Olivier de Rham
Nestlé Research Centre, Manufacturing, Milk and Nutrition Business Unit, Avenue Nestlé 55, 1800 — Vevey, Switzerland
This paper covers specific aspects of the use and production of food composition data in the food industry. Two types of information are stored by manufacturers: quantitative information on an extensive list of nutrient and non-nutrient components (including contaminants, stored separately), and qualitative information about suitability for diets to reduce or eliminate components. The aim of collecting such information is usually to check product compliance with regulations or internal specifications and norms, with a special emphasis on nutritional labeling. Control of costs, processing, storage stability, taste and texture constraints are important goals along with provision of product information to consumers. The origin of the information is usually analytical, calculated or borrowed from published food composition tables, depending on availability, regulatory requirements, product type, and cost of analysis. The meaning of the information is different depending on whether natural products are processed without intentional modification of composition, or whether international alteration of composition occurs, thus engaging the responsibility of the processor to guarantee the declared level. However, considering the natural variability in food composition, the 20 per cent tolerance margin usually accepted tends to discourage the declaration of the actual average value when liability is engaged. A clear understanding of these constraints is important to ensure a fruitful collaboration between industry and other interested parties in the use of food composition data.
The problem of an unequivocal description of a food in a table is a familiar one. The translation of food names needs not only a very good familiarity with the relevant languages, but also an intimate experience of the cultures and their local variants.
• Foods and Food Classes
Food Names and Descriptions
The situation is also difficult with commercial product brand names, as they often have no meaning in themselves. To complicate the situation further, the same brand name can be used for different products in various parts of the world, or conversely the same food product may have different brand names. A detailed description of the food in these cases is necessary, as well as the use of the food classes and subclasses, and internally the use of codes.
Food Classification
The interest to widen the classical list of food classes (and subclasses if needed) is multiple. They help to select an ingredient for a given aim from a suitably selected screen listing of the corresponding group(s) of ingredients. They allow the origin of a nutrient in a product or in a diet to be traced. They help in checking the coherence and validity of food composition data.
Table I. List of food ingredient classes
| Animal | Plant | Refined products |
| Meat | Cereals | Oils, fats, shortenings |
| Fish and seafood | Vegetables (leaves, stalks, flowers, fruits, bulbs, sprouts) | Proteins, hydrolysates, amino acids |
| Egg | Roots | Starches |
| Milk and milk products | Mushrooms | Sugars and sweeteners |
| Algae | Dietary fiber concentrates | |
| Oilseeds | Enzymes | |
| Herbs and spices | Microbiological starters | |
| Tubers (potatoes) | Acids, alkalis, salts | |
| Pulses (dry beans, grain legumes) | Vitamins | |
| Fruits | Thickeners, emulsifiers, antioxidants, preservatives | |
| Berries | Processing aids | |
| Nuts | Colours, aromas and flavours | |
| Cocoa | Water | |
| Coffee and surrogates | ||
| Tea and herbal teas | ||
| Wine, spirits, vinegar | ||
| Yeast and yeast products |
Whether a food can be attributed in a table to more than one class of products is an important question. The answer is almost always negative in the printed version of food composition tables as this would mean a waste of space, but could easily be positive in computerized databases with the necessary programming precautions.
• Ingredients and Ingredient Classes
Besides the traditional food ingredient classes found in published food composition tables, food manufacturers use additional raw materials for product manufacture:
industrial “raw” materials, often already processed to some extent, e.g. peeled, cut, pureed, deboned, cooked, dehydrated, freeze-dried
food ingredients not consumed as food in themselves and not usually accessible to consumers, constituting additional raw material classes. These include refined food components (e.g. fiber concentrates, starches, proteins, hydrolysates, amino acids, medium chain triglycerides, hydrogenated fats, trans-esterified fats), pure chemicals (acids, alkalis, salts), enzymes, microbiological starters, vitamins, additives (antioxidants, emulsifiers, thickeners, preservatives), colors, aromas and flavors (see Table I).
Out of these materials, a large number of intermediate products, subrecipes or premixes are produced, that can be either transient in processing, stored for later use, or purchased as such. These subrecipes or premixes also constitute an “ingredient” class.
• Products and Product Classes
Manufactured products may be classified according to consumption characteristics that are familiar to the consumer, rather than on the basis of the major ingredient which is sometimes very difficult to define. Minimally processed foods (with only sugar, salt, fat or water added or removed) can be classified within the class of their major ingredient. However, manufactured product classes go much further than that: beverages, breakfast items, starters, snacks, main dishes, garnishes, fast foods, side dishes, sauces, desserts, infant foods and clinical nutrition products (see Table II).
• Quantitative Information
Components Contents
Quantitative data on the contents of a great number of components including both nutrients and non-nutrients can be required by technologists or marketing specialists. The interest for each individual component is very variable according to the type of product concerned and the aims of the demand. Most requests do not ask for more than the proximate composition (“big 4”: energy, protein, fat, carbohydrates) or slightly more (“big 8”: the “big 4” plus dietary fiber, sodium, cholesterol, saturated fat), and possibly one or two vitamins and minerals, but there may be additional requests.
Dairy product specialists insist on milk fat and non-fat milk solids. Chocolate manufacturers have special requirements related to cocoa solids and fruit content. Yoghurt producers are interested in the transformation of lactose to lactic acid. Broth and stock producers ask for glutamate figures, meat and sausage manufacturers need carnitine values, coffee and cocoa beverage processors want data about methyl xanthines. In addition, there are national differences due to local legislation.
