- ENVIRONMENT AND FISH HEALTH WATER QUALITY FOR AQUACULTURE
By J. EDMONDSON
INSTITUTE OF AQUACULTURE UNIVERSITY OF STIRLING-SCOTLAND
- DIAGNOSIS OF BACTERIAL DISEASES
By M.T. HORNE
SCOTLAND
- BACTERIAL INFECTIONS IN GILT HEAD SEA BREAM (Sparus aurata)
By PROF. DR. HALUK ERYGUVEN
TURKEY
- DIAGNOSIS OF VIRAL DISEASESs
By M.T.HORNE
SCOTLAND
- LIMPHOCYSTIS DISEASE OF SPARUS AURATA IN MARINE CULTURE AT AEGEAN SEA
AND MEDITERRANEAN COASTS OF TURKEY
By AKIN GANDAN
TURKEY
- A HISTOLOGICAL STUDY OF CARP POX (Viral epithelioma) DISEASE IN TURKEY
By G. TIMUR
TURKEY
- NUTRITIONAL DISEASES OF FISHES
BY DR. ATTIA EL HILI HEDIA
INSTOP - SALAMBO - TUNISIA
- SEA BASS, SEA BREAM AND FLAT OYSTER PREDOMINANT PATHOLOGICAL EASES
IN NADOR LAGOON
BY TALBAOUT MUSTAPHA
MAROST - MOROCCO
- SOME IMPORTANT ASPECTS OF THE PATHOLOGY INVERTEBRATE SPECIES
BY FRANCISCO RUANO
PORTUGAL
- EXAMINATION OF FISH FOR PARASITES-EQUIPMENT
By Dr. C. SOMMERVILLE
SCOTLAND
- EXAMINATION PROCEDURE
By Dr. C. SOMMERVILLE
SCOTLAND
By J. EDMONDSON
SCOTLAND
INTRODUCTION
This document is a collection of notes from lectures on environment and disease given on the MEDRAP II training project in disease diagnosis and prevention for technicians working in aquaculture. These notes are intended to act as a reference for the participants of the course.
The intention during the day is to give an introduction to the diagnosis and prevention of disease caused or induced by environmental conditions. Poor environmental conditions may promote disease in two ways; firstly by making fish more succeptible to infectious disease, and secondly, by direct toxicity. In the first case an autopsy will show the infectious disease which cases mortality but not the stress promoting factors which led to the disease outbreak.
In the second case an autopsy will provide little information as the symptoms of different environmental diseases are virtually identical. Therefore, because of the difficulties in diagnosing environmental diseases, emphasis during the day is placed upon knowledge of the environmental conditions and behaviour of fish before and outbreak of disease. This historical information is essential for accurate diagnosis and correct remedial action.
The day is separated into four lectures and a practical which is to be held in the afternoon:
Introduction to water quality
Environment and health
Water quality management
Sampling and analysis methodology
1. INTRODUCTION TO WATER QUALITY
The aim of this lecture is to introduce and discuss some of the general concepts regarding water quality and aquaculture. it hopefully will prepare you for the following lectures which deals with tolerances and responses to particular water quality parameters.
GENERAL RELATIONSHIP
The relationships between an organism, its environment and disease are complex. This is summarised in the figure belows, which shows an organism in a state of equilibrium with its environment and with disease organisms, many of which are always present in the environment.
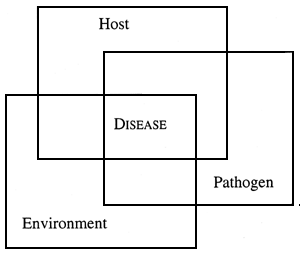
Disturbing this equilibrium by changing the environment may result in a stress (see Lecture 2) response in the fish, making it more vulnerable to fish disease. Some common stressors in farmed aquatic animals are:
poor water quality
overstocking
handling of fish
disease treatments
The most extreme response to stress is mortality, but below this level there may be several other responses, such as:
- changes in behaviour (e.g. hiding at the bottom of a tank, swimming near the inflow) or appearance (darkening or lightening of skin).
- poor appetite, poor food conversion, poor growth.
- reduced reproductive potential (eg low egg fecundity, spawning success)
- reduced tolerance to pathogens
- reduced ability to tolerate further stress
These last two are important because they emphasise:
That fish disease may follow any non-lethal but stressful environmental change.
that a combination of two or more factors, for example, high ammonia and handling, can be much more damaging to fish than one factor alone.
In many forms of aquaculture there is a fairly good general idea of the environmental factors which cause stress and will increase the likelyhood of disease outbreaks. However it is difficult to define what aspects of the environment cause specific stress to fish and to separate short term effects from the effects of long term exposure.
- In many ways, the aquatic environment is less stable than conditions on land (i.e. fluctuations can and do occur);
- Aquaculture is largely dealing with poikilotherms which are unable to regulate body temperature and thus maintain homeostasis;
- It is difficult to understand what aquatic animals need to keep them happy. As a farmer, it is also difficult to monitor and to react to changes in the aquatic environment.
(e.g. ammonia will affect the gill's structure with long term exposure, this may reduce the fish available
area for gass exchange and in low oxygen conditions the combined effect will result in stress
although the actual levels of ammonia and oxygen will not be toxic to the fish.)
When these sorts of combined effects are considered throught out the fishes life, it can be seen that
it is a complex problem to resolve the different environmental factors.
Whilst long term effects are difficult to define, we have a fairly good idea about short term effects from short term toxicity tests (e.g. LC50 tests). These tests can help us estimate the king term toxicity effect but it is difficult to extrapolate to an aquaculture situation because of the complex combination of factors which are always acting upon the fish.
Variability of the environment is a problem in aquaculture. Whereas a fish may be able to adapt to long term exposure, a large fluctuation. Such as diurnal changes in oxygen, may be more critical. This is particularly important in the early life stages of the organism.
Water quality within an aquaculture system is determined by three principal factors:
- The name of the supply; (exogenous-dependent on water supply)
- The nature of the system; (endogenous - produced or affected by the fish)
- Management. (some control over above factors particularly the system)
Those factors largely determined by the water supply and those largely determined by the system and its management are shown below. The water supply depends on location (altitude, latitude, geology). Water quality in the farm depends on the quantities of uneaten feed, FCR, and the fates of these wastes (i.e. how quickly and gently they are removed from the system or processed into harmless compounds),
Water supply
The following parameters are largely determined by the nature of the water supply and are not significantly affected by most fish farm systems.
pH
temperature
alkalinity
salinity
biocides
suspended solids in inflowing water
disssolved gases in inflowing water
background nutrient levels
metals
hardness
Aquaculture systems and water quality
The following water quality parameters may be significantly affected by the aquaculture operation.
dissolved oxygen
ammonia
nitrite
biochemical oxygen demand (BOD)
carbon dioxyde
suspended solids
phosphorus
These factors are directly amenable to management (will be discussed later). They are related to the biomass of the stock, their activity, and the level of feed intake.
The gills of fish are a major interface with the environment where most of the exchanges between a fish and its environment take place. The surface area of the gills is approximately twice that of the skin. As such they play an important role in mediating environmental effects.
Gill pathology is important in diagnosis of water quality problems. However it is essential to have a clinical history (e.g. if fish are dying from lack of oxygen there may be no change in the gill because of the speed at which death is occuring, observations of water quality and fish behaviour, such as gasping, would be needed for diagnosis.)
Smears and squashes of fresh tissue or histology can be done. Smears and squashes may show over production of mucus or bacterial proliferation as well as parasites. If the appearance of the gill is uniform then it is likely that water quality is a problem (although extensive parasitic infection may give an uniform appearance.)
With histological examination it is very important to get good fixation from freshly killed fish. Briefly, the gill is composed of secondary lammellae protruding from primary lamellae. The secondary lamellae consists of a blood space covered by an epithelium. Pillar cells bridge the space to hold the two sides together. For gas exchange there is only a single or double epithelial distance for the gas to travel across. A thin layer of mucus covers the outer surface of the epithelium. Mucus cells, inflammatory cells, lymphocytes and salt cells are generally found embedded in the primary lamellae at the base of the secondary lamellae.
It is very easy to produce artefacts when looking at gill pathology. Common artefacts are separation of lamellae and epithelia, sloughing of epithelia. Telangiectasis may occur as an artefact due to killing a fish by a blow on the head. Haemorrhaging may occur when the fish is caught and killed. Debris on the gill surface may be due to vomited stomach contents.
There are basically two types of change in the condition that can occur.
Firstly acute changes that can occur when there is a high level of irritants present in the water (e.g. treatment overdose, acid rainfall, toxic algal boom.) The acute changes that occur are normally: sloughing of epithelia, necrosis of individual cells in gill epitheleum, surface hypertrophy (oedema within the cells), spongiosis (oedema within and between cells), telangiectasis (swelling of he ends of gill filaments and a filling up with blood following rupture of the pillar cells in the secondary lamellae.)
