight and water temperature in respect of sea bass (D. labrax) (source: O. Guerin-Ancey, 1976).
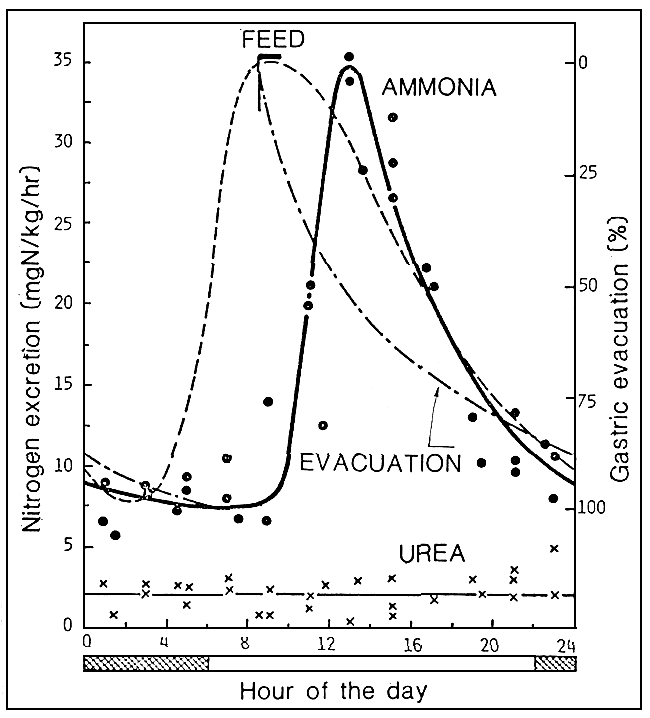
Fig. 3. Daily trend in respect of ammoniac excretion and urea in salmon (O. nerka). The graph also show oxygen consumption (source: J. R. Brett and C. A. Zala).
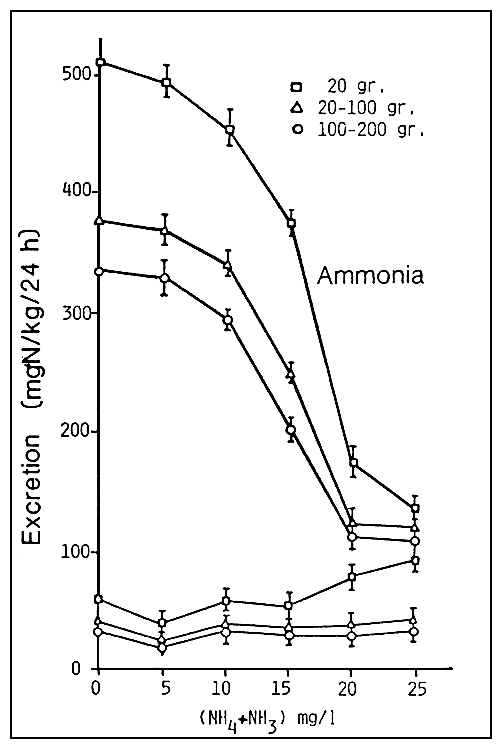
Fig. 4. Excretion of ammonia and urea versus the total ammonia concentration in the water in respect of sea bass (D. labrax) at different stages of development (source: O. Guerin-Ancey, 1976).
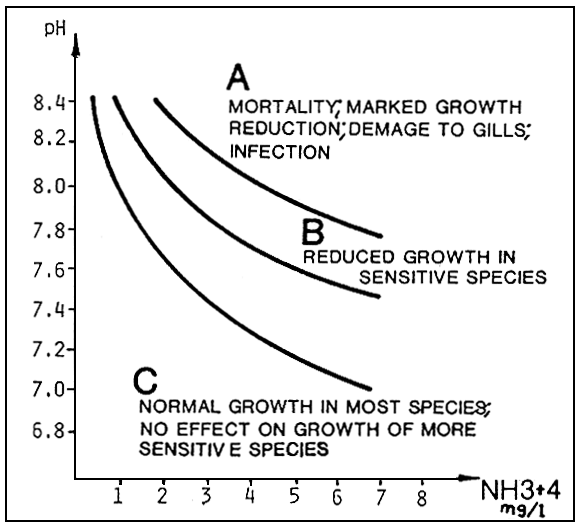
Fig. 5. Relationship between total ammonia and pH, at a temperature of 15°C, for levels of 0.07. 0.05. and 0.1 mg/l of non-ionized ammonia. The curves are valid within a salinity range of 0–36%, with an approximate value of 0.3 mg/l of total ammonia-nitrogen (source: G. Scarano, 1983).