Dietetic product specialists are most demanding, including not only a list of vitamins and minerals, but also occasionally figures for fat and sugar substitutes with reduced energy value. Breast milk substitutes and clinical nutrition products require a long list of vitamins, minerals and trace elements, and some amino acids and fatty acids. The figures for fatty acid classes are being requested more and more often.
Table II. List of food product classes
| Simple productsa | Dairy | Evaporated milk, milk powder, cheese, yoghurt |
| Meat | Preserved meats, sausages | |
| Fish | Frozen fish | |
| Cereals | Bread, pasta, bakery products | |
| Vegetables | Frozen prepared vegetables | |
| Tubers | Potato flakes, frozen croquettes, chips, crisps | |
| Beverages | Alcoholic | Wine, beer, cider, drinks, punch, cocktail, spirits, liquors |
| Soft | Fruit juices, vegetable juices, dairy beverages, milk modifiers | |
| Cereal drinks | Coffees, teas, water, mineral water, sodas | |
| Breakfast foods | Butter, margarine, jam, spreads, cheese | |
| Bacon and sausages, eggs, milk, yoghurt, fruits, fruit juices | ||
| Starters | Soups, broth, stock, “hors d'oeuvres” | |
| Snacks | Savory, salted | Appetizers, cocktail foods, chips, salted peanuts, crepes |
| Sweet | Ice-creams, chocolates, cereal bars | |
| Pastry, confectionery, sweets, biscuits | ||
| Yoghurts, fruits | ||
| Main dishes | With meat or fish | |
| Without meat or fish | ||
| Garnishes | Potatoes, cereals, pasta, bread, doughs | |
| Vegetables, legumes | ||
| Fast foods | Hot | Hamburgers, pizzas, quiches |
| Cold | Sandwiches, savoury pies | |
| Side dishes | Salads, cheese, bread, spreads | |
| Sauces | Salad dressing, mayonnaise | |
| Meat sauces | ||
| Seasonings, condiments, spices | ||
| Desserts | Fruits, dairy desserts, custard | |
| Cakes, tarts, sweet pies, fried desserts | ||
| Infant foods | Starter milks, follow-up milks | |
| Infant cereals, baby foods | ||
| Clinical nutrition products | Enteral | |
| Parenteral | ||
a Simply processed, with only salt, fat, sugar or water added
At Nestlé, the INFOODS tags (1) as such are not used, but instead a similar system of conventional abbreviations and standard units, to avoid confusion and errors arising during data transfer.
Contaminants
Data on contaminants contents is quantitative, and it is tempting to put it in the same database as the other components. But food composition data should have a predictive value, i.e. be valid in the future as well as in the past with the correct safety margins, whereas contamination levels vary greatly from batch to batch and cannot predict the values for future crops. The data should be stored with batch identification in a separate database that does not allow the usual averaging done in food composition data. This is so, for example, for aluminum levels in the ingredients for infant formulas.
• Qualitative Information
Elimination Diet Suitability
Elimination diets are sought for various reasons, either medical, religious or philosophical. For example, gliadin, milk protein, soy, must be eliminated from the diet of people allergic to these foods, meat from the diet of vegetarians, animal products from that of vegans, pork from kosher or halal products. This information is qualitative (present or absent) rather than quantitative (numeric).
Such qualitative criteria are delicate to handle. An error (which could have very serious consequences making somebody very ill) is easily made, whereas an occasional low figure for, say, protein on the label of the same product is harmless.
The decision whether an ingredient or a product is acceptable or not in a given elimination diet depends on a number of complex rules, especially for religious diets. In addition, in order to reach a decision on the suitability of a product all its ingredients must be correctly identified for the given criterion, the recipe must be strictly followed, and the processing (contamination, recycling, incompatibilities, processing equipment and conditions, processing-induced components) must be properly addressed.
Qualitative information is not suitable for components like sodium, cholesterol, lactose, sugar, phosphate, caffeine. In these cases, the aim is a reduction diet with a maximum acceptable daily intake and not an elimination diet. Such parameters are quantitative factors and must be handled as such.
Ingredients and Additives Usage
The recipes database gives quantitative information on the ingredients used in a product. This information is usually passed to the public in a simplified form of the qualitative list of ingredients. The additives are in this respect no different from the other ingredients. “Additives-free lists” can therefore only be obtained from the recipes database, and this information does not belong to the food composition databse.
A frequent confusion ignores the fact that some additives are at the same time natural molecules. The absence of such an additive in the recipe does not imply the absence of that molecule in the product. Such a molecule is then a normal food component to be handled quantitatively. For example, claiming “no-phosphate added evaporated milk” or “no glutamate-added tomato ketchup” suggests that these products are, respectively, phosphate-free and glutamate- free, which is incorrect. Furthermore, at the same time such claims reinforce and perpetuate erroneous beliefs, ideas and fears about additives among consumers.
• Quality of the Information
Aims of Information
Composition data in the food industry are usually produced to satisfy quality assurance aims: compliance with regulatory or scientific composition standards, and with internal norms and specifications. In recent years, a great deal of this activity has been geared to nutritional labeling as new regulations appeared in the US (2) and in the EEC (3) with compliance dates in 1994, and this will continue in the near future.
In addition, it is often necessary to control one or the other parameter of food composition to ensure optimal process control, taste, texture, flavor, convenience, price, shelf life. Only in a few cases does the publication of food composition information brochures and the transmission of data to dietitians or database compilers receive priority.