Secondly chronic changes will occur in the fill when subject to low levels of irritants (e.g. suspended solids, repeated chemical treatments chronic ammonia toxicity etc.) In chronic cases the first change is usually and increase in mucus production. This is damaging because gas exchange now has to take place through a much thicker layer and the excess mucus may cause secondary bacterial problems-bacterial gill disease. This is not a specific bacterial disease but a problem of water quality which then allows secondary bacterial proliferation, particularly with myxobacteria. over a period of time the epithelia in the gill may become fused together at the tips of the secondary lamellae and gradually the epithela along the secondary lamellae multiply a condition called hyperplasia. This is more serious than mucus proliferation because even after treatment a fish will never completely recover its normal gill structure.
Gill pathology may give some indication of whether a disease is due to water quality and if so what parameter is involved (e.g. algae or debris may be found on the gills, damage to tips of secondary lamellae may indicate water quality problems.) However, it is virtually impossible to distinguish between the effects of different water quality parameters, knowledge of water quality is therefore needed for accurate diagnosis.
2. ENVIRONMENT AND HEALTH
The aim of this lecture is to outline in more detail the specific relationship between different water quality parameters and fish health. The first section discusses how stress by less than ideal environmental conditions can promote the onset of infections disease. The second discusses the action of different water quality parameters.
STRESS AND INFECTIOUS DISEASE
STRESS
All organisms have tolerance rages for environmental parameters. In figure below this is illustrated for temperature. There is an optimum range, outside of which is a zone of stress. Beyond this the animal crosses what is termed an incipient lethal level, beyond which death swiftly occurs.
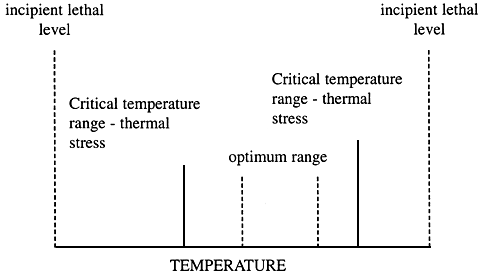
These boundaries, although somewhat species-specific, are not fixed, but vary, depending on:
- age
- previous exposure
- genetic makeup
- other environmental parameters
- other factors (e.g. nutritional status).
What do we mean by stress? There are many definitions of biological stress, but all incorporate some notion of a stimulus acting on a biological system and the subsequent reaction of the system.
Stress may be acute or chronic. Acute stress is defined as one in which the duration of the stress-minutes or hours-is considerably shorter than the physiological response, components of which may last days or even some weeks. Chronic or continuous stress is unavoidable (thus making the stress response ineffective or even dangerous). The animal must acclimate to it, albeit at reduced performance capacity if it is to survive.
Acute stresses handling and disease treatment, both of which last only a few minutes, but fish have to be handled from time to (e.g. grading, transport), or treated for disease.
THE EFFECT OF WATER QUALITY ON PATHOGEN NUMBERS
20 genera of bacteria have been isolated from fish, of which at least 15 species are recognised as actual or potential pathogens (i.e. causitive agents of disease). Most of these organisms are naturally occurring and widely distributed, living on dead or decaying organic matter which is universally present in aquatic systems. An increase in the level of organic matter and an increase in the numbers of saprophytic organisms can follow.
The nature of water as medium allows easy transfer of pathogens between the cultured organisms and from wild fish. The high densities of culture organisms means a large of potential hosts.
In summary-an aquaculture system can induce stress (and hence reduce the effectiveness of the fishes immune system) whilst at the same time promoting an increase in the numbers of pathogens. It should therefore be remembered that environmental conditions have a vital role in the onset of communicable disease.
The following section discusses the action of different water quality parameters, how they cause stress and, if at toxic levels, mortality.
THE ACTION OF DIFFERENT WATER QUALITY PARAMETERS
Temperature and Salinity
Teleost fish are poikiolothermic and so their rate of metabolism immunological response, and reproduction changes along with changes in temperature. In addition other factors such as the solubility of gases in water, biological oxygen demand, toxicity of pollutants, and growth of fish pathogens changes with temperature. Different species have an optimum temperature range, outside of which is a zone of stress. Below and above certain temperature death occurs.
Sea bass are found in water between 5°C in the winter and 27°C in summer. Temperature is spawning areas are less diverse (10–12.5°C). Maturation does not occur above 18°C.
Salmonids will generally survive underneath thin ice cover and at temperatures up to 25°C. However, above about 18°C the solubility of oxygen becomes limiting and it is necessary to starve the fish to reduce oxygen consumption, both by the fish themselves and also as a result of breakdown of wastes. Incubation of eggs should take place a temperatures below 13°C. With time fish can aclimitise to temperature to a certain extent-a sudden increase therefore may be more stressful than moderately high temperature over a long period.
Fish such as sea bass and rainbow trout can grow in a wide range of salinities. e.g. Sea bass are found in all salinities from fresh to full seawater. Maturation does not occur in very low salinity. Rainbow trout can be grown in fresh water but higher salinities results in better grown in fresh water but higher salinities result in better growth (salmon need salinities >30ppt). Marine fanning has the advantages of more stable environmental conditions, higher winter temperatures, and greater water supply. However, young and sexually mature fish may suffer stress as they maintain their osmotic balance. This may result in disease.
Dissolved oxygen
Dissolved oxygen is one of the basic water quality requirements for fish. Most fish obtain oxygen from water, although some, such as the snakehead, Ophicephalus striatus and catfish, Clarias batrachus, can survive in waters without oxygen (anoxic) by breathing air. Such fish are known as air breathers. Although air breathers can survive in anoxic waters, experience suggests that they are vulnerable to dieseases in conditions where dissolved oxygen remains continuously low.
There are three main physical factors affecting the amount of oxygen a water can hold (ie the solubility of oxygen in water).
| 1. | Temperature | Water holds less oxygen at higher temperatures |
| 2. | Salinity | Water holds less oxygen at higher salinities |
| 3. | Atmospheric | Water holds less oxygen at low atmospheric pressure pressures (eg at high altitude) |
| Temperature (°C) | Solubility of oxygen in freshwater (ie at 100% saturation) (mg/l) | ||
| 0 ppt | 30 ppt | ||
| 0 | 14.60 | 11.90 | |
| 2 | 13.81 | 11.29 | |
| 4 | 13.09 | 10.73 | |
| 6 | 12.44 | 10.22 | |
| 8 | 11.83 | 9.75 | |
| 10 | 11.28 | 9.32 | |
| 12 | 10.77 | 8.92 | |
| 14 | 10.29 | 8.55 | |
| 16 | 9.86 | 8.21 | |
| 18 | 9.45 | 7.90 | |
| 20 | 9.08 | 7.60 | |
| 22 | 8.73 | 7.33 | |
| 24 | 8.40 | 7.07 | |
| 26 | 8.09 | 6.83 | |
| 28 | 7.81 | 6.61 | |
| 30 | 7.54 | 6.39 | |
| 32 | 7.29 | 6.19 | |
| 34 | 7.05 | 6.01 | |
| 36 | 6.82 | 5.83 | |
| 38 | 6.61 | 5.66 | |
| 40 | 6.41 | 5.50 | |
Other environmental factors which influence the dissolved oxygen in water include:
Phytoplankton blooms: during blooms dissolved oxygen will fluctuate during the day due to photosynthesis, with maximum concentrations during late afternoon and minimum concentrations at dawn. Dissolved oxygen will also decrease during the death of blooms due to bacterial respiration.
Organic loadings: bacterial oxidation of organic matter removes oxygen from water.
Respiration of fish and other aquatic vertebrates and invertebrates.
The basic requirement of fish for dissolved oxygen varies considerably, depending on several factors:
Species-oxygen requirements vary between species
Size-fry and juvenile fish normally require more oxygen per unit weight than adult fish
Activity-exercised fish require more oxygen than resting fish
Temperature-fish normally require more oxygen as temperature increases( this may cause problems as the water holds less oxygen at higher temperatures)
Feeding-oxygen requirements increases after feeding because oxygen is required to digest the food
Stress-stressed fish require more oxygen (this may cause problems if fish are stressed at times of low oxygen or poor water quality)
Typical oxygen requirements range from:
resting fish: 100–500 mg DO/kg wet weight/hour
active fish: 300–1500 mg/kg/hour
Some examples of times when the oxygen requirements of fish may exceed the available dissolved oxygen are given below:
After feeding-take care not to feed in the afternoon or evening in heavily loaded pond systems
After adding organic manure to ponds-organic material will consume oxygen during decomposition
Early morning in pond systems
During the death of phytoplankton blooms-decomposition requires oxygen
Increases in temperature-fish require more but there is less in the water (also bacterial respiration may increase at such times)
Decreased water flow in more intensive systems.
The first indication of possible oxygen stress may be change in fish behaviour, with fish crowding at the surface or near the pond inflow, gasping for oxygen. If sustained over a period of time, low dissolved oxygen may result in significant sublethal and lethal effects.
