WIM TACKAERT and PATRICK SORGELOOS
INTRODUCTION
Artemia biomass and cysts can be produced in intensive as well as semi-intensive and extensive conditions. Intensive production is performed in indoor tank systems under completely controlled conditions (see Chapter 12). Semi-intensive and extensive production refer to the culture of Artemia in outdoor conditions. The former is performed in small and managed salt pond systems (mostly seasonal solar saltpon29 ds); e.g. with some degree of control over salinity, water retention time, and feed availability. The latter (extensive production) consists of the harvesting of mostly natural Artemia populations from large biotopes with year-round high salinity conditions such as large solar salt operations or salt lakes.
Since Artemia is highly susceptible to predation, a major prerequisite to semi-intensive and extensive production is the availability of brine of sufficiently high salinity that is free from fish and invertebrates. In most saltpond locations/natural biotopes, this situation is reached at salinities from >80 to 100 g/1, although situations have been reported where fish and insects were still present at salinities of >100 to 130 pp.
Natural populations of Artemia are widely distributed over five continents in a variety of isolated biotopes such as inland salt lakes, coastal lagoons and especially coastal salterns associated with commercial solar salt production.2.3 A recent list of natural Artemia sites compiled by Vanhaecke et al.3 extends to over 350 localities.
In most natural Artemia populations, densities are low, mainly as a result of food limitation due to a low nutrient content of the water. The few exceptions which have higher productivity are those biotopes (mostly large solar saltworks) which are located in highly europhic areas (e.g., near population centers, estuaries, or mangrove areas) such as the Leslie Saltworks in the San Francisco Bay, and the salinas of the Bohai Bay in China.4 Production estimates for a few natural Artemia biotopes are given in Table 1. As a result of their generally low productivity, most of the natural biotopes offer opportunities for extensive harvesting of Artemia biomass only. Production of cysts in these biotopes, especially when the local ecological conditions are fairly stable, occurs only occasionally or is erratic. Moreover, since the quality of Artemia cysts differs from strain to strain and eve from harvest to harvest5.6 it is imperative to determine its nutritional value for specific application in aquaculture, prior to commercial development.
A schematic outline of a typical solar saltwork is given in Figure 1, sea water flows over a series of successive ponds in which salinity gradually increases as sea water evaporates. During this process, salts with low solubility precipiate as carbonates and later as gypsum (see Figure 2). Finally, when the sea water has evaporated to approximately one tenth of its original volume,mother brine is transferred to the crystallizers where pure sodium chloride is deposited. Artemia is only found in the evaporation ponds of intermediate salinity, i.e., from approximately 90 g/1 (=upper tolerance level of predators) to approximately 200 to 250 ppt. At elevated salinities Artemia die as a result of either starvation because of increased energy associated with hyperosmoregulatory physiology7 and/or increased toxicity of the brine due to drastic changes in ionic composition caused by gradual precipitation and enrichment of different salts.
Very extended solar salt industries (i.e., highly mechanized operations of hundreds to thousands of hectares each with individual evaporation ponds of up to hundreds of hectares) are localized in climatic zones with high evaporation rates and restricted rainfall (e.g., various regions in Australia, South America, Mexico, United States, China, southern France, and southeast Italy) and are usually in production (though not necessarily harvesting) on a year-round basis. In contrast, numerous small cottage-scale units for artisanal solar salt production are in production in the tropical-subtropical belt only during a restricted period of the year, i.e., cycles of 3 to 6 months during the dry season when conditions are favorable for making salt.
Table 1: Production Estimates for Natural Artemia Populations
| Site | Maximum Country | production | Period | Ref. |
| Lake Rezaiyeh | Iran | 1.2 aduslts/l | 68 | |
| Sivash Salt Lakes | U.S.S.R. | 400/l | 69 | |
| Slagbaai | Bonaire, | 200–360/l | Oct.–June | 70 |
| Netherlands | ||||
| Antilles | ||||
| Mono Lake | California | 4 adults/l | ||
| 12 nauplii/l | June–Sept. | 71 | ||
| 400/l | Aug.–Sept. | 72 | ||
| Great Salt Lake | Utah | 10/1 | 73 | |
| 100–200g dw/m2 | per year | 74 | ||
| Salin de Giraud | Camargue, France | 10–100/l | March–Oct. | 75 |
| 0.02–0.2 g/l ww | ||||
| 16,000/m2 | 76 | |||
| Long Island Salina | Bahamas | 25–100/l | May–Sept. | 51 |
| Alviso Salt Ponds | California | 13 g/m3 dw | summer | 77 |
| San Francisco Bay saltponds | California (harvest) | 5 kg/ha ww | per week | 78 |
| Crimea Salt Lakes | U.S.S.R. | 250 kg/ha | October | 79 |
| 3000 kg/ha | June | |||
| Burgas-Pomorije saltworks | Bulgaria | 2.75 g/l adults ww | ||
| 0.93 g/l juveniles | June–Sept. | 80 | ||
| 0.5 g/l nauplii | ||||
| Lake Grassmere | New Zealand | 4.2 g dw/m3 | Nov.–May | 81,82 |
| 4.9 g dw/m3 | Nov.–April | 81,82 |
some data compiled from Persoone and Sorgeloos.83
The large operations can only be managed with regard to Artemia presence, especially to control the opportunistic dispersion of Artemia3 when better hydrobiological conditions are required for solar salt production.4.8.10 As a result, the big-pond systems can be tapped for extensive harvesting only. The small units are much more versatile and provide realistic possibilities for human-managed production of Artemia biomass and cysts through inoculation with selected strains, through salinity control, pond retention times, food availability, predator presence, etc. This chapter will cover the basic principales and strategies for semi-intensive production of Artemia in seasonal saltponds.
II. SEASONAL PRODUCTION OF ARTEMIA IN SMALL SOLAR SALTFARMS
A. Typical characteristics of seasonal saltfarms
Seasonal saltfarms generally have an area of less than a few hectares, while individual ponds can be as small as a few hundred m2. The ponds are usually shallow with a water depth of <10 cm. Sea water of mangrove or estuarine origin is usually supplied by tidal inflow, although some farms use pumps, windmills, and/or manually operated waterscoopers to permit better manipulation of water levels.
During winter (e.g., in China, southern Spain, and Sicily, and Sicily) or monsoon season (e.g., in Central America and Southeast Asia) salt production is abandoned. In monsoon climates, salt evaporation ponds are enventually converted into paddy fields or shrimp/fish ponds (e.g., Southeast Asia). In some farms deep brine reservoirs (pickle ponds) are available for storage of the brine which remains available at the end of the dry season (e.g., Philippines and Indonesia); at the onset of the dry season, brine is pumped back into the evaporation ponds and allows a faster start-up of salt production.
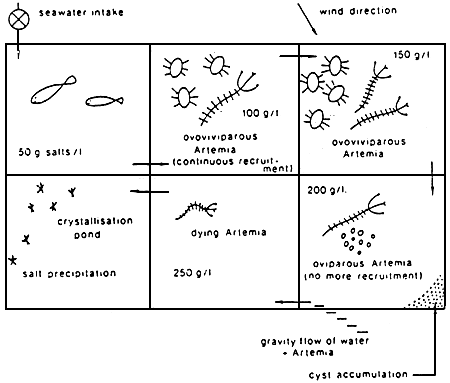
Figure 1. Schematic diagram of a solar salt operation with natural occurence of Artemia. (From Sorgeloos, P., Léger, P., Lavens, P., and Tackaert, W., Aquacult Dév. Cahiers Ethologie Appliquée, 7, 43, 1987. With permission.)
B. Pond modifications
Successful production of Artemia in seasonal solar-salt field involves minor pond modifications to increase the water depths in the future Artemia ponds (in the salinity range of 100 to 180 g/l) to a maximum level of about 40 to 50 cm (preferably 70 to 100 cm). High water depths are essentiel not only to prevent lethal high temperature conditions for Artemia, but also promote the development of phytoplankton which through its shading effect inhibits the development of phytobenthos. In contrast to phytoplankton, the latter is undersirable because it is too large to be ingested by Artemia and as it starts to float, it may further reduce evaporation and eventually contaminate the harvests of Artemia biomass and cysts as well as the salt. Since Artemia is a planktonic organism, deep ponds can also sustain a larger production per surface area than shallow ponds. High water depths and associated phytoplankton result in a dark colloration of the brine which is also benficial for salt production since it enhances the evaporation efficiency through increased absorption of solar heat. Higher water depths can easily be achieved by digging and inner perimeter ditch and using the soil from the ditches for heightening the dikes (Figure 3). However, the water levels in the Artemia ponds will then be higher than in the nonmodified upstream evaporation ponds, which implies that the brine has to be relifted (by pump or windmill) into the first Artemia pond from where it further gravitates into the following downstream ponds.