Origin of the Information
Food composition data are usually analytical (hopefully on multiple representative samples), calculated from the recipe, or borrowed from an external database. Whether one or the other source is to be preferred or rejected depends on the local constraints which may include, e.g. regulations, availability, facilities, costs, and needs for accuracy.
Variability
The biological nature of the materials used (e.g. ripeness, variety, soils, fertilizer use, rearing and feeding practices, post-harvest practices) introduces a large variability in the composition of ingredients. In addition, there is a variability in recipes, including intentional small variations for various technological, taste, price or regulatory reasons and non-intentional ones for practical reasons.
The cumulated variability of food composition is mastered to a certain extent by technologists, but can only increase as international comparisons are made. This raises the question of when two foods are close enough to be considered one and the same in the database. When the need for accuracy is low, such cumulations are easily accepted, whereas every batch must be recorded separately when high accuracy is needed. It is up to the user to decide the degree of precision that is needed and to handle the database accordingly.
The food industry composition data compiler enjoys some advantages over other database compilers. Purchase specifications are used for a defined list of raw materials. Fixed, precise and controlled recipes and processes are used. Manufactured products are controlled for various reasons (e.g. legal, standards of identity, cost, storage stability, taste and texture), which reduce the variability of the final composition.
In full cream milk powders we measured a relative standard deviation of 5 to 10 per cent for minerals, and 15 to 25 per cent for vitamins. It is probably greater for trace elements as well as in other less standardized products. The legislation usually allow for a 20 per cent tolerance. This is by far not large enough for minerals and vitamins, and the declared values are therefore often below the real average content, to allow a safety margin. This is unfortunate for dietitians, but largely unavoidable.
Meaning of the Information
Dietetic products are sold on the basis of medical advice, and their nutritional value is of prime importance. The technologist is required to produce a food that matches a predefined composition, and this is assured by standardization of every single batch of production. Action is taken to guarantee the required levels that are declared on the label, and the producer is responsible for ensuring that the declared figures are adhered to under normal circumstances. This is also the case when a component level is controlled (addition or reduction), but needs “overages” or safety margins in the declared values.
Normal, everyday food products on the other hand do not require such accuracy of composition data. In this case, the processor discloses what nature has produced, as influenced by processing. A yearly production average is in this case the most significant nutritional figure. One half of the production will have a content lower than the average and the other half a higher one. In order to ensure that the vast majority of packages correspond to the declaration (including possible losses during storage), the declaration might be below the actual average value for those perceived as healthy nutrients, while for those nutrients negatively perceived, such as sodium, the declaration might be higher than the actual average value.
• Conclusions
Food composition information that exists in the food industry is not primarily aimed at dietitians, nutritionists or database managers, and some frustrations are predictable. The cost of producing information for external use as well as the cost of its dissemination are high and may be restrictive. The risk of misunderstanding and misuse of this information is important to highlight, especially if its currency is not assured by regular updates. At the same time, the protection of that part of the information which is proprietary is a necessity.
Understanding the constraints and the reasons for producing such information will foster a common understanding of its meaning and limits, and will facilitate the dialogue and improve its use. It is in this context that a collaboration is possible and fruitful.
• References
(1) Klensin, J.C., Feskanich, D., Lin, V., Truswell, A.S., & Southgate, D.A.T. (1989) Identification of Food Components for INFOODS Data Interchange, UNU Press, Tokyo.
(2) Mermelstein, M.H. (1994) Food Technol. 48, 62–71
(3) EEC (1990) Council Directive of 24th Sept. 1990 or Nutritional Labeling of Foodstuffs 90/496/EEC. Official Journal of the European Community L276/40–44 of 6/10/1990.
Janine Lewis, Simon Brooke-Taylor, Fay Stenhouse
National Food Authority, PO Box 7186, Canberra, ACT 2610, Australia
The National Food Authority was established in August 1991 as a reform to the food standards setting system. Although development and variation of food standards is its primary role, the Authority is also responsible for the national references on nutrient composition and for food safety surveillance. Three database providing the supporting information systems for these activities are described. The food composition work of the Authority (and the federal Department of Health before it), has focused on revision of the national reference on nutrient composition and the release of the data in a number offormats. Nutrient data are produced mainly from an ongoing food analytical program and managed by means of a computer system, the Australian Nutrient Data Bank. These data enable the Authority to estimate the levels of nutrients in food, and the probable nutritional impact on foods of changes to the compositional and technological aspects of food standards. The published food composition tables also provide a valuable data source for industry when formulating nutrition information for labeling of foods. The Authority also has responsibility for the Market Basket Survey which identifies whether pesticides and contaminants are at levels which pose health risks to consumers. The last completed survey in 1990 concluded that Australian intakes of pesticides and contaminants were well below international limits recommended by the World Health Organization. To facilitate a uniform interpretation, implementation and enforcement of the Food Standards Code, the Authority is developing a national food safety information database, which will link all agencies involved and provide an information network to enable effective use of resources for a rapid national response to public health emergencies related to food.
The National Food Authority (NFA) was established in August 1991 with the proclamation of the Commonwealth National Food Authority Act 1991 (the Act), as an independent and expert body with responsibility for the development, variation and review of Australia's food standards.