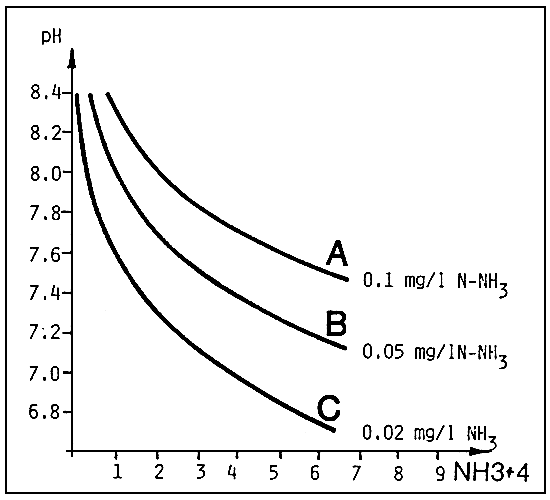
Fig. 6. The same as fig. 5, but calculated for a temperature of 25°C (source: G. Scarano, 1983).
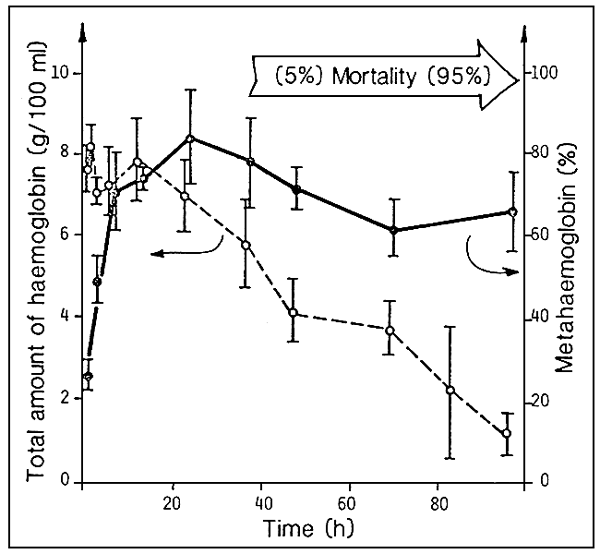
Fig. 7. Time trend in respect of haemoglobin in sea bass (D. labrax) during exposure to a lethal concentration of nitrose nitrogen (source: G. Scarano et al., 1984).
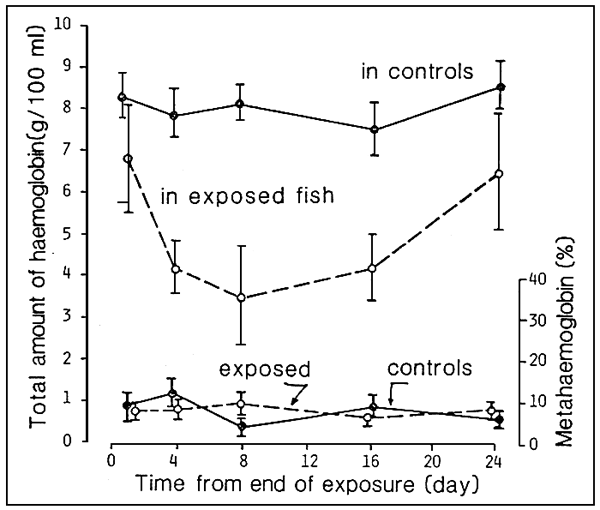
Fig. 8 Onset of haemolytic anemia in sea–bass (D. labrax) following brief exposure to lethal concentrations of nitrite (Source: G. Scarano and M. Saroglia, 1984).