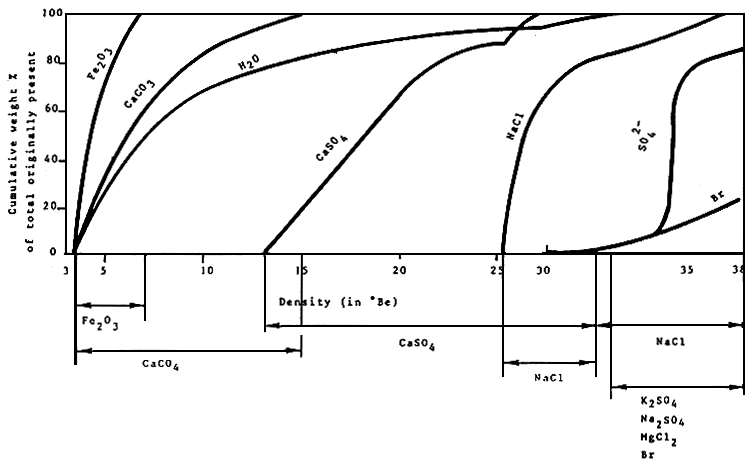
Figure 2. Deposition of salts during concentration of sea water (from Bradley, personal communication).87
C. Preparation of ponds
Prior to Artemia production, it is recommended that ponds be completely emptied to expose bottom soil for a period of one to two weeks, followed by raking the upper layer of the soil to enhance mineralization of accumulated organic matter, Fish left in remaining mud holes may be killed by the use of rotenone or tea-seed cake or by application of lime in combination with ammonium sulphate. 11.12 Coastal saltfields may be located in mangrove areas associated with acid sulphate soils containing pyrite. Upon exposure to air, pyrite is oxidized to iron oxides and sulphuric acid, especially in newly excavated ponds. The release of the acid entails very low pH values, resulting in aluminuium and iron leaching from the soil. The latter conditions are unfavorable for most aquatic organisms including Artemia; phytoplankton production is also inhibited though stripping of phosphorus from the water.13 In a number of cases, soil acidity may be visually observed; i.e., air-exposed soils turn yellow to brownish-red. Addition of lime neurtralizes the acid soil conditions, allowing for a better bioavailability of nutrients, which in turn enhances phytoplankton growth and production of Artemia. Lime, furthermore, aids in decomposition of pond muds, long term buffering of pH (which is essential in ponds where heavy organic fertilization is applied), and in the killing of undesirable fish eggs and pests through the toxic action of caustic components. There are several forms of lime : (1) calcium oxide, CaO, or quicklime has a neutralizing efficiency of 173% CaCo3, and is used for fast action in ponds with very low pH, in the range of 3.5 to 5.0; (2) calcium hydroxide, Ca(OH2), or hydrated lime has a neutralizing efficiency of 135% CoCo3 and also acts quickly to increase the soil pH; (3) calcium carbonate, CaCo3, or agricultural lime (ground limestone) acts relatively slow and therefore may be used for long-term acidity control. CaCo and Ca(OH)2 may be used in newly excavated ponds while CaCO3 is used in older, more stabilized ponds. To raise the pH by 0.1 unit, about 500 kg of CaCO3 is applied per hectare. 13 Lime is applied to dry pond bottoms although CaCO3 at low rates not exceeding 400 kg/ha, may also be used in ponds filled with water. Best results are obtained by spreading lime over the entire bottom or surface. Dikes should also be limed to prevent acidic runoff in case of rainfall.
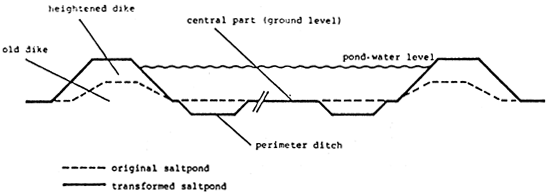
Figure 3. Longitudinal section through a modified Artemia pond. (Modified from Tackaert, W., Léger, P., Lavens. P., and Sorgeloos, P., El cultivo del Camaron, Langostino y Congrejo en el Mundo : Bases y Technologias (The Aquaculture of Shrimp, Prawn, and Crawfish in the World: Basics and Technologies), Chavez justo, C. and Sosa Nishizaki, O., EDS., McGraw-Hill, Mexico, 1989. With permission.)
D. Intake of sea water and increase of required salinities and water depths
Most artisanal saltfarms are designed to permit intake of sea water by tide. Some are provided with sluices in order to obtain the maximum water level in the reservoir as close to the high-tide level as possible. Even then, and especially in the case where modified Artemia ponds are operated at higher water depths, it is that the brine can travel through the entire system by gravity alone. Pumps or windmills (see Figure 4) are required to supplement or take the place of the tidal gates. While water depths in the reservoir should always be as high possible (to maximize supply of the brine to the downstream ponds), evaporators and Artemia ponds should initially only be filled to a level of 10 to 15 cm, in order to ensure maximum evaporation and to create high water temperatures harmful to predators. Predators should fruthermore be avoided by screening the intake water upon filling the ponds. If the pH of the water in the modified Artemia ponds is still lower than 7.0, it is advisable to wash out the remaining acidity by repeated flushing of the pond with sea water. If the pH is higher than 7.0, low water levels in the Artemia ponds are allowed to evaporate until a salinity of about 100 g/1 is reached : i.e., high salinity in combination with high temperatures obtained in thin waterlayers will eliminate copepods and other predators. At this point, gradual intake of sea water is restarted at a rate to maintain the slainity in the Artemia ponds around 100 g/1 be continue until the desired levels in the Artemia ponds (50 cm or more) are reached. While some predators (e.g., Cyrinodon variegatus and Aphanius fasciatus) are able to adpt to gradullay increasing salinities, they will not resist the severe salinity shock created by the above practice of water intake. As soon as the Artemia ponds are filled to their maximum level, the rate of sea water intake is adjusted to maintain these depths. Consequently a density gradient typical for a normal salt operation will be established in the successive Artemia ponds and evaporators. Between the Artemia ponds, the brine is perferentially bottom-drawn not only to prevent temperature and salinity strafication but also to enhance disribution and release of organic matter which accumulates on the bottom.

Figure 4. Windmill used in seasonal solar salt farming in Thailand. (From Sorgeloos, P., lavens, P., Léger, P., Tackaret, W., and Versichele. D., Manual for the culture and Use of Brine Shrimp Artemia in Aquaculture. Artemia Eeference Center, State University of Ghent, Belgium, 1986. With permission.)
E. Fertilization
1. General Requirements
Before introducing Artemia in the ponds, enough particulate food should be present in the water to guarantee high population productivity. Water with a green-brown color and a transparency of less than 20 cm mostly contains high concentrations of organic detritus particules and/or algae that can be used as food by the Artemia. This is generally the case in saltfarms associated with managrove areas or eutrophic estuaries. The availability of the nutrients from mangrove of estuaries can be maximized by pumping at low tide. 14 In this situation of good food availability, fertilization is not required, at least not before the introdcution of the Artemia nauplii. Water with only a slight coloration and a high transparency (>30 cm) is not productive enough and requires fertilization to increase the availability of natural food, 3 to 7 days prior to inoculation of Artemia.
Since the goal is to stimulate phytoplankton and not phytobenthos, it is essential to apply the fertilizer only to ponds already filled to maximum water levels. IN flow-through system, it is best to fertilize the low salinity ponds as we have often experienced difficulties in initiating a phytoplankton bloom when fertilizing high salinity ponds; i.e., chemical interactions limit the nutrient availability for a restricted number of algal species. In the latter systems, phytoplankton-rich water is ultimately drained into the high salinity ponds. Two kinds of fertilizers or a combination of both can be used: (1) organic fertilizers, such as dried chicken manure, and (2) inorganic fertilizers (commercial products used in local agriculture) with a high nitrogen and phosphorus content. Generally inorganic fertilizers stimulate phyoplankton growth more rapidly, while organic fertilizers act more slowly but provide a long-range effect since they first have to be degraded by bacterial action to release plant nutrients. In addition, some organic fertilizers such as dried ground chicken manure will easily disperse into the water column. Since they still contain up to 20% protein, they may also act as a direct food source for Artemia. Organic products are cheaper than inorganic fertilizers but much more bulky, and therefore involve more labor in their use. Moreover, organic fertilizers, especially when not properly distributed, may accumulate and decay at the pond bottom and create anaerobic zones resulting in oxygen deficiency, acidity, and toxicity through production of hydrogen sulphides.
Optimal rates of application are difficult to predict since they will vary from location to location due to climatic differences and quantitative/qualitative fertility of the local soil and water. Morales 86 reported that minimum concentrations of nitrogen and phosphorus in the water in order to obtain blooming of phytoplankton should be 1 to 2 mg/1 and 0.1 mg/1, respectively. Different fertilization programs also favor different types of food in the ponds; e.g., plankton is favored by a high ratio of nitrogen to phosphorus whereas organic manures, which are usually high in phosphorus, enhance the growth of undersirable filamentous algae. The fertilization program in Artemia ponds to be adjusted to optimize the availability of phytoplankton. The following doses can be recommended as a guideline. These concentrations have proven to be effective but other application rates and combinations of both organic and inorganic fertilizers are not excluded.
2. Organic Fertilizers
Best results to date have been obtained with chicken manure. Although cow and goat dung have been successfully used in some cases, chicken manure, in contrast to some other manures, such as from cattle (low nitrogen to phosphorus ratio : 1.5 ; high contents of undigested insoluble material) is more effective in inducing a phytoplankton bloom since it has a relatively high N/P ratio (3.5) and good dispersiblity, proving a larger surface for bacteria breakdown. In addition, it does not accumulate on the pond bottom since it is highly soluble. Van der Zanden15 reported a significant increase in the development of phytobenthos or «lab-lab» whenever cow dung was used.
Chicken manure needs to be dried and sieved for removal of debris, bran, feathers, etc., and it is preferable to grind it to increase its availability as a driect food source for Artemia. Nonetherless, good results have been reported by Jumalon et al. 11 when using chicken manure suspension (1:1 ratio of manure to sea water).
Dry chicken manure is applied at rates of 0.5 to 1.25 ton/ha at the start with dressings of 100 to 200 kg every 2 to 3 days.
3. Inorganic Fertilizers
Combination of 100 kg/ha mono-ammonium-phosphate (N:P:K ratio expressed in weight percentage of 16:20:0 and 50 kg/ha ammonium-nitrate (33:0:0); weekly dressings of 50 and 25 kg ha, respectively.
Combination of 50 kg/ha di-ammonium-phosphate (18:46:0) and 50 kg/ha urea (44:0:0); weekly dressings of 25 and 20 kg/ha, respectively.
100 kg/ha urea (44:0:0) weekly dressings of 40 kg/ha (e.g., in ponds where natural levels of phosphorus are high).
Recently a by-product from the industrial prodcution of monosodium glutamate derived from cassava or sugar cane molasses has been succesfully used as a cheap fertilizer for Artemia ponds. Application rates of up to 2500 1/ha have been found very effective in inducing dense phyoplankton blooms. In view of its acidity the effluents of the monosodium glutamate fermentation should be applied in small quantities but on a frequent basis.
Both organic and inorganic fertilizers should be very evenly spread over the pond surface. Slow dissolving pelleted fertilizers (e.g., 16:20:0) are first made into concentrated solutions (prepared overnigh) or are placed on a platform in areas of active water flow (e.g., near the brine intake), 15 to 20 cm below the water surface in order to ensure a more even release and mixing and prevent any trapping of nutrients into the soil.