• Functions of the National Food Authority
The Act specifies a list of 13 statutory functions that the NFA is required to perform. More than half of these functions do not relate directly to the Authority's primary role of developing and revising food standards, but focus on food safety research and education, and coordination of food recalls and food safety information. In summary, the functions are:
development and review of food standards
co-ordination of food surveillance
research and surveys
food safety education
co-ordination of food recalls
development of assessment policies on imported food
advice to the federal minister
development of codes of practice for industry
incidental functions.
Three of the functions have or will result in ongoing activities that are dependent on the databases of the Authority. They are:
researching the nutrient composition of the Australian food supply
monitoring the pesticide and contaminant levels in the food supply
establishing a system of food safety surveillance.
• Nutrient Composition Database
The responsibility for government-sponsored nutrition composition activities was transferred to the National Food Authority upon its formation. However, these activities have been conducted in Australia since the 1930s under the auspices of the federal Department of Health. Up to the 1970s, the Department issued three major revisions to the food composition tables, which were compilations of data from a number of sources mainly from overseas but including some Australian data. During the 1970s, there was a growing awareness and call for Australia to have a more comprehensive and contemporary set of food composition tables that reflected the Australian food supply (1).
The first step towards this goal was achieved in 1978 when it was decided to revise Australia's nutrient composition data completely by establishing a program to analyze the Australian food supply progressively. At that time, a Working Party of Australia's peak health advisory body, the National Health and Medical Research Council, was formed to devise a plan for the collection of food composition data including the priorities for analysis of foods and nutrients, and to review the format for publication. Their recommendations included extending the range of nutrients and foods, particularly for take-away and ethnic foods, and for publication of the tables to be in a loose-leaf format to enable easy incorporation of updates (2).
In establishing the program, it was decided that within the available funding, the data should be produced to serve nutrition and health goals before aiming to meet agricultural requirements. Thus, the overall direction of the revision program was generally to analyze one composite sample of food in preference to multiple samples, to collect data for primary produce prior to manufactured food products and to sample at the retail level on a regional rather than a national basis. After 13 years of the laboratory program, this approach has enabled Australian nutrient data to be produced on a broader range of foods than would otherwise have been possible given the funding constraints and the demand for comprehensive data in as short a time as possible.
Table I. Present range of constituents analyzed
| Proximate constituents | Minerals | Vitamins |
| Moisture | Sodium | Retinola |
| Total nitrogen | Potassium | α-carotenea |
| Total fat | Calcium | β-carotenea |
| Individual monosaccharides | Magnesium | β-cryptoxanthina |
| Individual disaccharides | Iron | Thiamin |
| Starch | Zinc | Riboflavin |
| Ash | Phosphorus | Niacin |
| Dietary fibera | Copper | Vitamin Ca |
| Cholesterola | Manganese | α-tocopherol |
| Fatty acidsa | Chloride | Vitamin B6 |
| Organic acidsa | Fluoride | Vitamin B12a |
| Selenium | Biotin | |
| Sulphur | Pantothenic acid |
a Analysis of these nutrients depends on the type of food
The initial laboratory work was undertaken at universities in Sydney. The majority of the work during the first five years of the laboratory program was conducted by the Wills and Greenfield team at the University of New South Wales. Since 1985 however, the analyses have been conducted by the South Australian division of the Australian Government Analytical Laboratories in Adelaide.
From the commencement of the program, routine analysis of foods included:
moisture; total fat; total nitrogen; carbohydrate components — monosaccharides, disaccharides and starch; ash;
sodium; potassium; calcium; magnesium; iron; zinc
retinol, α- and β-carotenes, β-cryptoxanthin; thiamin; riboflavin; niacin; vitamin C.
Other components were analyzed appropriate to the foods, such as cholesterol, fatty acids and organic acids. Special programs were also conducted for amino acids and dietary fiber analyzed by the Englyst method (3).
For the first 11 years, the program focused on obtaining a broad nutrient profile of foods commonly available in the Australian food supply. By the end of this period, over 2500 foods had been analyzed for their nutrient composition. As opportunities arose and expertise became available, the range of analyzed nutrients gradually expanded from six to ten vitamins, and from six to 13 minerals. The range of nutrients which now comprises the routinely commissioned constituents is given in Table I.
Because of the expansionary approach described above, there were some gaps in the vitamin and mineral data for foods analyzed during the early part of the program, principally for primary produce and take-away foods.
In 1992, the laboratory program began to resample foods to analyze those nutrients which were not included in the first round of analyses. The focus of the program from now on will be to build up a more comprehensive profile on all foods previously analyzed. The sampling scheme for these analyses is being maintained as close as possible to the original specifications. The “catchup” program also provides opportunities for other nutrients with previously doubtful values to be reanalyzed. In addition to the “catchup” analyses, there will be an ongoing component which will analyze new foods entering the market.
With the continuation of the food analysis program, there is now the opportunity for more detailed information to be collected. This is considered to be particularly useful for staple foods. One example of this approach is a program currently in progress to collect and analyze composite samples of three of the most commonly consumed types of bread from each of the eight Australian States and Territories. These results will further extend the information base by enabling calculation of descriptive statistics such as standard error of the mean. This project is being conducted in collaboration with the Bread Research Institute of Australia.
Over the life of the program, the food industry and other organizations have participated with government in building up the store of nutrient composition data by contributing their own data, or funding specific programs that analyze their products according to agreed specifications. Successful cooperation has occurred with industry organizations such as the Australian Meat and Livestock Corporation, the Australian Dairy Corporation and the Bread Research Institute of Australia.