Guidelines for non-salmonids
| 0.3–0.8 mg/l | may be lethal to many fish species if sustained over a period of time (most pond fish species can tolerate near anoxic conditions for a short period at dawn provided such conditions occur for a short period of time) | |
| 1.0–5.0mg/l | may be sublethal effects, for example on growth, feed conversation, and tolerance to disease | |
| >5.0mg/l | warm water fish reproduce and grow normally |
Guidelines for salmonid fish
| 0.8–4.0 mg/l | may bed lethal to salmonids | |
| 4.0–6.0 mg/l | may be sublethal effects, eg poor growth, bad food conversion, reduced tolerance to disease | |
| >6.0 mg/l | salmonids normally grow and reproduce normally | |
| >7.0 mg/l | recommended for hatcheries |
Nitrogen
Nitrogen is a very important element in aquaculture. As a nutrient, it is an important requirement for phytoplankton growth. Ammonia and nitrite, two inorganic forms of nitrogen, are also toxic to aquatic organisms.
Nitrogen originates in the atmosphere (80% of air is nitrogen), but can be found in several different forms in water nitrogen gas, ammonia, ammonium, nitrate, nitrate and various forms of organic nitrogen.
Ammonia
Ammonia is usually the second most important water quality parameter after dissolved oxygen. The total ammonia concentration in water consists of two forms:
NH3 unionised ammonia (gaseous form)
NH4+ ionised ammonia (ammonium ion)
These two are in equilibrium according to the equation:

The unionised ammonia (UIA) fraction is toxic to fish. The concentration of unionised ammonia in water depends on the pH and temperature of the water. As a general rule, the higher the pH and temperature, the higher the percentage of the total ammonia that is in toxic unionised from. This effect is illustrated below:
Percentage of unionised ammonia in water of different pH and temperature
| pH | Temperaure (°C) | |
| 20 | 32 | |
| 7.0 | 0.4 | 1.0 |
| 8.0 | 3.8 | 8.8 |
| 8.2 | 5.9 | 13.2 |
| 8.4 | 9.1 | 19.5 |
| 8.6 | 13.7 | 27.7 |
| 8.8 | 20.1 | 37.8 |
| 9.0 | 28.5 | 49.0 |
| 9.2 | 38.7 | 60.4 |
| 9.4 | 50.0 | 70.7 |
| 9.6 | 61.3 | 79.3 |
| 9.8 | 71.5 | 85.8 |
| 10.0 | 79.9 | 90.6 |
| 10.2 | 86.3 | 93.8 |
Alternatively, the percentage of unionised ammonia in a given sample of total ammonia can be calculated from the equation:
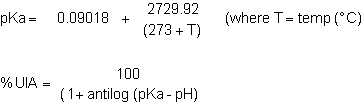
Amonia in water can originate from several sources:
Decomposition of organic matter-particularly after fertilising ponds with organic manure or inorganic ammonia based fertilisers. The decomposition of waste feed in intensive fish farming will also produce ammonia.
Industrial and domestic pollution
Excretion by aquatic organisms, particularly fish and shellfish in intensive aquaculture systems, Also, during fish transportation.
Denitrification-ammonia is oxydised to nitrite and harmless nitrate in oxygenated waters a (process known as nitrification). In deoxygenated waters, nitrate is converted to nitrite and ammonia (denitrification). Deoxygenation in heavily loaded pond system (such as intensive Clarias ponds in Thailand) can therefore lead to a build up of ammonia.
Death of phytoplankton blooms-high levels of ammonia in pond systems are commonly associated with the death of phytoplankton blooms.
The toxic effects of unionised ammonia on fish vary considerably depending on the fish species and environmental conditions. Some general guidelines are given below:
| 0.4–2.5 mg.l | Lethal to many fish species. Certain species, such Clarias batrachus have a very high tolerance to unionised ammonia with lethal concentrations around 3.4 mg/l | |
| 0.05–0.4 mg/l | sublethal effects depending on species, may include gill hyperplasia, reduced activity and growth, liver, kidney and brain damage. | |
| >0.02–0.05 mg/l | safe concentrations for many tropical and temperate fish species (salmonids are more susceptible). |
The toxicity of ammonia to fish is reduced at increasing salinity and at high dissolved oxygen and high carbon dioxide concentrations.
Nitrite
Nitrite is an intermediate product in the biological oxydation of ammonia to nitrate (nitrification). It is relatively low in most natural waters and healthly fish farming systems, but may reach high concentrations where there is organic pollution or oxygen is low.
Nitrite is highly toxic to fish. When nitrite is absorbed by fish it reacts haemoglobin to form methahaemoglobin. Methahaemoglobin is not as effective a carrier of oxygen as haemoglobin and therefore fish exposed to high levels of nitrite eventually die from lack of oxygen.
The main environmental factor which affect nitrite toxicity is chloride. The following guidelines have been developed for temperate water species by EIFAC :
Safe nitrite level (mg/l as N)
| chloride | salmonid | non-salmonid |
| 1 mg/l | 0.01 | 0.02 |
| 5 mg/l | 0.05 | 0.10 |
| 10 mg/l | 0.09 | 0.18 |
| 20 mg/l | 0.12 | 0.24 |
Lethal thresholds vary considerably Iron 1 mg/l for salmonids in low chloride waters to 152 mg/l for very tolerant species such as Clarias batrachus in high chloride waters.
Nitrate
Nitrate is the end product of the biological oxidation of ammonia and nitrite. It is effectively non-toxic to fish, except at concentration of <400 mg/l. Such concentration are unlikely in most water supplies.
Nitrogen gas
Water containing concentrations of gas above saturation levels known as supersaturated. Strictly speaking, all atmospheris gases can contribute to gas supersaturation, but because of its relative abundance in air, nitrogen gas in a major contributor to most gas supersaturation problems. These problems may occur under the following conditions:
Heating og water-the solubility of gases decrease with increasing temperature. Therefore, if saturated water is heated without allowing gas to escape, the water will become supersaturated. Mixing of waters of different temperatures can produce the same affect.
Ice formation-the solubility of gases is increased as water is cooled. As ice is formed, dissolved gases are expelled and concentrated in the remaining water. In shallow lakes, lethal dissolved gas levels may be formed under the ice.
Air entrainment-any time that air and water are in contact at pressure higher than aunospheric pressure, gas supersaturation may be produced (eg. spillways. air leaks in pipes).
Photosynthesis-dissolved oxygen production during algal blooms may result in lethal or sublethal gas supersaturation.
Pressure changes-reduction in pressure may result in gas supersaturation (eg in aircraft).
Gas bubble disease (or trauma) is classically characterised by gas bubbles in the blood, gills and other organs, resulting in various forms of tissue damage.
Safe levels: <105% saturation
Suspended solids
Suspended solids are normally defined as the solid material present in the water which is retained on a fine filter paper after filtration of the water sample. The mesh size of the filter influences the results and 0.45 um mesh is commonly used in most analyses. The suspended solids may also be measured indirectly using a Secchi dise (although this more correctly meaures turbidity).
Suspended solids may originate in the catchment area of a water supply through natural weathering of rocks and lend erosion or pollution. Within fish culture systems, suspended solids may come from phytoplakton blooms, uneaten food particles and fish faeces.
The effects of suspended solids depends on the nature of the solid. Abrasive particles such as wastes. from coal working or long-spind diatoms (algae) are more harmful to fish than soft materials.
The toxic effects of suspended solids are directed towards the sensitive gill tissues and gill damage, excessive mucus production and coughing and bacterial gill disease are all common responses to high suspended solids loads. The coating of eggs by suspended solids can also reduce efficiency of oxygen uptake and increase egg mortality.
Salmonids are extremely sensitive to suspended solids and he following safe levels are recommended:
| <20 mg/l | acceptable for ongrowing |
| <5 mg/l | essential for hatcheries |
Most tropical freshwater species such as the tilapias, many carps and catfishes are very tolerant of levels up to 10.000 mg/l, althoug effects will depend on the nature of the particle.
Suspended solids and turbidity may also be important in reducing the penetration of light into culture ponds, reducing the productivity and increasing the risks orf deoxygenation. Ponds with persistant turbidity problems (normally caused by fine clay mineral particles) should be treated with alum (25 – 45 kg/ha) or organic matter.
Acidity and alkalinity
pH
Definition
The pH of water is measure of the concentration of hydrogen icons in the water:
pH = - log (H+)
Note that because it is a logarithmic scale a one fold change in pH is equivalent to a 10 fold change in hydrogen ion concentration. pH is defined on a scale if 1 to 14; a pH <7 is acid and a pH >7 is alkaline.
Occurence of acid waters
Naturally acidic waters may be derived from waters draining peat swamps, acidic rocks or acid sulphate soils. Such waters may be particularly acid during floods, particularly in rain after a dry spell.