Although the fertilizer is generally applied directly to the Artemia ponds, several farmes are now working with separate «food production ponds» from which they feed the Artemia ponds; these may be low salinity ponds, 50 to 80 g/, which are not neccessarily integrated integrated in the brine circuit. Availability/production of phytoplankton in these ponds is maximized through intake of «green water» from fish/shrimp ponds or through supplemental fertilization with fecal droppings from a vertically integrated poultry farm.11.12
F. ARTEMIA STRAIN SELECTION
In view of the high degree of genetic variation 16 associated with the diversity of biometrical, and physiological characteristics 5.17 found among strains of Artemia the selection of the strain best adapted to the particular ecological conditions (espacially temperature regimes) of the saltfarm and/or most suitable to its later application in aquaculture farms, is very important. Strain selection can be based on the available data of grawth and production performance, 18.19 reproductive characteristics, 20 anion concentration tolerance,21 and especially temperature/salinity tolerance. 22 IN addition, whenever possible, a comparative bioassay culture test should be performed in closely simulaed conditions using the untreated brines of the habitat as culture medium. Strain selection might also be restricted by the intended application of the produced Artemia in local aquaculture; e.g., if small nauplii are needed for the production of the early larval stages of fish and shrimps, a strain producing small cysts and nauplii is to be preferred. 23 On the other hand, if local aquaculture is primarily interested in Artemia biomass as a nursery/weaning or shrimp maturation diet, then a strain showing good growth and survival as well as dominant ovoviviparous reproduction characteristics will be the most interesting.
G. INOCULATION PROCEDURES
Inoculation of Artemia should be performed as early as possible in the brine circuit where no predators are found (i.e., usually at salinity levels of around 100 g/l). In flow-through systems with short pond-retention times, downstream ponds at higher salinity need not be inoculated since they will be gradually stocked with Artemia drained from the inoculated ponds. Although Jumalon and Robles24 reported an optimum Artemia production at an inoculation density of 50 anuplii per 1, Vu Quynh and Nguyen Ngoc Lam25 reported faster growth and maturation as well as higher fecundity at densities <20 nauplii per 1. From our observations it is clear that small inocula (10 to 20 nauplii per 1) are generally as effective (at least under normal temperature conditions) and more economical then large inocula since they exhibit a faster population increase when compared to the latter. The quantity of cysts needed to obtain the number of nauplii needed for inoculation (and taking into account a 30% mortality at stocking) is calculated from the pond volume and the hatching efficiency of the selected cyst batch.26 Cysts are preferably hatched close to the ponds. Optimal hatching conditions26 are often difficult to achieve under field situations; nonetheless, the following aspects should be taken into consideration:
Shaded or roof-covered area should be used to prevent excessive heating containers by direct sunlight.
Transparent, preferably funnel-shaped hatching containers, should be used. If flat-bottomed containers have to be used, they should be equipped with several aeration lines on their bottom so as to provide good mixing and aeration of the water.
Hatching containers should be illuminated during the might to provide the essential light stimulus27 especially when cysts are incubated in late afternoon or evening.
Air should be supplied from a blower, compressor, or several aquarium pumps powered by the net current, a generator, or batteries.
Clean 20 to 35 ppt sea water should be used, filtered through a fine filter cloth (100 μm).
Solium bicarbonate should be added at a rate of 1 g/l hatching medium so as to buffer the sea water.
Cyst densities should not exceed > 1 g/l, espacially under suboptimal hatching conditions.
It is essential to harvest the nauplii in the first instar stage. This is determined from subsamples taken at regular intervals or from hatching rate and synchrony data for the given strain or batch.28 Older instar stages will not survive the salinity shock when transferred from natural sea water into 100 g/l of salt water. After hatching, the nauplii should be screened over a 125 μm filter, thoroughly washed and transferred to clean sea water or pond water or pond water at half their hatching density. They are now ready for inoculation into the pond. If the pond is not within walking distance from the hatching site, aeration should be provided during transport (battery-operated pump or oxygen tank) to prevent mortality. If transport takes several hours, it is best to cool the nauplii container to 0 – 5°C using cooled sea water or adding ices bags. At these low temperatures the naupliar metabolism and motoric activity are strongly reduced without affective their viability29 Moderate aeration, e.g., with a battery-operated aquarium pump, has to be provided to keep the motionless animals in suspension.
In this way about 100 million nauplii can be successfully transported for a period of several hours in a 20 liter plastic bag packed in a cooled styrofoam box. The best time of the day to inoculate a pond is during late evening when the water temperature is low and will continue to drop until early morning. When the nauplii have been transported at low temperature it is essential to allow the temperature in the containers to rise so that the animals can resume their motoric activity before they are introduced in the pond. Under heavy wind conditions it is important to siphon the nauplii on the leeward side of the pond to avoid having them driven on shore by heavy wave action.
H. FACTORS AFFECTING POPULATIONS GROWTH
During the first days after inoculation it is very difficult to see nauplii since thet have lost their distinc orange color and tend to concentrate in the deeper marts of the pond. It is only when the nauplii have grown into adults that one may evaluate if the inoculation has been successful. In fertilized ponds, operated under optimal conditions (i.e., temperatures within the tolerance range for the selected strain; pH between 7.5 and 8.5; intermediate salinity levels of 100 to 150 g/l; and presence of sufficient quantities of particulate feed, especially phytoplankton) sexual maturity may already be attained 7 to 10 days after inoculation. 25.30 Under these conditions the parental and the first generations will generally reproduce by ovoviviparity, resulting in a fast increase of the population. The size to which the population will grow in determined by the carrying capacity of the pond. The principal factors which affect this carrging capacity are ponddepth, food availability (determined by the concentration of nturients in the water), and the frequency of water intake which will improve the water quality and result in an extra nutrient influx and better mixing of the nutrients which accumulate on the pond bottom. Aside from quantity, quality of the planktonic algal population may also affect the populations growth of Artemia. Green algae (e.g., Tetraselmis and Dunaliella and diatoms (e.g., Chaetoceros, Navicula, and Pleurosigma) are a much better food for Artemia than planktonic filamentous blue-green algae (e.g., Lyngbya and Oscillatoria). The latter are too big for Artemia to ge ingested and clog their thoracopods resulting in starvation to the Artemia. Filamentous blue-green algae might predominate in stagnant waters (since green algae and diatoms sttle) and in conditions of high concentrations of organic matter, high pH, and low CO2 levels.31.33 In this regard, Jumalon and Ogburn33 found a high correlation between the arrest of water intake rich in CO2, the collapse of green algal populations, and the development of planktonic filamentous cyanophytes. This implies that regular water intake is important in pond management; i.e., aside from affecting the amount of food, continuous flow also stimulates blooming of particular phytoplankton species more suitable for Artemia development.
1. FACTORS CONTROLLING CYST PRODUCTION
Although the factors controlling the mode of reproduction are not fully understood, oviparity in Artemia is generally considered to be induced by environmental stress.34,35 In pond systems, cyst production is often observed when the population is exposed to high salinities (e.g., when the Artemia reach the high salinity ponds). Salinity shocks have also been found effective in switching the population toward cyst production36.37 i.e., abrupt lowering or raising of the salinity through rapid intake of brine of a very different density or at heavy rainfall. In addition, low oxygen concentrations or considerable fluctuations in dissolved oxygen levels reportedly induce oviparous reproduction in Artemia.34.38 This condition stimulates the synthesis of hemoglobin and the consecutive excretion by the brown shell gland of its metabolic end product, hematin, which is the main constituent of the cyst shell of Artemia.39.41 Oxygen stress in pond conditions may be accomplished by raising the salinity and/or by increasing the rate of fertilization to induce blooms of phytoplankton, creating extensive diurnal fluctuations in dissolved oxygen.
Several authors postulated that the frequency of oviparity in Artemia is not only correlated with environmental stress, but is also influenced by the geographical strain of Artemia which has been used for inoculation.20.42.43 In this regard, Gajardo and Beardmore44 suggested that encystment in A. franciscana is under genetic control and is associated at least in part with the levels of heterozygosity found in the females. In view of the considerable interstrain differences in the distribution of heterozygosity16 care must be taken in selection a strain showing high heterozygosity levels; e.g., a strain inhabiting a variable and stressful environment, if cyst production is preferred. Even then, pond management should be directed toward creation of stress conditions to retain the genetic variability in the population and consequently prevent a decline in the cyst production.
It has been observed on several occasions in tropical habitats that newly introduced populations initially exhibit a high rate of oviparous reproduction, followed, however, by a drastic decrease in cyst production as soon as the population has become fully established (adapted to the new environment?) and/or the biotope has become completely stablizied, e.g., in Brazil,45 Thailand,12 and Vietnam.46 This phenomenon was recently been observed for the second time in Macau, Brazil: reappearance of cyst production at the end of 1987 and beginning of 1988 was associated with transformation of the local shrimp farm into evaporation ponds for salt production presenting a «new biotope» for Artemia. Bathélémy-Okazak and Hedgecok47 persumed that this decline in cyst production may be due to harvesting of the cysts leading to a removal of the genotypes predisposed towards oviparity form the population. They suggested that cyst production could possible be revived by reinoculation with a highly oviparous strain.
J. MONITORING OF ENVIRONMENTAL CONDITIONS AND FOOD PRODUCTION IN PONDS
A basic prerequisite for correct pond management implies regular evaluation of the environmental conditions of the ponds. The physico-chemical parameters to be monitored include(1) dissolved oxygen, readily measured with an oxygen electrode or by Winkler titration;48 (2) brine densities at the water surface and the pond bottom measured with a refractometer or with Baumé scale hydrometers (see conversion tables for brine density and degrees Beaumé, as corrections for temperature in Tables 2 and 3) pH-values using a pH meter; (4) air and water temperature at water surface and pond bottom with a minimum-maximum thermometer; and (5) water depth, read from a depth gauge.
Since maintenance of a healthy phytoplankton population is considered to be one of the most important keys for a successful Artemia production, regular monitoring of nutrient levels and associated standing crops of phytoplankton are important for proper pond management, i.e, rate of water intake, fertilization dressings, and biomass harvesting. The concentration of the major nutrients should be regularly controlled to ensure that no deficiency causing inhibition of algal growth is developing and/or that the ratio of N/P is not becoming too low. This involves analysis of reactive inorganic phosphate (the major form of phosphorus required for algal cells), reactive nitrate, and ammonia, preferable by standard colorimetric procedures.48.49 The phytoplankton population should be analyzed at least once a week. Phytoplankton densities may be determined from a representative sample by direct microscopic counting using a counting chamber. Whenever possible, species composition and cell size of the algal population should be determined, as the first may directly affect the nutritional value of the Artemia produced,5 and the latter determines whether the algal cells (especially when forming chains or colonies) are small enough (≤ 50 μm) for ingestion by Artemia.50 Records of phytoplankton species commonly found in nutrient enriched salinas were published by Davis51 and Wongrat.52 Other parameters to follow in respect to phytoplankton densities are water turbidity may be measured using a colorimeter or a Secchi disk. Procedures for measuring dry weight, chlorophyl, and primary productivity are described in Vonshack.53 Strickland and Parsons,48 and Boyd.54 In situations where the water contains little or no sill, a good correlation is found between the latter parameters and the water turbidity, the measure of which still is the easiest and most rapid way to determine changes in phytoplankton densities.