An integral component of the revision process was the development of a supporting computer system for data storage, processing and reporting. The Australian Nutrient Data Bank (ANDB) was a mainframe application developed from a pilot system in 1987. A series of enhancements were made to the data bank in 1990. One of these was to provide the capability to calculate foods from recipes using factors as estimates of the weight change on cooking and nutrient retention. There is some scope now for the Authority to undertake the supporting research into weight loss factors for recipe foods. With the transfer of responsibility for the food composition program to the Authority, the databank has been relocated from a mainframe to the Authority's smaller computer system. It is anticipated that there will be future opportunities for developments to be made to this system. From a compiler's viewpoint, the opportunity to improve the system regularly is most important. It enables refinements to be made and new features to be added as well as taking advantage of improvements in software and hardware technology to enhance efficiency and functionality.
The results of the laboratory program are now published in a number of formats. Australia's national nutrition reference is a series of loose-leaf volumes entitled Composition of Foods, Australia (4). There are now six volumes in the series providing data on some 1430 foods. Volume 7 is presently in preparation and will add a further 100 foods, mainly restaurant dishes originating from other countries.
Additional formats are available to meet the needs of a range of data users. A summary database NUTTAB for use in software applications has been produced for public use since 1989, although a predecessor of this database was used to analyze the national dietary surveys conducted in the 1980s. The currently available version NUTTAB91–92 (5) is the third publicly available update to the database and provides data on 28 nutrients and energy for some 1580 foods. A revised version is planned for release after publication of Volume 7 of the food tables series. The additional foods in this database are those from British sources which have continued to be included from the original dietary survey database. Two condensed versions of the tables also have been released. Nutritional Values of Australian Foods (6) was produced to meet the needs of educators and students in food, biology and health courses at upper secondary and tertiary levels while Food for Health (7) is a simplified guide for the general public which also includes general dietary advice. The two latter publications have proved to be among the most popular books released by the government publishing service.
The food composition tables are also used as a reference by the food industry for food labeling purposes. Part A of the Australian Food Standards Code (8) sets out the labeling regulations, including those for nutrition labeling, with which manufacturers must comply. When a label carries a nutrition claim, a nutrition information panel must be displayed containing, as a minimum, information on the energy, protein, fat, total carbohydrate, total sugars, sodium and potassium content of that food. Other nutrients which may be claimed and listed in the panel include amino acids, starch, cholesterol, fatty acids, and dietary fiber. The information given in the panel should represent the average quantity of the listed components allowing for seasonal variability and other known factors which could cause actual values to vary. These average quantities can be determined from three sources of information:
the manufacturer's analysis of the food
calculation from the actual or average quantity of nutrients in the ingredients used
calculation from generally accepted data.
The term' generally accepted data' is a very broad one, but now that there are Australian data on a significant range of foods and ingredients, it is being increasingly used as a reference for labeling purposes. Manufacturers need to be aware of the different modes of expression between the data tables and the Code requirements. For example, the Code specifies that total carbohydrate content should be determined by difference rather than by analysis. The Australian tables report carbohydrate data by direct analysis. A carbohydrate by difference value can be readily calculated from those data tables because a value for ash is provided routinely, together with the other proximate data that are required.
The relevant food standard which controls the claims for the vitamin and mineral content of foods is currently being revised. The term “average quantity” previously defined in the general labeling provisions is proposed to be used as the basis for determining the amount of claimed vitamin or mineral. The Australian database is and will continue to be a valuable source of information for this purpose.
Although provision of nutrient composition data is not the National Food Authority's primary role, there is a profound appreciation of the important contribution made by these data to the improved knowledge of food and its relationship to health and disease in Australia. The Authority is committed to the ongoing program of laboratory analysis of Australian foods and the publication of contemporary and comprehensive nutrient composition data for the benefit of health professionals, educators, consumers, the food industry and the development of public health policy and programs.
• Market Basket Survey Database
The Australian Market Basket Survey (AMBS) is conducted in Australia every two years to estimate the levels of pesticides and contaminants including heavy metals and natural toxins in the Australian food supply. Responsibility for the AMBS was transferred to the Authority upon its establishment, from the federal Health Department.
The survey comprises the collection and preparation to table-ready state of a specified list of foods for analysis of particular chemical residues. These foods are selected to represent all major groups of foods in the Australian food supply. Foods are collected over one year according to one of three standard protocols and prepared according to a set of instructions. Core foods are sampled once every season and in every capital city; regional foods are sampled in every capital city but only in one season; and national foods, such as corn flakes, which are not expected to vary between regions, are sampled in only three capital cities in one season. The results are expressed on a per kilogram wet weight basis and entered into the AMBS database. The number of foods sampled has been increasing gradually over the years in which the surveys were conducted. In the 1990 survey, 53 foods were sampled, while 62 foods were sampled in the 1992 survey.
The laboratory results for each sample are identified on the database by the food name, its location and the season of the year in which it was collected. The information from each survey is maintained in separate files on the database. Currently, data from the 1985, 1986, 1987, 1990 and 1992 surveys are stored on the database. The survey was run annually until 1988 when a review was held which recommended that the survey be conducted biennially. The maximum range of the residue data for one food from the 1992 survey is given by the following example. Twenty-four samples of grilled lamb chops were analyzed for residues of 57 compounds. They were three heavy metals, 13 organochlorines plus three metabolites of organochlorines, 22 organophosphates, six synthetic pyrethroids, two fungicides and one class of fungicides, six herbicides and polychlorinated biphenyls. Analyses were conducted also on specific compounds often found in particular types of foods. For example, seafood was analyzed for mercury, peanut paste for aflatoxins and potatoes and potato crisps for solanine content. The database is sufficiently flexible so that it can store such data when they are commissioned from time to time.