Pollution from mining and various industries (eg rubber and palm oil processing) may also be acidic. Effluents from such industries may also contain heavy metals and other acidic anions (eg chromic acid) which may themselves he extremely toxic to fish. Metal joins are more soluble in acidic waters and therefore all acid waters may contain metals which may be more toxic to fish than the acidity itself.
Occurrence of alkaline waters
Naturally occurring alkaline waters are usually derived from calcium and silica rich areas. In addition, legal blooms may result in very alkaline pH values.
Pollution from soft drink and brewing industries may also be extremely alkaline.
Physiological effects of alkaline waters
The optimum pH range for most freshwater fish species is from pH 6 to pH 9. Outwith this range, there are increasingly severe effects on fish production, because of increasingly toxic effects on the fish themselves and because, of adverse effect on pond productivity.
The direct toxic effects on fish at alkaline pH may star at pH 8 (artificial spawning trials of Sarotherodon aureus have failed at pH > 7.6) but continuous exposure to pH>9 is required before there are any toxic effects on most species. As a general guide:
| pH | effect | |
| >11 | lethal to all fish species except sometimes in ponds with very high levels of dissolved oxygen | |
| 10–11 | lethal to many fish species if exposed over a long period or time. Sublethal effects may include gill damage and damage to the lens and cornea of the eye (often seen as an opagueness in the eye) | |
| 9–10 | sublethal effects on many species. |
The toxic effects of high pH may also be made worse by the presence of metals (eg zinc) and by increasing toxicity of other compounds (eg ammonia) at high pH.
Physiological effects of acid waters
Acidic waters between pH 6.0 and 5.0 are not normally directly toxic to fish unless fish are acclimated to alkaline pH or the concentration of free carbon dioxide is greater than 20 mg/l or the water contains large amounts of iron or aluminium.
Increasing levels of H+ result in a gradual breakdown of the gill and epidermis in fish which results in increasing loss of body salts and difficulties in taking up oxygen. Eventually fish may die due to osmotic disturbance and/or lack of dissolved oxygen. The effects on the gills can be seen as swelling or destruction of the epidermal cells and excessive mucus production.
As a general guide:
| pH | effect | |
| <4.0 | direct mortality may occur in many fish species | |
| 4.0–5.0 | sublethal effects may include a loss of body salts, gill damage, reduced spawning success, poor growth and lowered resistance to disease in many fish species | |
| 5.0–6.0 | poor and productivity. |
There are many factors affecting the toxicity of acid to fish. Some of the most important factors are outlined below.
Carbon dioxide: high free CO2 increases the toxicity of acids.
Calcium, magnesium, sodium and chloride: the primary effect of acidity is to disrupt the ionic balance of fish. Thus, an increase in the concentration of these cations will help to protect fish from harmful effects of acids. Calcium is particularly important
Species, size, age, acclimation of fish: the fry stages on hatching fish are normaly most vulnerable to acids. Some acid ponds can be successfully used for fish culture if fingerlings rather than fry are stocked. Fish may also be acclimated to low pH if exposure is gradual. Rapid changes in pH are most damaging to fish, particularly if the fish are acclimated to high pH.
Many of the effects previously attributed to H+ ious are now known to be due to aluminium.
Alkalinity
Alkalinity refers to the concentration of bases in water ana capacity of the water to accept acidity (ie its buffering capacity). In most waters, bicarbonate and carbonate are the predominant bases.
Waters with a low alkalinity (total alkalinity < 20 mg/l as CaCO3) have a very buffering capacity and consequently are very vulnerable to fluctuations in pH (eg during rainfall and phytoplankton blooms). Such fluctuations amy be directly harmful to fish populations. Low alkalinity ponds also tend to be much less productive than high alkalinity ponds although ponds with alkalinities greater than 300 mg/l as CaCO3 may also be unproductive because of limitations to carbon dioxide availability at such concentrations.
The ideal range for alkalinity is 20 – 300 mg/l as CaCO3
Total hardness
The total hardness of water is made up of the cations of alkali earth metals, mainly calcium and magnesium ions. The total hardness concentrations should be similar to the total alkalinity in most waters because calcium and magnesium are commonly bound to the main alkalinity bases, bicarbonate and carbonate. When the total hardness of a water exceeds the total alkalinity, some of the calcium and magnesium is bound to anions other than bicarbonate and carbonate (eg sulphate and chloride). When the total alkalinity exceeds hardness, some of the bicarbonate and carbonate is associated with sodium and potassium rather than calcium and magnesium.
In most waters the alkalinity is more important than the total hardness. A total hardness of greater than 20 mg/l as CaCO3 is considered satisfactory for pond productivity and should help to protect fish against the harmful effects of pH fluctuations and metal ions.
Carbon dioxide
Carbon dioxide is a gas which is highly soluble in water, but because there is only a small amount in the atmosphere, the concentrations in most waters is low.
Carbon dioxide has an acidic reaction with water.

Because of this reaction, pure water in equilibrium with the atmosphere has an acidic pH. At 25°C. the pH of pure water is 5.7. As a general rule. Carbon dioxide will not cause the pH to drop below 4.5 and any pH below 4.5 must be due to mineral acidity.
Carbon dioxide can be found in three closely related forms in water:
CO2 - free carbon dioxide
HCO3 - bicarbonate ion
CO3 - Carbonate ion
The concentration of each depends on pH:
| CO2 | % of total CO2 in each form in relation to pH | |||||||
| pH | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| CO2 | 99.5 | 95.4 | 67.7 | 17.3 | 2.0 | 0.2 | - | - |
| HCO3 | 0.5 | 4.6 | 32.2 | 82.7 | 97.4 | 94.1 | 62.5 | 14.3 |
| CO3 | - | - | - | - | 0.6 | 5.7 | 37.5 | 85.7 |
Free carbon dioxide is the form that is toxic to fish. Consequently high toxic concentrations are normally only found in natural or acidic waters.
Most natural waters contain low concentrations of free carbon dioxide (>6 mg/l). However, carbon dioxide may reach high levels in the following circumstances:
Acidic ground water
In ponds with large phytoplankton populations carbon dioxide may reach high levels during:
- the death of a bloom
- at night due to phytoplankton respiration
- during cloudy weather
In ponds heavily loaded with organic manure of feed. Levels in Thai Clarias ponds commonly reach 30–40 mg/l.
Fish transportation - fish excrete carbon dioxide and consequently high concentrations may build up when a large biomass of fish is enclosed in a small volume of water. CO2 build up is worse when fish are transported in enclosed bags containing oxygen. Open tanks with aeration are less likely to suffer from CO2 problems.
In natural waters high concentrations may occur after herbicide treatments High concentrations of free dioxide can be harmful to fish. Carbon dioxide hinders the uptake of dissolved oxygen. Consequently the effects of high CO2 are made worse at low dissolved oxygen concentrations. In air breathers, such as Clarias and Ophicephalus, 90% of carbon dioxide is excreted across the skin and gills into the water. It is thought that high external concentrations will interfere with this exchange causing respiratory problems and stress. Some general guidelines may be given as follows:
| 50–60 mg/l | : | lethal to many fish species with prolonged exposure |
| 12–50 mg/l | : | sublethal effects may include respiratory stress and the development on kidney stones (nephrocalcinosis in some species). |
Hydrogen sulphide
Hydrogen sulphide is produced by bacteria in anoxic waters (ie waters deficient in dissolved oxygen). It is common in aquaculture systems where there is a heavy organic load (eg it heavily fertilised ponds or below intensive cage farms).
Two forms of hydrogen sulphide exist in water:
HS ionised sulphide ion
H2S unionised hydrogen sulphide gas
Unionised hydrogen sulphide gas is TOXIC to fish
Analytical techniques measure TOTAL SULPHIDE (as ammonia). The proportion of this total sulhide which is in the toxic hydrogen sulphide gas form is related to pH:
% total hydrogen sulphide in the toxic gaseous form at 25°C
| pH | % |
| 5.0 | 99.0 |
| 5.5 | 97.0 |
| 6.0 | 91.1 |
| 6.5 | 76.4 |
| 7.0 | 50.6 |
| 7.5 | 24.4 |
| 8.0 | 9.3 |
| 8.5 | 3.1 |
| 9.0 | 1.0 |
Most fish species are extremely sensitive to H2S gas. Toxic levels are:
| 0.002–0.4 mg/l | sublethal effects, including gill damage, depending on species |
| 0.01–5.3 mg/l | lethal effects, depending on species |
WATER QUALITY CRITERIA
| Salmonids | Sea bass and bream | |
| Dissolved oxygen | >6 mg/l > 7 mg/l (hatcheries) > 5.5 mg/l (rainbow trout) | >3 – 4 mg/l |
| Ammonia (unionised form) | <0.02 mg/l (unionised form) 0.002 mg/l (hatcheries) | <0.1 mg/l |
| Nitrite | 0.01 mg/l (soft water) 0.06 mg/l (hard water) (as NO2 - N) | |
| Nitrate | <40 mg/l | |
| Carbon dioxide | <6 mg/l free CO2 | |
| pH | 6.5–8.5 ideal | |
| Suspended solids | <5 mg/l (Hatcheries) <25 mg/l | found in very turbid Waters |
| Hydrogen sulphide | <0.002 mg/l | |
| Aluminium | <0.1 mg/l (pH 5–6) | |
| Cadmium | <0.001 mg/l | |
| Chromium | <0.025 mg/l | |
| Copper | <0.005 mg/l (soft water) <0.01 mg/l (hard water) | |
| Iron | <0.5 mg/l | |
| Lead | <0.001 mg/l (ideal) | |
| Mercury | <0.00005 mg/l | |
| Nickel | <0.01 mg/l (soft water) <0.05 mg/l (hard water) | |
| Zinc | <0.03 mg/l (soft water) <0.5 mg/l (hard water) |
SUMMARY - WATER QUALITY AND FISH DISEASE
| factor | disease link |
| stress | all fish more susceptible to disease following one or more stress events |
| Wastes | myxobacteria, fungal infection, gill disease (chronic, acute), Aeromonas |
| solids hardness | myxobacteria gill disease soft water - chronic kidney disease hard - soft water - poor transfer |
| Carbon dioxide temperature | nephrocalcinosis high temp - more stress - less DO Aeromonas >16–18°C for salmon myxobacteria - low T, high wastes |
| nutrients | algal blooms site dependant, gill damage, direct toxicity |
3. WATER QUALITY MANAGEMENT
Water quality within an aquaculture system is determined by three principal factors:
- The nature of the supply;
- The nature of the system;
- Management.