Table 2 : Conversion Table for Various Units of Salinity
| Density (g/ml) | Degree Beaumé (°Be) | Salinity (g/l) | Density | Degree Beaumé | Salinity | Density | Degree Beaumé | Salinity | Density | Degree Beaumé | Salinity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.020 | 2.8 | 28.6 | 1.061 | 8.4 | 1.102 | 13.4 | 1.141 | 17.8 | |||
| 1.021 | 3.0 | 1.062 | 8.5 | 1.103 | 13.5 | 1.142 | 17.9 | ||||
| 1.022 | 3.1 | 1.063 | 8.7 | 1.104 | 13.6 | 1.143 | 18.0 | ||||
| 1.023 | 3.3 | 1.064 | 8.8 | 1.105 | 13.7 | 1.144 | 18.1 | ||||
| 1.024 | 3.4 | 1.065 | 8.9 | 1.106 | 13.8 | 1.145 | 18.2 | ||||
| 1.025 | 3.6 | 1.066 | 9.0 | 1.107 | 14.0 | 1.146 | 18.3 | ||||
| 1.026 | 3.7 | 1.067 | 9.2 | 1.108 | 14.2 | 1.147 | 18.5 | ||||
| 1.027 | 3.8 | 1.068 | 9.3 | 1.109 | 14.3 | 1.148 | 18.6 | ||||
| 1.028 | 4.0 | 1.069 | 9.4 | 1.110 | 14.4 | 159.5 | 1.149 | 18.7 | |||
| 1.029 | 4.1 | 1.070 | 9.5 | 99.4 | 1.111 | 14.5 | 1.150 | 18.8 | 222.1 | ||
| 1.030 | 4.2 | 42.4 | 1.071 | 9.6 | 1.112 | 14.6 | 1.151 | 19.0 | |||
| 1.031 | 4.4 | 1.072 | 9.7 | 1.113 | 14.7 | 1.152 | 19.1 | ||||
| 1.032 | 4.5 | 1.073 | 9.9 | 1.114 | 14.9 | 1.153 | 19.2 | ||||
| 1.033 | 4.7 | 1.074 | 10.0 | 1.115 | 15.0 | 1.154 | 19.3 | ||||
| 1.034 | 4.8 | 1.075 | 10.1 | 1.116 | 15.1 | 1.155 | 19.4 | ||||
| 1.036 | 4.9 | 1.076 | 10.2 | 1.117 | 15.2 | 1.156 | 19.5 | ||||
| 1.037 | 5.0 | 1.077 | 10.3 | 1.118 | 15.3 | 1.157 | 19.6 | ||||
| 1.038 | 5.1 | 1.078 | 10.5 | 1.119 | 15.4 | 1.158 | 19.7 | ||||
| 1.039 | 5.3 | 1.079 | 10.6 | 1.120 | 15.5 | 175.1 | 1.159 | 19.8 | |||
| 1.040 | 5.4 | 56.4 | 1.080 | 10.7 | 114.1 | 1.121 | 15.6 | 1.160 | 19.9 | 237.8 | |
| 1.041 | 5.5 | 1.081 | 10.8 | 1.122 | 15.7 | 1.161 | 20.0 | ||||
| 1.042 | 5.7 | 1.082 | 11.0 | 1.123 | 15.8 | 1.162 | 20.2 | ||||
| 1.043 | 5.8 | 1.083 | 11.1 | 1.124 | 15.9 | 1.163 | 20.3 | ||||
| 1.044 | 6.0 | 1.084 | 11.2 | 1.125 | 16.0 | 1.164 | 20.4 | ||||
| 1.045 | 6.1 | 1.085 | 11.3 | 1.126 | 16.2 | 1.165 | 20.5 | ||||
| 1.046 | 6.2 | 1.086 | 11.5 | 1.127 | 16.3 | 1.166 | 20.6 | ||||
| 1.047 | 6.4 | 1.087 | 11.6 | 1.128 | 16.4 | 1.167 | 20.7 | ||||
| 1.048 | 6.5 | 1.088 | 11.7 | 1.129 | 16.5 | 1.168 | 20.8 | ||||
| 1.049 | 6.6 | 1.089 | 11.8 | 1.130 | 16.6 | 190.6 | 1.169 | 20.9 | |||
| 1.050 | 67 | 70.6 | 1.090 | 11.9 | 128.6 | 1.131 | 16.7 | 1.170 | 21.0 | 253.7 | |
| 1.051 | 6.8 | 1.091 | 12.0 | 1.132 | 16.8 | 1.171 | 21.1 | ||||
| 1.052 | 7.0 | 1.092 | 12.1 | 1.133 | 16.9 | 1.172 | 21.2 | ||||
| 1.053 | 7.2 | 1.093 | 12.3 | 1.134 | 17.0 | 1.173 | 21.3 | ||||
| 1.054 | 7.3 | 1.094 | 12.4 | 1.135 | 17.1 | 1.174 | 21.4 | ||||
| 1.055 | 7.5 | 1.095 | 12.5 | 1.136 | 17.3 | 1.175 | 21.5 | ||||
| 1.056 | 7.6 | 1.096 | 12.6 | 1.137 | 17.4 | 1.176 | 21.6 | ||||
| 1.057 | 7.7 | 1.097 | 12.7 | 1.138 | 17.5 | 1.177 | 21.7 | ||||
| 7.9 | 1.098 | 12.81 | 1.139 | 17.6 | 1.178 | 21.8 |
Table 3 : Temperature Corrections (to 20°C) For Density Readings of Concentrated Sea Water
| Density range (g/ml at 20°C) | |||||||
|---|---|---|---|---|---|---|---|
| Tem (°C) | From 1.00 to 1.05 | 1.05 1.10 | 1.10 1.15 | 1.15 1.20. | 1.20 1.25 | 1.25 1.30 | |
| 10 | Subtract correction from measured density | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.003 |
| 11 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.003 | |
| 12 | 0.001 | 0.002 | 0.002 | 0.003 | 0.003 | 0.002 | |
| 13 | 0.001 | 0.002 | 0.002 | 0.003 | 0.002 | 0.002 | |
| 14 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | |
| 15 | 0.001 | 0.002 | 0.002 | 0.003 | 0.003 | 0.002 | |
| 16 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | |
| 17 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| 18 | - | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | |
| 19 | - | - | 0.001 | 0.001 | - | - | |
| 20 | - | - | - | - | - | - | |
| 21 | - | - | 0.001 | 0.001 | 0.001 | - | |
| 22 | Add correction to measured density | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| 23 | 0.001 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | |
| 24 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.002 | |
| 25 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | .002 | |
| 26 | 0.002 | 0.002 | 0.003 | 0.003 | 0.003 | 0.003 | |
| 27 | 0.003 | 0.003 | 0.004 | 0.004 | 0.004 | 0.004 | |
| 28 | 0.003 | 0.003 | 0.004 | 0.005 | 0.005 | 0.004 | |
| 29 | 0.004 | 0.004 | 0.005 | 0.005 | 0.005 | 0.005 | |
| 30 | 0.004 | 0.004 | 0.006 | 0.006 | 0.006 | 0.006 | |
| 31 | 0.004 | 0.005 | 0.006 | 0.006 | 0.006 | 0.006 | |
| 32 | 0.005 | 0.006 | 0.006 | 0.007 | 0.007 | 0.007 | |
| 33 | 0.005 | 0.007 | 0.007 | 0.007 | 0.007 | 0.007 | |
| 34 | 0.006 | 0.007 | 0.007 | 0.008 | 0.008 | 0.008 | |
| 35 | 0.006 | 0.007 | 0.008 | 0.008 | 0.008 | 0.008 | |
K. MONITORING OF ARTEMIA PRODUCTION PERFORMANCE
Precise estimates of Artemia densities are difficult to make because of the heterogenous distribution in ponds which is influenced by wind, water temperature, light, pond depth, etc.55.56.57 Nevertheless, rough estimates of Artemia densities, among other field data, may provide a valuable tool to assess the rate of biomass harvesting. Water samples should be taken at weekly intervals from fixed stations scattered throughout the pond, in the ditch as well as in the central part or along fixed transects. Sampling is preferentially done as early as possible in the morning when Artemia are more uniformly distributed.24.25 Samples may be taken with a specific sampler24 or with a variety of containers such as beakers, buckets, etc. When using the latter sampling procedure, it is advisable to thoroughly mix the water column so to stir up bottom-dwelling Artemia58 The number of samples to be taken from a pond depends on the distribution of the Artemia as well as on the volume of the sample and the abundance of Artemia. This may be estimated by calculating the cœfficient of variance (CV, variance/mean density), i.e., the lower the CV value, the more precise the sampling.
Table 4: Examples of population Composition in Artemia Pond at Various Time (Athrough H)
| Sampling time | Nauplii | Juveniles | Preadults | Adults | Cysts |
|---|---|---|---|---|---|
| A | + | - | - | - | - |
| B | - | - | ++ | - | |
| C | ++ | - | - | + | - |
| D | + | + | + | + | - |
| E | + | - | - | ++ | - |
| F | - | - | - | ++ | - |
| G | - | - | - | ++ | + |
| H | + | + | + | + | + |
Of essential importance in ponds management (e.g., rate of biomass harvesting,fertilizer addition, pond retention time, etc.) is the population composition, which provides valuable information on the population dynamics of Artemia in the ponds. The population composition should be analyzed from representative samples taken at weekly intervals from several places in the pond (e.g., combined with the density sampling). Samples containing large numbers of animals may be subsampled until they contain 200 to 300 animals. The Artemia categorized into five classes: cysts and/or nauplii (Instar I–IV), juveniles (Istar V–VII), larvae with developing thoracopods), preadults (adults size but not yet reproductively active), and adults. These classes may be distinguished under a dissection microscope or by pouring the Artemia over three successive filter screens with mesh sizes of about 500, and 125 μm which respectively retain adults and preadults; juveniles; and nauplii and cysts. The adults and preadults can easily be separated by eye. The (relative) presence of each Artemia class is expressed as percentage of the total number of Artemia counted in the plankton sample or is evaluated as follows: - absent; + present; ++ dominant presence. Of further interest is the evaluation of the reproductive activity of the females; i.e.,empty or full broodsacs and nauplii or cysts bearing. Table 4 shows a typical example of the population composition in an Artemia, pond at various time intervals. The population changes over one-week intervals, from A through D, reveal a very healthy population; e.g., inoculation (A), growth up to preadult and adult stage (B), first generation of nauplili released (C), and continuous reproduction and good growth conditions (D). However, a population composition remaining for consecutive weeks (E) eventually evolves into a situation which reveals food-limiting conditions (F); e.g., initial algal concentrations are still sufficient for the adults to ensure reproductive activity but too limited for the nauplii which have a lower feeding efficiency than adults; subsequently (F) food becomes too scarce even for the adults. When oviparity is the dominant mode of reproduction no population recruitment is observed (G), in heavily fertilized ponds a mixed reproductive activity is often observed (H).