These results are used to estimate the total dietary intake of these substances by six age/sex categories covering adults and children, and thus to assess the safety of the Australian diet with respect to these substances. The intake data are derived by reference to the consumption patterns given in the most recent national dietary surveys. These values in grams/day are entered on to a spreadsheet and an estimate of the total dietary intake of each substance is then calculated for each age/sex category. Dietary intakes at the 95th percentile of energy are determined. For the 1992 survey, intakes at the mean intake of energy were also determined. These results and the summary data on the distribution of levels of pesticides and contaminants found in the sampled foods are provided in regularly published reports of each survey (9). The estimated dietary intakes are also compared to the internationally accepted safe limits which are the Provisional Tolerable Weekly Intakes (PWTIs) for contaminants and Acceptable Daily Intakes (ADIs) for pesticides.
The results of surveys over the last 23 years have indicated where additional vigilance is required in the food production chain as well as estimating the overall levels of these substances. This important monitoring function has shown to date that Australia's food supply has generally safe levels of presticides and contaminants and these levels have been improving since monitoring began.
• Food Safety Information Database
The coordination of food surveillance information is a statutory function of the Authority. Currently there is no national system to collate these types of data. Most of the States and Territories however, are collecting and maintaining information on the results of food inspection and monitoring activities within their jurisdictions. Unfortunately, the data are variable in scope and are stored in a number of formats, some of which are more technologically advanced than others. The types of information currently collected include surveys of the microbiological and contaminant levels in food, food complaints, recalls and prosecutions.
Soon after the formation of the Authority, the NFA Advisory Committee (NFAAC), comprising representatives of all State and Territory Health Departments, the federal Health Department and the Australian Quarantine Inspection Service, was formed. At its first meeting, the Committee expressed general support for the concept of a national food surveillance database and agreed that a consultant should be employed to undertake a feasibility study of the project.
The feasibility report points to significant overall savings in resources at the State and Territory level, as well as the broader benefits of a shared and online system. It is proposed that a fully automated national system will include:
an information base to facilitate the allocation of resources efficiently and effectively by reducing unnecessary duplication in sampling activities and assisting to redirect effort to areas best serving public safety objectives.
on-line access to current national information from a range of agencies. This will assist agencies plan activities and allocate resources, and facilitate rapid response to food safety concerns. It will also promote uniform enforcement of standards by permitting agencies immediate access to up-to-date information on investigations in other jurisdications
availability of printed information on food safety issues, plans, trends, and contacts
potential savings in information technology development and support. These are greatest for those States supporting existing systems and for States and Territories which intend to develop their own food safety related computer systems.
It is expected that the development of a national food safety information system, which by providing a unique information base of food recall, survey and sampling data, will offer significant benefits to regulatory agencies.
The Food Safety section of the Authority is currently collating information on food surveillance procedures of the States and Territories to enable the identification of relevant fields for the database and levels of security required. It is anticipated that a trial will be undertaken shortly between the Authority and a State or Territory, to gain experience in the transfer of food surveillance data, prior to any large scale implementation.
• References
(1) Greenfield, H., & Wills, R.B.H. (1981) Food Technol. Aust. 33, 101–130
(2) English, R. (1990) Food Aust. 42, S5–S7
(3) Englyst, H., Wiggins, H.S., & Cummings, J.H. (1982) Analyst 107, 307–318
(4) Commonwealth Department of Community Services and Health/National Food Authority (1989-) Composition of Foods, Australia, Australian Government Publishing Service, Canberra
(5) Commonwealth Department of Community Services and Health (1991) NUTTAB 91–92, AGPS, Canberra, ACT
(6) Commonwealth Department of Community Services and Health (1991) Nutritional Values of Australian Foods, Australian Government Publishing Service, Canberra
(7) National Food Authority (1991) Food for Health, Australian Government Publishing Service, Canberra
(8) Australian Food Standards Code, Australian Government Publishing Service, Canberra
(9) National Health and Medical Research Council/National Food Authority (1991) The 1990 Australian Market Basket Survey, Australian Government Publishing Service, Canberra
James T. Tanner
Office of Special Nutritionals, HFS-451, Center for Food Safety and Applied Nutrition, US Food and Drug Administration, Washington, DC 20204, USA
The Nutrition Labeling and Education Act of 1990 requires that all foods sold in the United States be nutritionally labeled. In response to the requirements of that Act, the Food and Drug Administration (FDA) published final regulations in January, 1993, on enforcement of nutrition labeling. In the regulations, provisions were made for the use of databases for labeling, and a manual was prepared to assist companies and organizations in this task. This manual gives generic information on how to develop and calculate from databases label values which will meet regulations that FDA is required to enforce. FDA does not prescribe how an individual company is to determine nutrient content for labeling purposes but does offer to review a database and to work with the manufacturer to resolve problems before taking any regulatory action. The compliance policy of FDA remains based on analysis of composite samples, performed using methods of the Association of Official Analytical Chemists (AOAC), or other validated methods if no AOAC method exists. FDA will then compare the label values with the results from laboratory analyses. The use of databases for labeling, the regulations of FDA for foods sold in the United States, the compliance policy of FDA, and the future uses of databases are discussed.