The water supply depends on location (altitude, latitutude, geology). Water quality in the farm depends on the quantities of uneaten feed, FCR, and the fates of these wastes (i.e. how quickly and gently they are removed from the system or processed into harmless compounds). The endogenous parameters (those produced or altered by the fish) can be controlled and managed to a certain extent. The different parameters are considered in turn below but first an outline of water quality management is given:
The essentials of good water quality management are:
Good site selection
Correct stocking biomass and density
Correct husbandry system
Monitoring
Planning
Remedial action
SITE SELECTION
Many factors are taken into consideration in site selection. Over emphasis on non-environmental factors such as price of land, road access and commercial opportunity may result in a site with a recurring environmental problem throughout the life of the farm which limits product on and causes stress to the cultured species. (e.g. land based farms with an inadequate water supply, cage farms in highly enclosed bays with poor water exchange).
STOCKING BIOMASS AND DENSITY
If a system is overloaded with an excessive biomass deterioration of water quality will result in an increase in stress and therefore disease. Holding capicity must be calculated for the particular system as some environments are more sensitive than others. In calculating holding capacity it may be possible to identify the parameters which will limit production and which may be the likely cause of health problems.
CORRECT HUSBANDRY SYSTEM
Some basic requirements for husbandry systems are:
- to provide for the environmental requirements of the stock
- to be able to observe and examine the stocks response in the production system
- control of the system so that conditions can be manipulated if possible
- failsafe in use i.e. if a breakdown occurs there is not an immediate fish kill
- easy as possible to operate e.g. feeding, cleaning, grading, disease treatment harvesting, removal of moribund/dead fish
- work economically-expensive systems may force the farmer to increase product leading to overstocking poor environment and disease problems
- hatchery systems should be compact with easy handling, observation and treatment eggs and fry
Without the correct system water quality problems will result, which will increase stress the incidence of disease.
some advantages and disadvantages of holding systems are listed below:
| PONDS | |
| Advantages | Disadvantages |
| Relatively low cost | Less easy to manage |
| Internal water reconditioning | Difficult to control water quality |
| Natural food supply | Stock handling relatively difficult |
| Easy to construct if location right | Possible dramatic changes in environment |
| Difficult to clean | |
| Generally lower production/unit area | |
| Difficult to adapt or move | |
| TANKS | |
| Highly controllable environment | Relatively high cost |
| Good self cleaning | Concentrated wastes may be discharged |
| Relatively high production/area | Design problems with scale-up |
| Easy to handle/observe stock | Note normally fail-safe |
| More uniform environment | Raceways wasteful of water with small fish - may require specific land gradient |
| CAGES | |
| Low cost | Difficult to control environment |
| Movable | Disease treatment often difficult |
| Relatively good water quality | Often difficult to observe fish |
| Easy stock management | Possible feed losses |
| Minimal need for land | More risk of physical damage |
| Rapidly developed | High labour input |
| Relatively high productivity | Wastes disposed around stock |
MONITORING
It is important to monitor environmental parameters on a regular basis, particularly at certain times of the year (e.g. particularly warm water, high biomass). This is important for two reasons. Firstly so that remedial action is possible if a problem can be seen to be developing (e.g. algal bloom, falling oxygen levels, high temperature. The type and frequency of sampling is discussed in the next lecture.)
PLANNING
In order to protect stock from a developing water quality problem, or to overcome an existing problem, good planning is essential. It this context planning means avoiding causing additional stress to fish at important times (e.g. not grading or treating when oxygen levels are low.) and readiness for deterioration of water quality so that remedial action can be taken quickly. (e.g. cage salmon farms in south west Ireland which experience toxic blooms of Gyrodinium aureolum increase water sampling at critical times of the year and ensure that aeration equipement is ready for emergency use.)
REMEDIAL ACTION
Where a particular water quality parameter is posing a problem the following streps can be taken:
Dissolved oxygen
Dissolved oxygen concentrations may be controlled by:
Aeration (or oxygenation)
Correct stocking and fertilisation of ponds (amounts and timing). Fertilisation can also be used to stop phytoplankton blooms from dying.
Increasing water flow
Good pond design - deep sheltered ponds are more vulnerable to deoxygenation than shallow open ponds
Ammonia
The toxicity of ammonia to fish is reduced at increasing salinity and at high dissolved oxygen and high carbon dioxide concentrations. Several techniques may be used to reduce the effects of ammonia on fish populations.
Improve overall dissolved oxygen concentrations by aeration - will also tend to decrease pH (hence reduce toxicity) and may blow off some of the gaseous unionised ammonia from the water.
Good pond management - healthy phytoplankton populations will remove ammonia from water. Care should be taken when using fresh manures high in ammonia (these can be left to dry for a few days if required, to allow ammonia gas to escape)
Stocking and feeding control and improved water flows in more intensive systems
Chemical treatment - salt has been show to reduce the toxicity of ammonia to Clarias. 200–300 kg/rai (I rai = 1600 m2) is commonly used to treat Clarias ponds in Thailand. Other forms of treatment for more intensive systems included ion exchange resins (zeolite) and addition of acid (commonly HCI) to reduce pH.
Biological filtration - may be used to treat water to convert ammonia to nitrite to harmless nitrate (nitrification). Essential ingredient of recycling aquaculture systems.
Nitrite
Problems with nitrite in fish culture can be avoided by:
correct stocking, feeding and fertilisation practices particularly by keeping ponds well oxygenated.
Addition of sodium chloride to ponds at 250 mg/l (succesfully used in Clarias batrachus culture in Thailand.
Biofiltration : biological conversion of nitrite to harmless nitrate.
Nitrogen gas
In order to avoid nitrogen gas supersaturation care should be taken in the following areas:
Heating of water - the solubility of gases decrease with increasing temperature. Therefore, if saturated water is heated without allowing gas to escape, the water will become supersaturated. Mixing of waters of different temperature can produce the same effect.
Ice formation - the solubility of gases is increased as water is cooled. As ice is formed, dissolved gases are expelled and concentrated in the remaining water. In shallow lakes, lethal dissolved gas levels may be formed under the ice.
Air entrainment - any time that air and water are in contact at pressures higher than atmospheric pressure, gas supersaturation may be produced (eg. spillways, air lakes in pipes).
Photosynthesis - dissolved oxygen production during algal blooms may result in lethal or sublethal gas supersaturation.
Pressure changes - reduction in pressure may result in gas supersaturation (eg in aircraft).
Acid and alkaline water
Treatment of alkaline water
Alkaline water can be treated in several ways:
Rapid fluctuation in pH caused by excessive phytoplankton blooms may be treated by ensuring the pond is welt limed and that the pond water has an alkalinity of greater than 20 mg/l as CaCO3.
Acid forming fertilisers
Addition of acid to water supplies (HCI and H2SO4 have been used: add small amounts and monitor pH to determine exact requirements)
Treatment of acid water
Acidic water can be treated in several ways:
Liming: the addition of calcium based material is preferable because calcium gives added protection to fish gills against the toxic effects of acidity
Salt water: sea water may be flushed through ponds in coastal waters to neutralise acidity.
Alkalinity
Ponds with low alkalinity can be treated with lime.
Hardness Total hardness can be improved by liming
Carbon dioxide
Carbon dioxide can be removed from water in several ways:
Vigorous aeration
Increasing pH by addition of calcium hydroxide (hydrated lime)

In ponds with low alkalinity, care must be taken not to overtreat because excess lime may cause the pH to rise which may be directly harmful to fish or increase the concentration of toxic unionised ammonia if total ammonia concentrations are high.