L. STRATEGIES FOR CULTURE MAINTENANCE
The information collected from the monitoring programs is used to make appropriate decision about pond management. Optimal conditions for biomass production are at the lower salinity levels (100 to 150 g/1) and under conditions of very regular food availability. When transparency levels are high (>30 cm), and pond nutrient levels become undetectable or fall below levels found in the intake water, fertilizer dressing or intake of nutrient-rich water (e.g., from a feed production pond) should be considered. During temperature/salinity stratification (which causes lethal high temperature for Artemia or when plankton blue-algae become dominant, the bottom flow of water from pond to pond should be maximized. Selection of desirable phytoplankton species and/or prevention of the development of undesirable cyanophyes which have the ability to fix atmospheric nitrogen my also be aided by supplemental fertilisation with specific nutrients; e.g., through application of nitrogen fertilizer during conditions where nitrogen is limiting but phosphorus is abundant, or through application of silicate in low salinity ponds to enhance the growth of diatoms, which are rich in the desired highly unsaturated fatty aids.
Sustained population growth also regular harvesting of the biomass so as not to exceed the carrying capacity of the pond. Insufficient harvesting may lead to a complete removal of the food (even in ponds with high nutrient levels) due to the high grazing pressure of increasing numbers of Artemia,eventually leading to a collapse of the population. Similarly, over-harvesting may reduce the grazing pressure on plankton algal blooms which may deleteriously affect the salt production.4.9 Ideally, The rate of biomass harvesting should approach the maximum sustainable yields. Since a precise estimation of the latter is impossible, the rate of biomass harvesting should assessed from estimation of density, population composition, fertility parameters, and phytoplanktonic standing crop (e.g., estimated by water turbidity levels). In situations where densities increase over time with a population composition showing all Artemia classes represented, combined with a dominant ovoviparous reproduction, biomass is being renewed at a high rate. In this case and when transparency levels increase, indicating that the primary production rate cannot sustain the grazing pressure of the Artemiapopulations. Frequent harvesting of biomass is recommended. If on the other hand, densities are decreasing and/or the population consists mostly of preadults and adults showing low fecundity and/or dominant oviparous reproduction, harvesting should cease.
In section II.I of this chapter we describe the factors which induce cyst production. Production of cysts may naturally occur in the high salinity ponds. It may also be induced, even at low salinity levels, by increasing the rates of fertilization or by applying through salinity shocks of 10 to 20 g/l rapid intake of low salinity brines. OViparity, however, should only be induced when the population density in sufficiently high since during conditions of dominant cyst production, recruitment will be inhibited eventually resulting in a gradual decrease of the population due to constant mortality.
M. HARVESTING AND QUALITY CONTROL
Produced cysts float at the surface and accumulate along the windward side of the pond. They can be easily collected from the water surface with a double-screen dip net (Figure 5). Cysts should be harvested as soon as possible after production (accumulation) in order to ensure maximum recovery and hatching quality because:
Cysts washed ashore are difficult to harvest as well as to clean; in addition they will dry and may eventually become airborne.
Cysts may be exposed to temperature > 40°C which are lethal or hydrated cysts.26
Cysts may be exposed to repeated hydration/dehydration cycles (rainfall, high humidity) and lose part of their energy reserves59 resulting in a decreased hatchability.26
In order to prevent the cysts being washed ashore and to facilitate harvesting, the winward pond corner or side should be steepened or lined with a cyst barrier, e.g., corrugated plastic. When winds develop heavy waves, foam is built up in which cysts are trapped and then blown away. In this case breakers should be installed in two or more rows parallel to the cyst barrier (Figure 6).
Harvested cysts may undergo an on-site cleaning by washing the harvesting product with saturated brine or water from the pond over screen with different mesh widths (e.g., 1000, 500, 125um) in order to remove debris larger and smaller than the cysts. This wet-dry product stored in brine or mixed with crude salt (for proper dehydration) may be an acceptable product for local use, provided it is consumed within a few weeks. For the production of high quality cysts with optimal hatchabilit, maximum purity, and storability, the following additional steps are however required: (1) density in brine to remove heavy debris in the same size range of the cysts; (2) rapid washing in fresh water to remove salt; (3) density separation in fresh water to separate full cysts from empty cysts and other small light debris — this step should not take longer than 15 minutes in order to prevent eleated hydration levels which may initiate cysts metabolism; (4) removal of excess water by squeezing or centrifuging the cysts; and (5) drying in order to reduce the water level in the cysts below the critical level of 10% (preferably between 2 and 5%) in order to arrest the metabolic activity in the cysts. Optimal cyst quality in terms or hatching efficiency, hatching rate, and energy content is obtained by fast and homogeneous drying of all cysts at temperatures just below 40°C.84 Among the different drying techniques, optimal results are obtained when the cysts are kept in continuous movement in the drying air; i.e., each cyst is dried individually at the same time. This may be accomplished in a fluidized bed dryer (Figure 7) or a rotary dryer (Figure 8). If the latter equipment is not available, cysts may also be dried on drying racks on which the cysts are spread in this layers of uniform thickness (few mm only). The drying racks are placed in the open air, protected from the sun to avoid temperatures higher than 40°C, or in a temperature-controlled room or even at 35 to 38°C provided with good air exchange. Homogeneous drying is enhanced by granulating the cysts upon distribution on the drying racks through a 3 mm screen and by regular brushing (initially every h) of the cysts. Table 5 shows the effect of drying conditions on the hatching efficiency of cysts in Lavaldue, France. For more details with regard to cyst processing and drying, we refer to Sorgeloos et al. 26
For long-term storage, cysts should be packed in air tight containers (cans) under vacuum or nitrogen.
Adult biomass may be harvested manually with a dip net (in small ponds) or with a conical net (see Figure 9) which can be towed over the entire pond. In highly eutrophic ponds optimal catches are made during the early morning after a clam night, when the dissolved oxygen concentration in the ponds is so low that the Artemia concentrate in very dense «blow-up» in the upper water layers where they perform so-called surface respiration. 26 Biomass may also be harvested by static nets installed at the pond gates where active brine-flows drain large numbers of Artemia. The end part of the harvesting nets should have a small mesh size (<100 μm) to prevent extrusion of the animals.
The net must be harvested at intervals of less than 1 h since the Artemia accumulated at the end of the filter sac are exposed to anaerobic conditions which they cannot survive for more than 2 h. The harvesting efficiency of static nets can be improved by harvesting during the night light attraction to lake advantage of the positive phototactic behavior of the brine shrimp. Harvested biomass may be temporarily stocked (up to one week) in nylon screen cages (e.g., 1.5 × 2.0 × 0.5m) with a mesh width of 800 mm, which are suspended in the culture ponds (see Figure 10). For long distance live transport theArtemia biomass may be packed at densities of 100 g/1 in plastic bags filled with one third cooled pond water (5 to 10° C) and two-third oxygen at atmospheric pressure. The bags are placed in a syrofoam box together with a few bags of ice (see Figure 11).
If not used directly, Artemia biomass should be frozen or dried after through washing with fresh water. Since Artemia is extremely prone to decomposition (due to proteolytic enzyme activity) it is essential to freeze the animals when still alive. In order to ensure optimal quality, the biomass should be frozen as quickly as possible in a blast or plate freezer (- 25°C or lower) in thin layers (maximum 1 cm thick) or small ice cube trays. A properly frozen product when thawed in water yields only intac animals and does not pullet the water by leaching of body fluids (see Figure 12 for quality control).
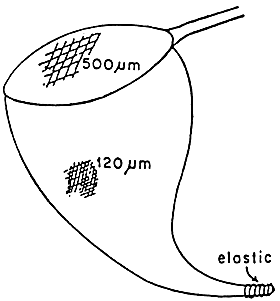
Figure 5. Double dip net cyst harvesting.(From Sorgeloss,P., Lavens, P., Léger, P., Tackaret, and Versichele, D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture. Artemia Reference Centre, State University of Ghent, Belgium, 1986. With permission.)
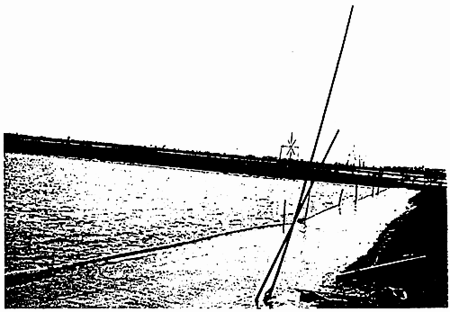
Figure 6. Floating bamboo poles used as wave breaker for the harvesting of Artemia cysts. (From Sorgeloos. P., Levens. P., Léger, P., Tackaert. W., and Varsichele. D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture. Artemia Reference Centre. State University of Ghent. Belgium 1986. With permission.)
N. SPECIFIC MODIFICATIONS FOR FURTHER OPTIMIZATION OF ARTEMIA PRODUCTION
Since the production of Artemia requires the availability of high salinities to exclude predators, the production season in monsoon climates is basically limited to the dry only. Neverthless, a significant extension of the production season may be realised by the installation of overflow devices (e.g., PVC turndown pipes and level controlled gates) in order to allow decanting of stratifying layers of rain water, combined by rigorous control of predators (e.g., through screening of intake water by means of a bag screen or semi-circular screen mounted to or surrounding the gate/pump). Year round production of biomass has been successfully applied in both the Philippines11 and Thailand12 at salinities of 60 to 80 and 70 to 90 g/1 respectively.