In 1973 the US Food and Drug Administration (FDA) promulgated regulations (1) that required nutrition labeling in certain circumstances. The agency took this action largely in response to recommendations of the 1969 White House Conference on Food, Nutrition, and Health (2).
• Overview of Database Use
The 1973 regulations required nutrition labeling only for certain foods, those with added nutrients or for which a nutrition claim was made in either labeling or advertising. Some foods such as fresh produce were specifically exempted. FDA encouraged manufacturers, however, to provide nutrition labeling voluntarily on a wider variety of food products, including the exempt foods.
Industry-wide databases were suggested as a possible means of reducing the cost of developing nutrition labeling for individual companies. In 1979 FDA, the US Department of Agriculture (USDA) and the Federal Trade Commission (FTC) encouraged this concept in a notice (3) published in the Federal Register, describing the agencies' policies and intentions with respect to numerous food labeling issues. In that notice, FDA, while not agreeing to approve databases, stated that it would work with industry to resolve any compliance problems that might arise for food labeled on the basis of a database that the agency had accepted. Specifically it stated, “If products bearing nutrition labeling in accordance with properly evaluated [FDA evaluated] nutrient databases and manufactured in accordance with food manufacturing practices are found not to be in compliance with applicable nutrition labeling regulations, the agency will work with the firms responsible for the product in question and with the appropriate authorities who are maintaining the applicable nutrient database to correct the problem before initiating compliance provision actions.” The policy given in that 1979 notice is the same policy that is in effect today.
With the Nutrition Labeling and Education Act of 1990 (4) expanding mandatory nutrition labeling to nearly all foods regulated by FDA, greater interest has been expressed in using industrywide databases for some food products. Some manufacturers of food products not currently labeled have expressed interest in using data available from other sources, for example, the open scientific literature, as the basis for labeling their products.
The policy of the Food and Drug Administration is that the choice of a data source is the prerogative and the responsibility of the firm or organization that provides a nutritionally labeled product. The firm or organization needs to be judicious in this selection, however, to ensure that the product labeling is in compliance with the regulations for that product. FDA has developed a manual (5) which will be of assistance in identifying data that are of sufficient quality to provide an adequate basis for nutrition labeling. In addition, guidance has been given on when average values may be used and when calculated values using the equations given in the manual should be used. The agency understands that most companies will not have sufficient information to meet the suggested criteria listed in the manual; however, we view this as the “gold standard” and hope that by making a diligent effort there will be sufficient analytical data available in five to ten years' time to comply fully with the different criteria given in the manual.
Historically, label values based on calculation of nutrient content from ingredients were considered unacceptable for a mixed product for the following reasons: 1) There are no quality indicators of the data for the components; 2) there are no indicators of the methods of analysis and sampling used to obtain the data for the components; 3) there are no indicators of the design and execution of quality control procedures used to monitor the sampling and analysis of the com Nutrition Labeling in the United States ponents; and 4) there are no indicators of nutrient loss during the processing and handling of the mixed product. In addition, inclusion of sugars as mandatory in nutrition labeling and a change in the definition of fat limit the use of most ingredient databases.
Table I. Formulas for calculating label values
| (1) | Class I nutrients (fortified): Computed value=mean-t(0.95;df)(1/k + 1/n) 1/2(s) |
| (2) | Class II nutrients (naturally occurring)≥80% label value: Computed value = [mean - t(0.95;df)(1/k + 1/n)1/2(s)](5/4) |
| (3) | Class II nutrients (naturally occurring): calories, sugars, total fat, saturated fat, cholesterol, and sodium, ≤120% label value: Computed value = [mean + t(0.95;df)(1/k + 1/n)1/2(s)](5/6) |
| where | mean = sample mean t(0.95;df) = 95th percentile of the t-distribution df = n-1 degrees of freedom n = sample size used to calculate the mean k = number of future units making up the future mean. |
Use of data from the open literature and use of ingredient databases present similar problems because the values given are generally averages based on an undetermined number of analyses. Average values based on numerous analytical values representing the different variables associated with a nutrient may be sufficient if they are within the range (per cent CV) represented by the coefficient of variation given in the manual. If the coefficient of variation is large, then the equation given in the manual should be used to ensure that the regulatory requirements can be met. This applies to indigenous nutrients only; for fortification nutrients the value must be at least 100 per cent of the label value. If average values are used, then the value will be correct only 50 per cent of the time. The manual gives equations for calculating label values for indigenous and fortification nutrients that would have the highest probability of meeting the regulatory requirements which the agency must enforce.
• Equations for Use in Calculating Label Values
For label values computed to reflect the variability of individual units, the onesided 95 per cent prediction interval is constructed to contain the result of a single (k = 1) future retail unit or to contain the mean of k = 12 future retail units. Suppose there are n individual values of a nutrient in the database, from which the sample mean and SD are computed. The calculation of a lower or upper limit of the one-sided 95 per cent prediction interval also depends on the nutrient classes (i.e., Class I or II). The computed limit for Class II nutrients is adjusted for the 20 per cent margin of allowance in the FDA compliance evaluation.
The computing formulas are given in Table I. Label values computed using these equations have the highest assurance of meeting FDA requirements.
• Class I and Class II Nutrients and Compliance Policy
Compliance with nutrition labeling is determined in the following manner: A collection of primary containers or units of the same size, type, and style produced under conditions as nearly uniform as possible, designated by a common container code or marking, or in the absence of any common container code or marking, a day's production, constitutes a “lot.”