Field trials have shown that approximately I mg/l of hydrated lime can remove 1.68 mg/l of free carbon dioxide.
Control of phytoplankton populations and organic loading by correct stocking feeding and fertilisation.
Pond design-open shallow ponds are less likely to suffer carbon dioxide problems that deep sheltered ponds.
Hydrogen sulphide
Sulphide is oxydised to sulphate in oxygenated waters. Keeping the system well oxygenated is the best way of stopping hydrogen sulphide being formed and of removing it from the system (particularly close to the sediments).
4. SAMPLING AND ANALYSIS
AQUACULTURE WATER QUALITY SAMPLING
AIMS OF SAMPLING
Evaluating suitability of a water supply for establishing a new fish farm (more important for sensitive species or sensitive stages of the life cycle, eg. salmonids, prawns, hatcheries).
Routine water quality monitoring, in order to ensure conditions are optimal for fish growth.
Assessing natural productivity of a lake (or marine site), as a basis for estimating fish yields, or for examination of the potential for cage culture (intensive or extensive).
Monitoring of effluent quality (increasingly important)
CHOICE OF PARAMETERS TO ANALYSE
Conservative parameters (eg major ions (hardness), conductivity, alkalinity, salinity) require less frequent sampling (although beware acidic catchments or marine and freshwater systems receiving large amounts of rainfall)
Variable parameters (eg dissolved oxygen, ammonia, carbon dioxide) may be very variable within aquaculture system and require more frequent sampling (weekly, daily, diurnal).
The parameters which should be investigated depend very much on the type of system and the critical parameters which you identified. In general, a more extensive sampling programme is used to identify critical parameters and subsequent sampling will concentrate on these. A good pre-development study should also identify future analytical requirements.
A guideline for preliminary sampling in different systems is given below:
| SYSTEM | PARAMETERS | SAMPLING FREQUENCY |
| Heavily loaded flow through systems, recycle systems | DO.NH3.NO2 pH.T.CO2 solids (salinity, if SW) | daily at critical times plus 24h runs occasionally |
| Intensive ponds | DO.NH3CO2T pH. Secchi disc NO2 PO4 chlorophyll (salinity, if SW) | daily at critical times. plus 24h runs checks during high loading or algal blooms |
| Freshwater cages | DO, NH3, NO2, PO4 chloruphyll, Secchi, pH, (sediments), T | weekly, plus DO, NH3 daily |
| Seawater cages | as above, plus salinity and H2S | as above, plus salinity daily |
| Pumped intensive systems | as heavily loaded flow though, plus gas saturation | as heavily loaded flow through |
| Effluent checks | pH, BOD, NH3, solids, Total p(cages), DO | high loading, depends on consent conditions |
SOURCES OF VARIABILITY
Spatial variability
horizonal variability, eg in lakes and ponds (depending on wind, inflow, outflow configuration)
vertical variability, eg during stratification
Temporal variability
temporal variability varies depending on the type of water supply; eg in terms of stability, bore hole supply > lakes (sea) ponds > rivers > streams. Variability is also affected by season, tides, climatic conditions.
BIOLOGICAL INDICATORS
Critical fluctuations in water quality may be missed by water sampling. biological indicators sometimes give a better indication of overall water quality.
Why and who should monitor
(a) Statutory bodies
Most countries have some body or other concerned with regulating discharges from industry or agriculture. For legislative purposes either statuatory bodies or properly equiped and recognised water quality laboratories should carry out monitoring to ensure accuracy.
(b) The farm
Monitoring should be carried out to provide information on water quality for management.
Partly to fulfill legal consent obligations, partly to optimize conditions for stock. Some farms prefer an outside agency to carry our work.
The important parameters depend upon the system and any critical parameters which may have been identified. Usually the following should be monitored regularly and careful records kept as they are most important for fish health.
Dissolved oxygen
Temperature
Ammonia/nitrite
Transparency (cage sites)
These parameters can be monitored with probes or kits.
Sediments around water-based farms can be monitored for indicative changes in a number of parameters such as:
- macrobenthic community structure (most sensitive, although time-consuming)
- sedimentary redox potential (quick, easy; used in conjunction with macrofaona)
- organic C and N (useful, but patchy due to distribution of wastes and benthic inverts; may lead to complicated interpretation and thus not recommended for routime monitoring)
- photographs of the sediment surface
It is sometimes useful for the farm to monitor redox potential and sediment appearance to check for outgassing or excessive waste accumulation.
WATER QUALITY - USEFUL REFERENCES
Alabaster, J.S. (1982) Report of the EIFAC workshop on fish farm efflnents. EIFAC Technical Paper, No. 41
Alabaster, J.S. and Lioyd, R. (1982) Water quality critcria for freshwater fish Second edition. Butterworths.
Beveridge, M.C.M. (1984) Cage and pen farning. Carrying capacity models and environmentsl impact, FAO Fisheries Technical Paper No. 255
Boyd, C.E. (1982) Water quality management for pond fish culture Developments in Aquaculture and Fisheries Science, Volume 9, Elsevier
Boyd, C.E. (1989) Water quality management and aeration in shrimp farming Fisheries and Allied Aquacultures Departmental Series No. 2. Alabama Agricultural Experiment Station, Aubum University, Alabama, USA
Colt, J. (1984) Computations of dissolved gas concentrations in water as a function of temperature, salinity and pressure Americal Fisheries Society Special Publication.
Colt. I. (1986) Gas supersaturation - impact on the design and operation of aquatic systems. Aquaculture Engineering. 5.49 – 85.
Exley, C and Phillips. M.J. (1988) Acid rain: Implications for the farming of salmonids. Recent AdvAnces in Aquaculture, volume 3 Croon Helm.
Sniezko, S.F. (1974) The effects of environmental stress on outbreaks of infectious diseases of fish J. Fish Biol 6. 197 – 208.
Thurston, R.V. et al (1983) A review of the EPa Red book: Quality Criteria for Water. Water quality section of the Americal Fisheries Society, Bethesda, MD.
Us Environmental Protection Agency (1977) quality Criteria for Water Office of Water and Hazardous Materials. U. S. Environmental Protection Agency. Washington, D.C. 256pp.
By M.T. HORNE
SCOTLAND
Routine monitoring, isolation and identification is part of normal, goods farming practice. If a farm has a disease problem:
Obtain information, before visit, of type of farm, size of farm, species concermed, any previous history of discase and any indicators of the quality of the farming practice at the site.
At the farm, obtain information which might be relevant to the occurrence of disease, For example, any rise or fall in temperature before the disease outbreak, any change in water quality (oxygen level, flow rater, suspended solids or pollution).
Any change in management , for example, grading, chemical treatments, fish movements, importation of new stocks, change in appetance or diet.
The farm records and farm staff should have this information.
This is all helpful because good quality fish, well managed, do not easily contract disease. Many discase problems are «secondary» caused by stress consequent to changes in the items listed above.
Look at the external sings of the fish behaviour abnormal swimming, «itchings» , clustering
at outlets, jumping excessively.
Chemical signs: haemorrhages, lession, gill conditions, parasites, binsters, fungal growth.
Similarly study the intenal appearance for these purposes and for sampling (below) use moribund or freshly dead fish. Do not forget to examine normal (non discased) stocs from the same farm.
Sampling:
Where possible use a field sampling kit or transport suitable individuals to the laboratory. Transporting dead fish except for very short journeys, is not so good.
Tissue samples, where live isolation of pathogen is not required, should be transported in a suitable fixative for either histopathology or electron microscopy.
If tissue samples are transported for live isolation waterproff them in polyester and transport on ice.
External sampling: The surface of a fish will always have many bacteria which will grow in culture media but which are not associated with disease. It may be necessary to sue a partially selective medium in addition to normal media.
The central, necrosed part of a lesion will have many such bacteria: look for the pathogen at the periphery whwre new tissue is being necrose. Gills may be sampled by a direct «print» to a glass slide (for staining and microscopy) or by a print onto growth medium followed by spreading for single colonies.
Internal sampling: Swap the ventral surface with a sterilant of heat. Cut with a fresh scalpel through the sterilised are. Avoid puncturing internal organs. Sample any affected areas. Sample also «healthy» tissue. Good, «routine» sample areas are the anterior kidney and the spleen. Sampling is done by the use of a bacteriological loop, be careful to be clean. Surface: sterilise the organs for sampling if there is extensive haemorrhaging since the blood will probably be contaminated.
Remove samples for histopathology after sampling for bacteria has been completed.
Identification in the laboratory.
For general growth use
- Nutrient broth/agar
ultra-violet microscope.
Frequently diagnosis is of lesser importance than recommendation of therapy. Here the antibiotic sensitivity of the isolate s required. Use commercially available testing discs which contain relevant levels of antibiotic and will give zones of lysis, indicating sensitivity, when placed on an agar plate culture of the pathogen. Rarely the MIC (Minimal Inhibitory Concentration) may be required. This is asseyed by growth in a range of concentration of the antibiotic.