In farms having brine reservoirs, salinity control during the rainy season may further be facilitated by the recirculation of surplus brine from these reservoirs into the Artemia ponds. In additions, this practice allows for maximal water exchange (essential for good photoplankton production) and salinity manipulation (e.g., salinity shocks for the induction of cyst production). Another specific modification beneficial for Artemia production under high temperature conditions involves the installation of shading platforms (e.g., made of coconut fronds). De los Santos et al. 60 reported that Artemia tend to concentrate under this shade to escape lethally high temperatures occurring on sunny days.
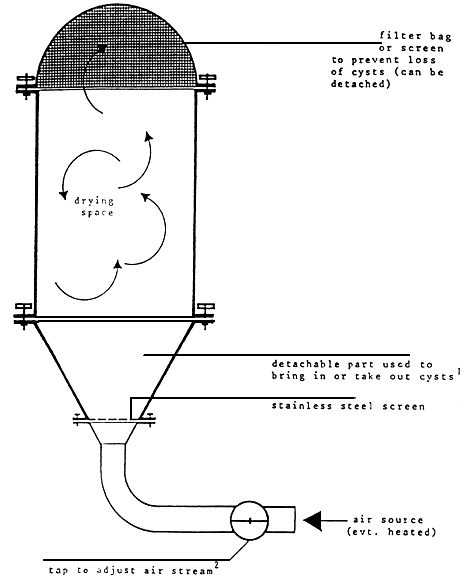
1 Conical shape results in differences in air pressure and assures better mixing of the cysts
2more pressure is needed at the start (heavy cysts containing mush water before dehydration) than at the end (light cysts which low water content) to keep the cysts suspended in the drying chamber.
Figure 7. Schematic drawing of fluidized bed dryer for Artemia cysts. (From Sorgeloos, P. Léger, P., Tackert, W., and Versichele, D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture Artemia Reference Center, state University of Ghent, Belgium, 1986 with permission.)
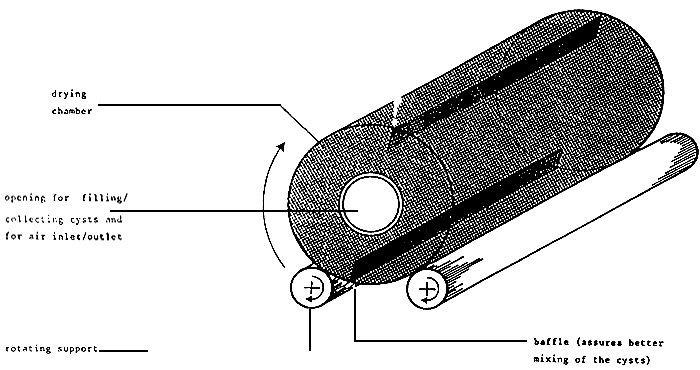
Figure 8. Schematic drawing of votary dryer for Artemia cysts. (From Sorgeloos, P., Lavens, P. Léger, P., Tackert, W., and Versichele, D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture Artemia Reference Center, state University of Ghent, Belgium, 1986 with permission.)
Table 5. The Effects of During Conditions on the Hatching Efficiency of Cysts from Lavalduc, Francea
| Drying conditions | Hatching effeciency (nauplii/g cysts) | |||
| Method | Tem (°C) | Thickness of cysts layer (cm) | Xb | SDb |
| Oven dryer | ||||
| 30 | 1.5 | 69,120 | 9,760 | |
| 30 | 0.5 | 149,600 | 10,240 | |
| (154,120) | (7,600) | |||
| 38 | 1.5 | 150,880 | 7,200 | |
| 38 | 0.5 | 181,360 | 9,600 | |
| (179,200) | (10,100) | |||
| Fluidized-bed dryer | 35 | 182,400 | 6,400 | |
| (181,960) | (6,920) | |||
| Control (unprocessed cysts) | 178,640 | (8,840) | ||
a Data complied from Sorgeloos et al.26
b In parentheses, data for same cysts but after 1-months storage under vacuum.
III. PRODUCTION FIGURES OF ARTEMIA IN FERTILIZED PONDS
Table 6 shows production figures of Artemia cysts and biomass in different man-managed saltfarms. Although the most successful farms yield 10 to 20 kg dry weight (dw) cysts and/ or 375 kg wet weight (ww) biomass/ha month, there is considerable variation from farm to farm, mainly as a result of differences in farm management. A survey of salt cum Artemia farms in Thailand in 1983, 61 revealed that poor production in the Samut sakorn and Phetburi area (see Table 6) were correlated with low pond water depths, inappropriate local conditions such as acidity of the soil, and insufficient fertilization. Poor farm management including lack of puming and application of cow dung instead of the previously used chicken manure resulted in the development of lab-lab and overall food-limiting conditions. These were also responsible for the sharp decrease in cyst productions in Vinh Chau, Vietnam (area not specified) in 1988 (29.1 kg ww) as compared to 1987 (120 kg ww).15 On the other hand, Vu Do Quynh and Nguyen Ngoc Lam25 found that the introduction of a flow-through type management in Cam Ranh Bay, Vietnam improved the cyst yield from 1.4 – 6.8 to 8.6 kg of dw cysts/ha/month.
Recently, biomass has been the production of preference (especially in Thailand) largely because it is easier to master than cyst production, which in most farms has remained inconsistent. Biomass production furthermore offers new local marketing opportunities; e.g., during the dry season in Thailand more than 3000 kg of locally produced biomass is being harvested and consumed on a daily basis as a starter feed in shrimp nursing.85
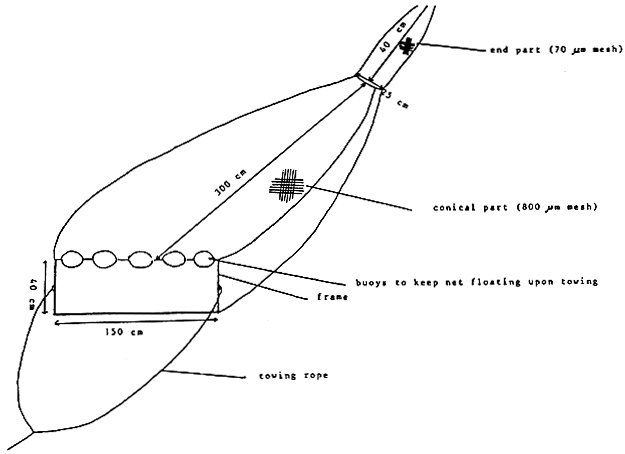
Figure 9. Conical harvesting net for Artemia biomass.

Figure 10. Storage net for Artemia biomass produced in seasonal ponds in S.E. Asia, (from Sorgeloos, P., Lavens, P., Léger, P., Tackaert, W., and Verishele, D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture, Artemia Reference Center, Salte University of Ghent, Belgium, 1986. With permission.)
IV. SOCIO-ECONOMIC ASPECTS AND BENEFITS OF SALT CUM AREMIA PRODUCTION
Seasonal solar-salt production as practiced in Southeast Asia, Central America, etc, is a labor-intensive activity generating employment for thousands of families. Its profitability, however, is usually limited, largely because of the low yields and the poor quality of the salt produced, owing to the small scale and artisanal manufacturing practices of this type of salt operation. in Viet Nam, for exemple, the mean annual income/worker in 1987/1988 was equivalent to 30 kg of rice. 15 In Thailand, the revenue of solar salt production has drastically decreased due to competition from rock-salt mining.12 In fact, in many countries (e.g., Thailand. Panama, and Costa Rica) hundreds of those family-operated saltfarms are being abandoned for socio-economic reasons.
The profitability of these seasonal saltfarms can be considerably improved by integrating Artemia production with solar-salt production, Based on a survey of five salt cum Artemia farms in Thailand, Vanhaecke61 estimated that the total cost required for pond modification and operation of one ha Artemia pond was about 2040 U.S. dollars. Assuming a production of 180 kg ww cysts and 500 kg ww biomas/ha (extrapolated from average production figures of farms that adopted proper pond modification and good biological management) at average market prices of $ 16 and $4.4/kg respectively, the average benefits from Artemia production amounted to $3040 and $3870 in the first year and $3870 in the following years of operation. This represents an additional income almost triple of that derived from salt.12
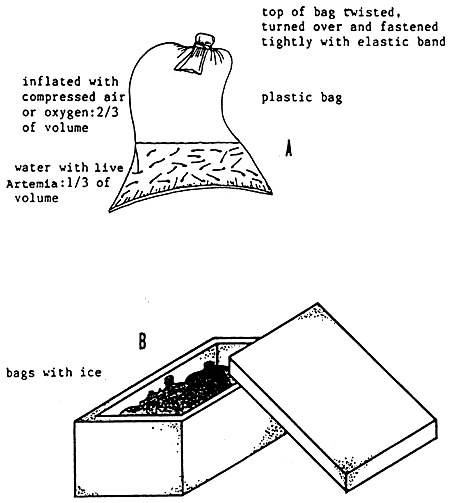
Figure 11. Live transport of Artemia - transport bag (A) and styrofoam box (B). )From Sorgeloos, P., Lavens, P., Léger, Ph. Tackaert, W. and Versichele, D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture, Artemia Reference Center, State University of Ghent, Belgium, 1986. With permission).
During recent years salt cum Artemia production has become a profitable business. The latest data for Thailand62 reveal annual revenues from Artemia biomass production of over$14,000 (for average production yields of 260 to 375 kg/ha/month on a year round basis and wholesale prices of about $4/kg). Integrated Artemia production is not only attractive from a socio-economic point of view, it also stimulates the development of local aquaculture (especially in those countries which do not have hard currency for importing Artemia cysts, e.g., Vietnam, Bangladesh, etc.) through the local availability of cheap Artemia products.
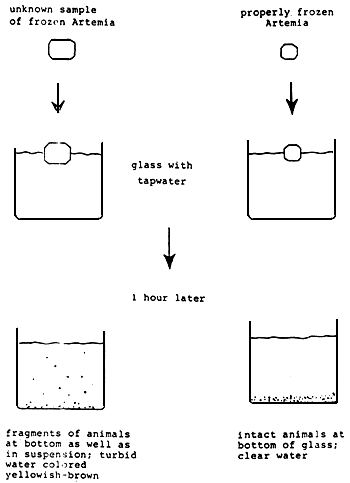
Figure 12. Quality control of Artemia biomass. (From sorgeloos, P., Lavens, P., Léger, P., Tackaert, W., and Versichele, D., Manual for the Culture and Use of Brine Shrimp Artemia in Aquaculture, Artemia Reference Center, State University of Ghent, Belgium, 1986. With permission).