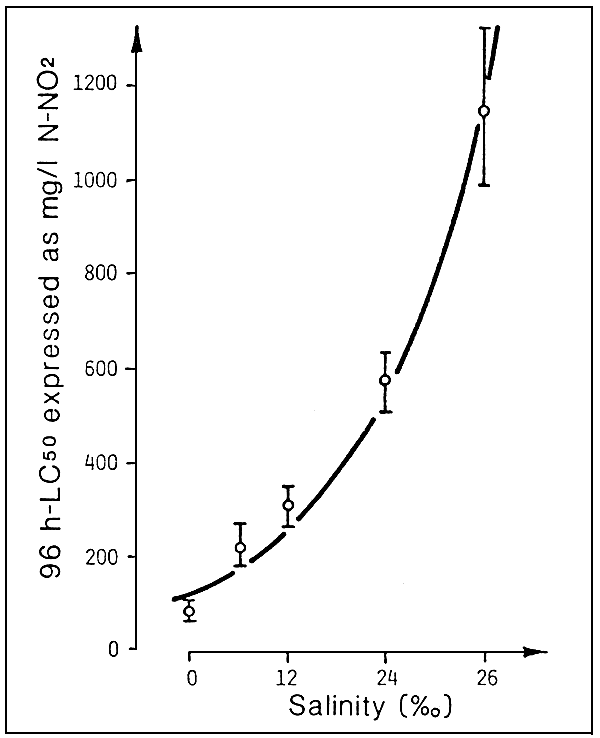
Fig. 9 Water salinity versus acute toxicity caused by nitrite in eels (A. anguilla). (Source: M. Saroglia and G. Scarano, 1984).
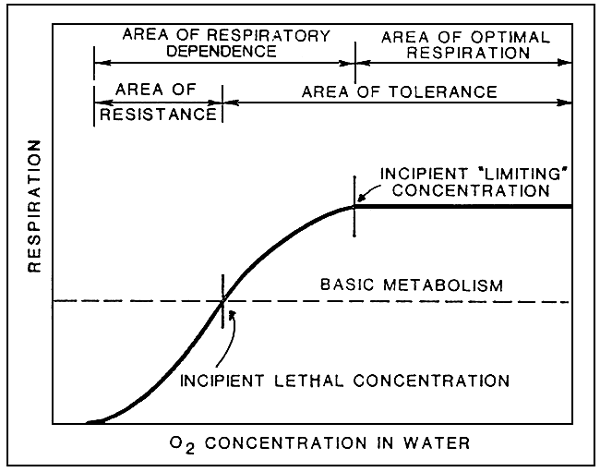
Fig. 10. Availability of oxygen in relation to respiration (Source: W.S. Hoar, 1966).
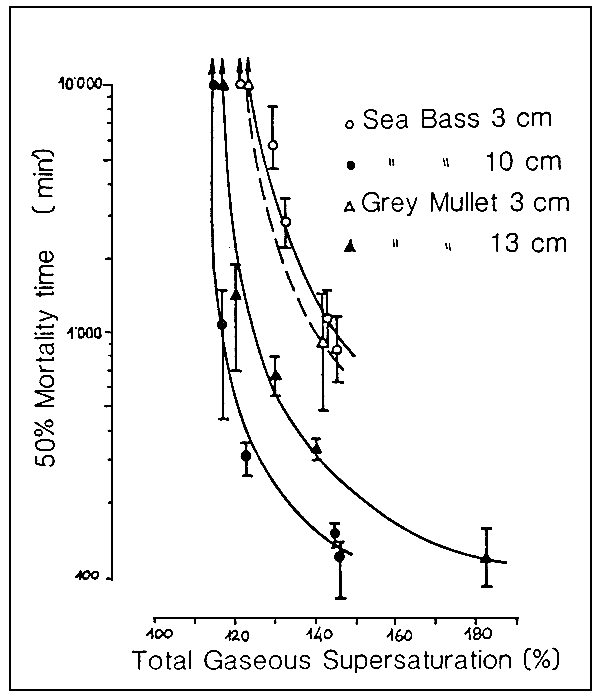
Fig. 11. Risk threshold in respect of gaseous supersaturation for sea bass (D. labrax) and grey millet (M. cephalus) at different stages of develoment. (Source: R.H.Gray et al., 1984).