Bacterial groups discussed:
VIBRIOS
AEROMONADS
GRAM NEGATIVE+PIGMENTED RODS
EDWARDSIELLA
- Tryptic Soy broth/agar
If marine bacteria are being isolated, ensure that the sodium chloride is a minimum of 0.5%. 1% salt is normal for initial isolation. It may be necessary to add some serum or blood, 1 – 5%.
There are many specialist agars such as Marine Broth/Agar or Cytophaga Agar which may be preferred. Some bacteria are more fastidious and may grow slowly for example Renibacterium., Where not bacteria are cultured, histopathology may indicate whether this is genuinely negative or is result od culture conditions. Incubation temperature may sometimes be a problem. For example, vibrio salmonicida grows poorly or not all above 14°C. It may also be useful in diagnosis, for example, vibrio alginolyticus and parahaemolyticus grow at > 35°C.
Selective media are not extensively use for priminary isolation but may be useful for diagnosis later or where there is heavy, non-specific contamination. Examples are Rimmler-Shotts medium for Aeromonas, Thio-citrate, bile salts medium for vibrio species and SKDM for Renibacterium.
Identification is made from:
Gross morphology of colonies on media.
Gross morphology of individual cells (microscopy) such as shape, size, motility, gram reaction etc,. The electron microscope is easy to sue and informative at this stage. At this stage a presumptive diagnosis is likely to be possible based upon the clinical signs, the farm history, gross cell morphology and rapid serological tests.
Biochemical tests based on the detection of enzymes, ability to utilise different carbon substrates, tolerance of physical and chemical conditions. Use proper diagnostic tables such as those in Bergey's Manual of determinative Bacteriology.
Serological tests depend on the availability of sera containing specific anti bodies. The commonest tests are simple, slide-agglutination, Ochterlony reaction and EIA. For detection and diagnosis of pathogens in histo-pathological tissue section immunofluorencense is valuable but requires the use of an.
By Prof. Dr. Haluk ERGUEN
TURKEY
Bacterial disease are responsible for heavy mortality in both wild and cultured fish.
The majority of fish pathogens are Gram-negative rods but there are some Gram-psoitive pathogens, including a few which are acid-fast.
With the growing interest in the development of fish culture, there is the importance of diseases as one of the major limiting factors in culturing of fish. In many countries, there has been considerable recent development of fish farming in warm sea water. From that reason, aquaculturists must be carefull for diagnosis and control of diseases.
In many countries, wild fry are still main source of culture material especially for extensive farming. We know that, this is in spite of the increasing prospects for hatchery reared culture stocks, since production of this stocks will remain limited by practical and socio-economic factors (the high cost of hatchery construction and operation and the expertise requirements).
The purpose of the studies of fish diseases, is to close the gap in knowledge on the diagnosis and control of diseases already affecting, or posing a potential health hazard to cultural gilt head sea-bream.
One of the important diseases of the Gilt head Sea-bream (Sparus aurata) is Vibrio spp.
Vibrio disease is a serious and economically important disease occurring during the warmer months in salt water.
The first external signs were small petechiae in the mouth region and on the opercula and at times on the ventral body surface immediately anterior to the pectoral fins. From that reason we called «Red pest» or «Red boil disease».
The incubation period varies with temperature, strain virulence and the degree of stress under which the fish are living.
They are Gram-negative curved rod 0.5 × 1.0–2.0 um.
Clinical pathology:
First signs of losses, affecting most susceptible fish, are often anorexia, darkening and sudden death.
Acutely affected fish show swollen, dark skin lesions which ulcerate to release blood coloured exudate. Ulcers may be very deep and necrotic.
Internally: main feature is enlargement and liquefaction of the spleen and kidney and petchlation of visceral and parietal peritoneum. Focal haemorrhages may also be seen on the surface of the heart and the gills are usually paler.
Histological examination of peracute cases, cardiac myopathy, renal and splenic necrosis and periorbital oedema. Acute cases show less severe cardiac lesions but are characterized by the skin lesion which comprise acute hypodermal inflammatory foci extending deep into the muscle.
Although these lesions are limited by the stratum compactum for some time, they eventually ulcerate. There is severe myofibrillar necrosis with the center of the lesion comprising an agglomerate of sarcoplasmic debris, macrophage nuclear baseophilic remanants and fibrin, with bacteria. In the liver there is focal necrosis and spleen and kidney. In the kidney the necrosis extends to the renal glomerulus, tubules and often to the endocrine cells of the internal tissue.
In chronic cases the severe haemolytic anemia induced by the lytic toxin of the vibrios results in heavy deposition of haemosiderin in the melanomacrophage centres of the remaining splenic and renal haemopoietic tissue.
Control of the disease:
Immunization and genetic selection have been show to improve the resistance of the fish. Vaccination is possible
We can be use Oxyletracycline, Suplhonamides and Nitrofuraus, but anorexic fish do not receive the drug.
Isolation of bacteria:
The two media routinely used for the first isolation were TSA (tryptic soy agar) and TCBS (thiosulfate citrate bile salts sucrose) agar.
Due to salt requirements of all the isolated, the media for isolation and for biochemical tests were prepared with 25% filtered natural sea water: The pH was adjusted to 7.2 – 7.4 with a 1 M NaOH solution.
Pseudomonas spp. and Aeromonas spp, are also identified in a few cases. They have also mentioned assoication of Myxobacteria with morbid gillrot and skin and fin necrosis in all cultured species of fish.
Pseudomonas spp.: it is found in soil and water and can often be isolated from decaying fish and spoilage-vulnerable foods.
It is usually motile, multitrichous polar flagella this organism grows well on ordinary nutrient media and usually produces diffusible fluorescent pigments, especially in iron-deficient media colonies are round and glistening and a fluorescent yellow colour may be noted in the adjacent medium.
This bacteria is usually associated with haemorrhagic bacterial septicaemia. The condition is usually clinically indistinguishable from aeromonad septecaemias.
Clinical pathology:
The haemorrhagia septicaemia may be acute or chronic. Large haemorrhagic skin lesions are the most commonly observed signs and heavy mortalities may ensure very shortly after the advent of lesions. At necrosis, in chronic cases fibrinous peritonitis have been described, and may be we can see ascites.
Histopathology:
Main foci of pathological change are the skin and haemopoietic tissues. In the skin the earliest changes, hyperaemia of dermal vessels with severe oedema extending into the lower epidermis. But ulceration follows and the lesions also extend down into the underlying muscle.
Spleen and kidney lesions are primarily interstitial and comprise rupture of melanomacrophage centers, necrosis of haemopoietic elements and presence of large number of melanin-granule-bearing macrophages within the renal blood sinuses.
Treatment:
Poor environmental conditions is the affective on the fish and this is the because of the pscudomonad infections.
We can be use Oxytetracycline with food but usually such infected fish will not feed.
If it is possible we can use kanamycin injection intraperitoneally. Also we must be change to possitive the environmental conditions.
Aeromonas spp: This organism is a Gram-negative red 0.7–0.8 um × 1.0–1.5 u. It is motile by means of polar flagella.
Culture:
This organism is readily isolated from the kidney or blood of affected fish on relatively unsophisticated media and by use of the Rimmler-Shotts selective medium it can usually be isoleted and typed in 24 hours.
It is cytochrome oxidase positive, chemo-organotrphie fermentative and respiratory.
This organism is usually associated with haemorrhagic septicemia of fishes, which are under stress for some other reasons.
The clinical feature and pathology are similar to those of psecudomonad septicaemia.
Clinical pathology:
Affected fish are usually under stress from some other factor and show darkening in colour, with large and irregular haemorrhages on the body surface and base of fins and ascites.
Internal organs are seen to be congested with haemorrhages over the viscera. Incision of the kidney and swollen spleen usually results in the semifluid contents dripping out.
Histopathology is indistinguishable from that of pseudomonad infection with the renal and splenic haemopoietic tissue reduced and the remaining cells necrotic. The intenstinal mucous membrane is usually necrotic and sloughed into the lumen. Focal necrosis is found in cardiac muscle, liver, gonad and pancreas.
The skin lesions beg in as severe oedema of the dermis and hyperacmia of the stratum reticulare, leading to spongiosis and ulceration of the epidermis followed by extensive hacmorrhagic necrosis down to the level of the muscle, but usually the lesions are more superficial than those of vibricosis.
Treatment:
Enviornmental improvement, reduction of the organic pollutants.
The condition can usually be controlled by treatment with antibiotics or polentied sulphonamides. But affected fish usually anorexic, paranteral treatment may be necessary.