Table 6 : Production of Artemia Cysts and Biomass in Man-Managed Saltfarms
| Artemia production (kg) | ||||
|---|---|---|---|---|
| Country | Location | Cysts | Biomass | Ref. |
| Thailand | Chonburi | 23.1 (dw/ha/month) | 52.5 (ww/ha/month) | 61 |
| Thailand | Samut Sakorn | 5.2 (dw/ha/month) | 61.7 (ww/ha/month) | 61 |
| Thailand | Phetburi | 3.0 (dw/ha/month) | 27.2 (ww/ha/month) | 61 |
| Thailand | Cha-Choengsao | 17.5 (dw/ha/month) | 14.4 (ww/ha/month) | 61 |
| Thailand | Samut Songkram | 15.3 (dw/ha/month) | 51.5 (ww/ha/month) | 61 |
| Thailand | 25.0 (ww/ha/month) | - | 12 | |
| Thailand | Tambon klong Tamru, Chounburi | - | 260–372 (ww/ha/month) | 62 |
| Thailand | Cha-Choengsao | 5.0 (ww/ha/month) | - | 58 |
| Philippines | Barotac Nuevo | 5.0–18.6 (dw/ha/month) | 29.4 (ww/ha/month) | 11 |
| Viet nam | Cam Ranh Bay | 1.4–6–8.6 (dw/ha/month) | - | 25 |
| Viet nam | Vinh Cahu | 3.2–3.4 (dw/ha/month) | - | 15 |
| Viet nam | Vung Tau | 5.0 (dw/ha/month) | - | 63 |
| China | Xuwen County | 74.6 (dw/ha/year) | - | 64 |
| Peru | Virrilla | 35.0 (dw/ha/month) | 0.06 (standing crop/m3 | 65 |
| Indonesia | Madura Island | 38.0 (dw/ha/month) | - | 66 |
| Jamaica | Portland Cottage | 8.2 (dw/ha/month) | - | 67 |
REFERENCES
SORGELOOS, P., Artemia Newsletter, No. 11, Artemia Reference Center, State University of Ghent, Belgium, 1989.
BROWNE, R.A. and MACDONALD, G., Biogeography of the brine shrimp, Artemia : distribution of parthenogenetic and sexual population, J. Biogeogr., 9, 331, 1982.
VANHAECKE, P., TACKAERT, W., and SORGELOOS, P., The biogeography of Artemia : an updated review, in Artemia Research and its Application, Vol. 3, Sorgeloos, P., Bengtson, D.A., Decleir, W., and Jaspers, E. Eds., Universa Press, Belgium, 1987, 129.
SORGELOOS, P., Brine shirmp Artemia in coastal saltworks - hydrobiological key to improved solar salt production - inexpensive source of food for vertically integrated aquaculture, in Atti del Convegnio Internationale «Conversione delle saline in acquaculture», D'Amelio, V. and Santulli, S., Eds., Libera Universa Trapani, Italy. 1988, 133.
LÉGER, P., BENGTSON, D., SIMPSON, K.L. and SORGELOOS, P., P., The use and nutritional value of Artemia as a food source, Oceanoger. Mar. Biol. Ann. Rev., 24, 521, 1986.
LÉGER, P., BENGTSON, D., ., SORGELOOS, P., SIMPSON, K.L., and BECK, A.P., The nutritional value of Artemia: a review, in Artemia Research and its Applications. Vol. 3, Sorgeloos, P., Bengtson, D.A., Decleir, W., and Jaspers, E., Eds, Universa Press, Wetteren, Belgium, 1987, 357.
CROGHAN, P., C., The mechanism of osmotic regulation in Artemia Salina (L) : The physiology of the branchiae, J. Exp. Biol., 35, 234, 1958.
DAVIS, J.S., Importance of microorganisms in solar salt production, in 4th Symp. Salt, Coogan, A.H., Ed., The Northern Ohio Geological Society, Inc., Cleveland, Ohio, 1977.
DAVIS, J.S., Experience with Artemia at solar saltworks, in the Brine Shrimp Artemia, Vol. 3 Persoone, G., Sorgeloos. P., Roels, O., and Jaspers, E., Eds,. Universa Press, Wetteren, Belgium. 1980, 51.
JONES, A.G., EXING, C.M., and MELVIN, M.V., Biotechnology of solar saltfields. Hydrobilogia, 82, 391, 1981.
JUMALON, N.A., ESTENOR, D.G., and OGBURN, D.M., Commercial production of Artemia in the Philippines, in Artemia Research and Its Application. Vol. 3. Sorgeloos, P., Bengtson, D. A., Decleir, W., and Jaspers. E., Eds., Universa Press. Wetteren, Belgium, 1987, 232.
TARNCHALANUKIT, W. and WONGRAT, L., Artemia culture in Thailand, in Artemia Research and its Applications, Vol. 3. Sorgeloos, P., Bengtson, D.A., Decleir, W., and Jaspers, E., Eds., Universa Press. Wetteren, Belgium, 1987. 202.
BOYD, C. E., Line requirements and application in fish ponds, in Advances in Aquaculture, FAO Technical Conference on Aquaculture, Kyoto, Japan, 1976. 120.
HAXBY, R. E. and TACKAERT, W., Workshop report : role of Artemia in solar salt operation in Artemia Research and Its Applications, Vol. 3, Sorgeloos, P., Bengtson, D.A., Decleir, W., and Jaspers, E., Eds., Universa Press, Wetteren, Belgium, 1987, 291.
VAN DER ZANDEN, J.J.G., Third report on the activities on the culture of Artemia salina and Macrobrachium rosenbergii in Can The and Vinh Chau in Southern Vietnam, Dutch Committee for Science and Technology for Vietnam, 1988.
ABREU-GOBOIS, A., A review of the genetics of Artemia, in Artemia Research and Its Applications, Vol. 1, Sorgeloos, P., Bengtson, D.A., Decleir, W., and Jaspers, E. Eds., Universa Press, Wetteren, Belgium, 1987, 61.
VANHAECKE, P., VERGELIKENDE studie van diverse geografische rassen van het pekelkreefje, Artemia ter verbetering van zijn gebruik in de aquakultuur, Ph. D. thesis, State University of Ghent, Belgium, 1983.
VANHAECKE, P. and SORGELOOS, P., International Study of Artemia. XIV. Growth and survival of Artemia Jarvae of different geographical origin in a standard culture test, Mar. Ecol. Progr. Ser., 3, 303, 1980.
VANHAECKE, P. and SORGELOSS, P., International Study on Artemia. XL VII. The effect of temperature on cyst hatching, larval survival and biomass production for different geographical strains of brine shrimp Artemia. spp., Ann. Soc. Zool. Belg., 199, 7, 1989.
BROWNE, R. A., SALLEE, S.E., GROSCH, D. S., SEGRETI, W. O., and PURSER, S.M., Partitioning genetic and environmental components of reproduction and lifespan in Artemia, Ecology, 65, 949, 1984.
BOWEN, S.T., FOGARINO, E. A., HITCHNER, K. N., DANA, G.L, CHOW, H.S., BUONCRISTIANI, M.R., and CARL, J.R., Ecological isolation in Artemia : population differences in tolerance of anion concentrations. J. Crust. Biol., 5, 106, 1989.
VANHAECKE, P., SIDALL, S.E., and SORGELOOS, P., International Study on Artemia. XXXII. Combined effects of temperature and salinity on the survival of Artemia of various geographical origin, J. Exp. Mar. Biol. Ecol., 80, 259, 1984.
VANHAECKE, P. and SORGELOOS, P., International Study on Artemia. IV. The biometrics of Artemia strains from different geographical origin, in the Brine Shrimp Artemia, Vol. 3, Persoone, G., Sorgeloos, P., Roels, O., and Jaspers, E. Eds., universa Press, Wettern, Belgium, 1980, 393.
JUMALON, N.A. and ROBLES, R. E., Sampling and stocking density studies for Artemia production in ponds, in Proc. 1 st Int. Warm Water Aquacult. Conf. 1983 (Crustaceans), Brigham Young University, Hawaii campus, 1983, 188.
VU DO QUYNH and NGUYEN NGOC LAM, Inoculation of Artemia in experimental ponds in Central Vietnam : an ecological approach and a comparison of three geographical strains, in Artemia Research and Its Applications, Vol. 3, Sorgeloos, P., Bengtson, D. A., Decleir, W., and Jaspers, E., Eds., Universa Press, Wetteren, Belgium, 1987, 253.
SORGELOOS, P., LAVENS, P., LÉGER, P., TACKAERT, W., and VERSICHELE, D., Manual for the Culture and Use of the Brine Shrimp Artemia in Aquaculture, Artemia Reference Center, State University of Ghent, Belgium, 1986.
SORGELOOS, P., First report on the triggering effect of light on the hatching mechanism of Artemia salina dry cysts, Mar. Biol, 22, 75, 1973.
VANHAECKE, P. and SORGELOOS, P., International study on Artemia. XVIII. The hatching rate of Artemia cysts - a comparative study, Aquacult. Eng., 1, 263, 1982.
LÉGER, P., VANHAECKE, P., and SORGELOOS, P., International Study on Artemia. XXIV. Cold storage of live Artemia nauplii from various geographical sources : potentials and limits in aquaculture, Aquacult. Eng., 2, 69, 1983.
TUNSUTAPANICH, A., Cyst production of Artemia salina in salt ponds in Thailand, in Giant Prawn Farming. New, M. G., Ed., Elsvier Scientific, Amsterdam, Netherlands, 1982, 233.
FOGG, G.E., The comparative physiology and biochemistry of the blue-green algae, Bacterial. Rev., 20, 148, 1956.
OLOFFSON, J.A., The role of microlayers in controlling phytoplankton productivities, in Developments in Hydrobiology. Vol. 2., Hypertrophic Ecosystems, Barica, J. and Mur. L. R., Eds,. Dr. W. Junk, The Hague, Netherlands, 1980. 83.
JUMALON N. A. and OGBURN, D. M., Nutrient flow and physico chemical profile studies of an integrated poultry - salt - Artemia - milkfish - scabass - shrimp pond production system, in Artemia Research and its Applications, Vol. 3. Sorgeloos, P., Bengtson, D. A., Decleir, W., and Jaspers. E., Eds., Universa Press. Wetteren, Belgium, 1987, 239.
LAVENS, P. AND SORGELOOS, P., Controlled production of cysts under standard conditions in a recirculating culture system, Aquacult. Eng., 3. 211, 1984.
LAVENS, P., Intensive productie en kwalietisonderzoek van Van Artemia adulten en hun nakomelingen, Ph. D. thesis. State University of Ghent, Belgium. 1989.
SORGELOOS, P., Artemia Newsletter, No. 11, Artemia Reference Center State university of Ghent, Belgium.
TARNCHALANUKIT, W., personal communication.
VERSTCHELE, D. and SORGELOOS, P., Controlled production of Artemia cysts in batch cultures, in The Brine Shrimp Artemia Vol. 3. Persoone, G., Sorgeloos, P., Roels, O., and Jaspers, E., Eds., Universa Press, Wetteren, Belgium, 1980, 231.
DUTRIEU, J., Quelques observations biochemiques et physiologiques sur le développement d'Artemia salina Leach, Arch. Zool. Exp. Gèn., 99, 1, 1960.
GILCHRIST, B.M. and GREEN, J., The pigments of Artemia, Proc. R.Soc., (Ser.B), 152, 118, 1960.
Fautrez, J. and Fautrez-Firlefyn, N., Contribution à l'étude des glandes coquilières et des coques de l'œul d'Artemia salina, Arch, Biol., 82, 42, 1971.
METALLI, P. and BALLARDIN, E., Radiobiology of Artemia : radiation effects nd ploidy, Curr. Top. Radiat. Res. Q., 7, 181, 1972.
DHERT, PH., Vergelijking van de kwalitatieve en kwantitatieve kenmerken van de voortplanting bij diverse Artemia rassen, M.S.c. thesis, State Unviersity of Ghent, Belgium, 1986.
GAJARDO, G.M. and BEARDMORE, J.A., Ability to swith reproductive mode in Artemia is related to maternal heterozygosity, Mar. Progr. Ser., 55, 191, 1989.
CAMARA, M.R. AND DE MEDEIROS ROCHA, R., Artemia culture in Brasil : an overview, in Artemia Research and its Application. Vol. 3, Sorgeloos, P., Bengtson, D.A., Decleir, W., and Jaspers. E., Eds., Universa Press, Wetteren, Belgium, 1987, 195.
VAN DER ZANDEN, personal communication.
BERTHÉLÉMY-OKAZAKI, N.,J. and HEDGECOCK, D., Effect of environmental factors on cyst formation in the brine shrimp Artemia, in Artemia Research and Its Applications, Vol. 3, Sorgeloos, P., Bengtson, D. A., Decleir, W., and Jaspers, E., Eds., Universa Press, wetteren, Belgium, 1987, 167.
STRICKLAND, J. D. and PARSONS, T.R., A Practical Handbook of Seawater Analysis, Bull. Fish. Res. Board Can., 1972. 167.
GRASSHOFF, K., Methods of Seawater Analysis, Chemie Verlag. New York, 1976.
DOBBELEIR, J., ADAM, N., BOSSUYT, E., BRUGGEMAN, E., and SORGELOOS, P., New aspects of the use of inert diets for high density culturing of brine shrimp, in The Brine Shrimp Artemia, Vol. 3, Persoone, G., Sorgeloos, P., Roels, O., and Jaspers. E. Eds., Universa Press, Wetteren, Belgium, 1980. 165
DAVIS, J.S., Biological communities of a nutrient enriched salina, Aquat. Bot., 4, 23, 1978.
WONGRAT, L., Biological analysis of Artemia from salt and salt cum Artemia farm, in Artemia Newsletter, No. 4, Artemia Reference Center, State University of Ghent, Belgium, 1987.
VONSHACK, A., Laboratory techniques for the cultivation of microalgae, in CRC Handbook of Microalgal Culture, Richmond, E., Ed., CRC Press, Boca Raton, FL, 1986, 117.
BOYD, C.E., Water Quality in Warmwater Fish Ponds, Alabama Agricultural Experiment Station, Auburn University, Auburn, Alabama, 1988.
MARCHANT, R. and WILLIAMS, W.D., Population dynamics and production of brine shrimp, Austral. J. Mar. Freshwater Res., 417, 1977.
LENZ, P.H., Ecological studies on Artemia : a review, in Artemia Research and its Applications, Vol. 3. Sorgeloos, P., Bengtson, D. A. Decleir, W., and Jaspers, E. Eds., Universa Press, Wetteren, Belgium, 1987. 5.
WEAR, R.G. and HASLETT. S.J., A minimal strategy for assessing Artemia biomass harvestable from production salinas, in Artemia Research and Its Applications, Vol. 3. Sorgeloos, P., Bengtson. D. A., Decleir. W., and Jaspers, E., Eds., Universa Press. Wetteren, Belgium, 1987, 217.
VOS, J. and TUNSUTAPANICH, A., Detailed report on Artemia cyst inoculation in Bangpakong. Chachoengsao Province. FAO/UNDP Field Document, THA/75/008, 1979.
RAKOWIEZ, M., Notes on Artemia salina, in Prawn Farming Systems Techniques, Food Institute. East-West Center, Honolulu, HA, 1975.
DE LOS SANTOS, C., SORGELOOS, P., LAVINA, E., and BERNARDINO, A. Successful inoculation of Artemia and production of cysts in man-made salterns in the Philipines, in The Brine Shrimp Artemia. Vol. 3. Persoone, G., Sorgeloos, P., Roels, O., and Jaspers. E., Eds., Universa Press. Wettern, Belgium. 1980. 159.
VANHAECKE, P., Report mission to Thailand, Feb. 22 - March 13, Artemia Reference Conter, State Unviersity of Ghent, Belgium, 1983, unpublished.
TARNCHALANUKIT, W. and WONGRAT, L. personal communication, 1989.
TACKAERT, W., unpublished data.
TANG SENMING, personal communication. 1989.
SALGADO LEU, A., Preliminary trials of extensive culture of Artemia in Peru, in Artemia Research and Its Applications. Vol. 3. Sorgeloos, P. Bengtson. D.A., Decleir, W., and Jaspers, E., Eds., Universa Press, Wetteren. Belgium, 1987. 289.
YAP, W., personal communication, 1989.
HENRY, M., personal communication. 1989.
PARKER, G.H., Lake Urmi. Amer. Natural., 315, 1900.
GUN'KO, A.F., Methods of increasing the output of sturgeon hatcheries by controlling the temperature regime during incubation and by using Artemia as food for young sturgeon. Tr. Azovsk. Nauchn. Issled Inst. Rybn. Khoz, 5, 73, 1962 (in Russian).
ROOTH, J., The flamingos in Bonaire (Netherland Antilles), Habitat, diet and reproduction of Phoenicopterus ruber. Uitgave Natuurwetenschappelijke Suriname en de Nederlandse Antillen, Utrcht, The Netherlands, 41, 1965.
MASON, D.T., Limnology of Mono Lake, California, U. Calif. Public. in zool., 83, 1, 1967.
LENZ, P. H., Ecology of an alkali-adapted variety of Artemia from Mono Lake, California (U.S.A.), in The Brine Shrimp Artemia, Vol. 3, Persoone, G., Sorgeloos, P., Roels, O,, and Jaspers, E., Eds., Universa Press, Wetteren, Belgium, 1980, 79.
WIRICK, C.D., Dunaliella-Artemia plankton community of the Great Lake, Utah, M.S.c. thesis, University of Utah. 1972.
STEPHENS, D. W. and GILLESPIE, D. M., Phytoplankton production in the Great Salt Lake Utah, and a laboratory study of algal responses to enrichment, Limnol. Oceanogr., 21, 74, 1976.
ISENMANN, P., Observations sur la mouette pygmée (Larus mimutus) en Camargue de 1971 à 1974, Terre et Vie, 29(1), 77, 1975.
BRITTEN, R. H. AND JOHNSON, R., An ecological account of a Mediterranean salina : the Salina de Giraud. Camargue (S. France), Biol. Conserv., 42, 185, 1987.
CARPELAN, L.H., Hydrobiology of Alviso salt ponds, Ecology, 38, 375, 1957.
BAKER, M, J., Autoecology of Artemia : factors influencing hemoglobin synthesis and cyst production, M.Sc. thesis, San Francisco State College, CA, 1966.
VORONOV, P. M., Seasonal abundance and biomass of Artemia salina and its eggs in saline lakes of the Crimea, in Problems of Rational Marine Fisheries and Reproduction of Marine Fish and Shellfish, Proceedings of All-Union Research Institute of Marine Fisheries and Ocenography (VNIRO), 94, 170, 1973 (in Russian, English abstract).
LÜDSKANOVA, J., Die Entwicklung von Artemia salina l. in den Teichen der Salzgarten von Burgas and Pomorije, Arch. Hydrobiol., 74, 473, 1974.
HASLETT, S.J. and WEAR, R, G., Biomass estimation of Artemia at Lake Grassmere, Mariborough, New Zealand. Aust. J. Mar. Freshwater Res., 36, 537, 1985.
WEAR. R.C. and HASLETT, S.T., Studies on the biology and ecology of Artemia from Lake Grassmere, New Zealand, in Artemia Research and its Applications. Vol. 3, Sorgeloos, P., Bengtson, D. A., Decleir, W., and Jaspers, E., Eds., Universa Press Wetteren, Belgium, 1987, 101.
PERSOONE, G., and SORGELOOS, P., General aspects of the ecology and biogegraphy of Artemia, in The Brine Shrimp Artemia, Vol. 3. Persoone, G., Sorgeloos, P., Roels, O., and Jaspers, E. Eds., Universa Press, Wetteren, Belgium, 1980. 51.
VANHAECKE, P. and SORGELOOS, P., International Study on Artemia. XVIII. The hatching rate of Artemia cysts - comparative study, Aquacult. Eng., 1, 263, 1982.
SORGELOOS, P., Booming of shirmp hatcheries in Thailand, in Artemia Newsletter, No. 11, Artemia Reference Center, State University of Ghent, Belgium, 1989.
MORALES, J., personal communication, 1989.
BRADLEY, H. L., personal communication, 1987.