REFERENCE:
COLORNI. A. PAPERNA. L. GORDIN, H., (1981) Bacterial infection in Gilt-Head Sea Bream (Sparus aurata) Cultured at Elat. Aquaculture, 23,257–267
ROBERTS. R. J., (1978) Fish Pathology. Bailliere Tindall. London
SHOTTS.E.B.JR., BOLLOCK.G.L., (1975) Bacterial Diseases of fishes; Diagnosis Procetures for Gram-Negative Pathogens. Jurnal of the Fisheries Research Board of Canada Vol:32,8
By M.T.HORNE
SCOTLAND
Viruses are genctic material, RNA or DNA, protected by a protein coat made up of identical sub-unit called capsomeres. They reproduce by invading a host cell and controlling its metabolism with their own genetic material. Because the infected cell is functioning in an approximately normal fashion there are no chemical treatment available for treating disease routinely: control is by avoidance, vaccination and culling.
Sampling procedures and field work :
Similar to those given for bactriology.
Laboratory procedures : (Sample preparation)
Samples for storage may be frozen at-20°C or-70°C although occasionally some viruses may be damaged by this. Samples for histopathology are fixed in formalin: samples for electron microscopy in glutaraldehyde. Other fixatives are available some of which have dual purpose.
For the isolation and growth of the virusis, homogenise the tissue sample in phosphate buffered saline (PBS), centrifuge or filter to remove cell debris. If the sample is not to be used immediately add antibiotics, for example, 100 i.e. Pencillin, 100 mg/ml Streptomycin and Nystation. If lonf term storage is planned also add 50% by volume glycerol and store at-20°C or-70°C.
Laboratory Procedures : (Host cells)
Several cell times are available commercially Rainbow trout gonad (RTG-2) and Fathead Minnon (FHM) are the commonest. Failure to isolate virus may be to the choice of an unsuitable host. It is also possible to prepare cell lines for other species of fish if required. In outline, tissue is homogenised in PBS and the integrity of the tissus destroyed by incubation with trypsin, Cells are harvested and re-trypinised. After final harvesting they are seeded into growth medium.
Laboratory Procedures : (Media)
There are many commercially available growth media and manufacturer's manuals should be consulted to determine which are most suitable for your purpose. In general terms such media are based on: balanced mineral salts, essential and non-essential amino acids, essential vitamins, antibiotics foctal calf serum and glutamine.
Laboratory Procedures : (Isolation of virus from fish samples)
Select the host cells and grow as a monolayer to 60–80% confluence. Change the mediaum and add the tissue extract. Allow the virus to absorb for 1 hour at 20°C. discard the medium and the sample and add fresh growth medium. culture and examine the cells daily for cutopathic effects (CPE). The most important of these are rounding of the cells, the formation of multinucleated cells and the formation of plaques due to cell lysis. These may be seen directly with a suitable substage microscope or by staining for normal, light microscopy.
Laboratory Procedures : (Indentification and quantification of virus)
Each of these should be studied in detail to obtain protocols and technique.
Serum neutralization:
Serum specific for the virus suspected, sometimes together with other viral sera, are added
to the wells of a tissue culture, microtiobic plate at an unform concentration Samples of
the supernatant from the cultures under scrutiny are diluted across the plate. Positive and
negative controls are incorporated. The plates are incubated and examined daily for signs
of CPE.
There are many variations on this procedure designed to investigate or quantify other parameters. For example, the use of an uniform virus those against sera taken from fish suspected of disease may be used to determine the presence of virl-specific antibodies in blood samples.
Enzyme immunoassay (EIA) :
Virus specific antibodies are coated onto microtitre plates-normally 96 well or strips-and
a postcoat added to prevent non-specific adsorption to the plastic. Tissue extracts from tissue
samples are added to the well and incubated for 1–3 hours. Positive and negative
controls are used. The sample is discarded and the wells washed. At this stage any antigen
(virus) present will have adhered to antibody. A second virus specific antibody, conjugaled
with an enzyme, (the «conjugate») is added and this will also combine with viral antigen
if it is present. This is then detected by the addition of the enzyme's substrate with a
colour dye (or «chromogen») coupled to it. The reaction of the enzyme with its substrate
relases colour indicating a positive (virus present) result. By diluting the reagents across
the plate and comparing with the control (virus positive) rows the concentration of virus
in the fish tissue sample may be quantified.
Complement Fixation (CF)
This test which, like the others, may be modified to show different parts of the virus host
interaction, depends, on the utilization of complement by the antigen/antibody reaction.
Complement is the name given to a cascade system of enzymes working as a unit equimolar
proportions in the blood. Viral antigen and antibody are allowed to react, in the presence
of complement, in a microtitre plate. The use of the complement gives no direct
visual effect. The amount of complement used is measured indirectly by the use of a
second antigen/antibody reaction which does give a visible effect i.c. Sheep red blood cells
and their specific antibody
Viruses discussed
Infections Pancreatic Necrosis (IPN)
Viral Haemorrhagic Septicaemia (VHS)
Infectious Haematopoietri Necrosis (IHN) - Lymphocystis.
By Akin CANDAN
TURKEY
Lymphocystis disease has been reported in almost one hundred marine and freshwater fish species, since Lowe (1874) described it in Platichthys flesus from Britain.
Lymphocystis disease is the only viral disease of Sparus aurata that has been reported until now. This first case in Turkey is the third report of the disease in Sparus aurata (Paperna et.al., reported the disease in Israel in 1982 and Menezes et.al., reported in Portugal in 1987).
Lymphocystis disease in a chronic, slowly developing viral disease of connective tissue cells. Only cells infected with the virus become hypertropic others near or attached to affected cells, remaining unaffected. The tumorous growths are not malignant. The disease usually is not fatal to infected fishes.
The etiological agent of Lymphocustis disease belongs to the lridovirus family. The Lymphocystis disease virus is a large. complex virus with a deoxyribonucleic acid genome. The nucleocacid is icosahedral shaped. The viral diameter is with a rang of 200 to 300 u.m. The virus is located in the cytoplasm of infected cells, where it stimulates formation of bars of inclusion substance. virions can be found in large numbers in contact with the inclusion substance.
The prevailing water temperatures in the Acgea Sea water range annually from 17 to 28°C and the salinity is about 33%. In a period of 18 months, 14 fish farms were checked our periodically.
For the diagnosis, tissues with lesions were analysed under light and electron microscope and the existance of the virus particles in the cells were observed.
In March 1990, Lymphocystis infection began from one farm in Bodrum Yalikavak and spread to Marmaris Bozburun within 12 months. The clinical picture of the disease is chjaracterised by whitish nodules, which aggregates of hypertropic cells, scaltared on the fins and skin of affected fishes.
In heavily infected fish aggregates of hypertorphic cells, 2–3 mm. in diameter, covered a large portion of the body and the entire surface of the caudal and pectoral fins.
For light and electron microscopy studies, isolated, nodules of hypertrophic cells were fixed in 3% glutar aldehyde for 6 h.at 4°C washed extensively in buffer and postfixed in 1% OSO4 for 1 h. After dehydratation the tissue was embedded and blocked.
In size (150–250) mm. in diameter) and structure, Lymphocystis infected cells from S. aurata were in agreemant with data from other hosts such as flatfish (Russel 1974) sciaccids (Christmas and Howse 1970 and Paperna et al., 1982)
All organelles are enlarged in the lymphocystis cell: a large vesiculated nucleus with irregular ring and large nucleolus and large ribbonshaped basophilic inclusions at the peripheral zone of the cytoplasm.
The virus particles were 180–230 um. in diameter. the capsid was hexagonal and was surrounded by amorphous layer of fuzzyhalo.
Mortality levels of lymphocystis disease are quite low, but in heavy culture conditions of some farms in Bodrum peninsula the mortality was more than 10% among the small fish.
Maintenance of fish in high densities, frequent handling, transfer and netting, which always results in minor injuries and abrasions to fish skin, could undoubtedly promote transmission of the virus and at the same time stress the fish, hence reducing their tolerance to infection.
REFERENCES:
CANADA, A (1991) Determining of the seasonal bacterial and viral diseases in Gilt Head sea Brean) (Sparus aurata) I., 1758) and the development of the treatment methods. (Ph. D. Thesiz).
MENEZES, J., RAMOS, A and PERETRA, T.G. (1987) Lymphocustis diseas an outbreak in Sparus aurata from Rin Formoza south coast of Portugal. Aquaculture vol. 67,222–225. PAPERNA, I., ILANA SABNAJ.I., COLORNT, A., (1982) An otubreak of lymphocystis in sparus aurata l in the Gulf of A Qaba, Red Sea. Journal of fish diseases 5.433–437.
RUSSELL, P.H., (1974) Lymphocystis in wild plaice Pleuronectes platessa L. and flounder, Platichthye flesur L. in Btitsh coastal waters: a historpatholotgical and scrological study, Journal of fish Biology 6, 771–778.
YAMAMOTO, T, MAC DONALD, R.D. CILLESPIS, D.C., and KELLY, R.K. (1976) viruses associated with lymphocsutis disease and dermal sarcoma of walleye (Stizestedion vilerum), Journal of the Fisheries Research Board of Canada 33,2408–2419.