The sample for nutrient analysis shall consist of a composite of 12 subsamples (consumer units), one from each of 12 different randomly chosen shipping cases, taken to be representative of a lot. Unless a particular method of analysis is specified, composites shall be analyzed by appropriate methods as given in the Official Methods of Analysis of the Association of Official Analytical Chemists, 15th Edition (1990) (6), or in the supplements issued annually. If no AOAC method is available or appropriate, other reliable and appropriate analytical procedures should be used.
Two classes of nutrients are defined for purposes of compliance: Class I nutrients: added nutrients in fortified or fabricated foods; and, Class II nutrients: naturally occurring (indigenous) nutrients. If any ingredient which contains a naturally occurring nutrient is added to a food, the total amount of such nutrient in the final food product is subject to Class II requirements unless the same nutrient is also an added ingredient.
A food with a label declaration of a vitamin, mineral, protein, total carbohydrate, dietary fiber, other carbohydrate, poly- or monounsaturated fat, or potassium shall be deemed to be misbranded under Section 403(a) of the Federal Food, Drug, and Cosmetic Act (the Act) (7) unless it meets the following requirements:
Class I vitamin, mineral, protein, dietary fiber, or potassium: The nutrient content of the composite is at least equal to the value for that nutrient declared on the label
Class II vitamin, mineral, protein, total carbohydrate, dietary fiber, other carbohydrate, poly- or monounsaturated fat, or potassium: The nutrient content of the composite is at least equal to 80 per cent of the value for that nutrient declared on the label
Provided: That no regulatory action will be based on a determination of a nutrient value that falls below this level by a factor less than the variability generally recognized for the analytical method used for that food at the level involved (8).
A food with a label declaration of calories, sugars, total fat, saturated fat, cholesterol, or sodium shall be deemed to be misbranded under section 403(a) of the Act if the nutrient content of the composite is greater than 120 per cent of the value for that nutrient declared on the label. Again, the same statement on analytical variability applies.
Reasonable excesses over labeled amounts of a vitamin, mineral, protein, total carbohydrate, dietary fiber, other carbohydrate, poly- or monounsaturated fat, or potassium are acceptable within current good manufacturing practice. Reasonable deficiencies under labeled amounts of calories, sugars, total fat, saturated fat, cholesterol, or sodium are acceptable within current good manufacturing practice.
Compliance with these provisions may be provided by use of a database that has been compiled by following FDA guideline procedures and submitted to FDA for approval and with foods that have been handled in accordance with current good manufacturing practice to prevent nutrient loss. Guidance in the use of databases may be found in the FDA Nutrition Labeling Manual — A Guide for Developing and Using Databases (5).
• Nutrient Database Development and Use
The development of a database (a collection of related information) is a complex task that consists of several general steps such as development of a sampling plan, collection of the samples, analysis of the laboratory samples, and statistical analysis and interpretation of the results. Each of the steps can be performed in several different ways, and decisions made regarding the alternatives may directly affect the available resources, the quality of the data, and the risk of making incorrect decisions.
The process of developing a sampling plan involves the resolution of a series of interrelated tasks that may be broadly classified as follows:
defining the sampling objective
defining the target product population
developing the sampling frame
selecting the sampling methods (i.e., stratified multistage plan)
selecting the analytical methods.
To increase the chance that the data will be of the desired quality, it is essential that these tasks, as a minimum, be given careful consideration, and that specific questions be addressed and resolved in the planning stage of the data collection effort.
In using a database for the purpose of labeling, consideration has to be given to:
the variability of the factors that influence nutrient content
the distribution that the nutrient values follow
the statistical methodology that is applied in deriving label values.
To resolve these tasks effectively, information on the variability of the nutrient levels in the product is needed. Variables such as variety or geographic growing area for fruits and vegetables need to be determined. For mixed products and/or products requiring processing, the nutrient content may change during processing or during storage before sale. Information on the variability of the analytical method for the nutrients of interest is also needed.
If sufficient information is not available, it will be necessary to perform a pilot study or perform a literature search to obtain the necessary information before developing the sampling plan.
A database that would be adequate for the purpose of nutrient labeling will reflect a satisfactory degree of data quality, and hence database accuracy. Data quality (determined by the amount of error that is contained in data) depends primarily on the effectiveness of the activities stated earlier for database development. The accuracy of a database depends on its data quality, which is expressed in terms of four characteristics:
precision (magnitude of the error of the estimate)
representativeness (how accurately samples reflect the population)
comparability (similarity of data from different sources)
completeness (amount of information collected).
It is necessary that satisfactory degrees of these characteristics be reflected in the data.
• Calculation of Label Values
Once an acceptable amount of analytical data of satisfactory quality has been accumulated, a value must be determined for the label, which will reflect the nutrient content of the product. This number may be calculated in several ways.
The first and perhaps the most straightforward way is to use the mean of the analytical data. If the analytical data are within certain limits indicated by the coefficient of variation (usually 11 per cent or less), then the mean value may be used. This applies only to Class II nutrients. The coefficient of variation is the standard deviation times 100 divided by the mean. The reason for using the coefficient of variation is that numbers which are applicable to all concentrations can be given in the guidelines.
For nutrients that are highly variable, i.e., that have coefficients of variation higher than the maximum limits given in the guidelines, there is an equation for Class I nutrients (which must be at least 100 per cent of the label claim) and two for Class II nutrients, one for those nutrients that must be 80 per cent of the label claim, or greater, and one for those nutrients that may not exceed 120 cent of the label claim.
Once the nutrient content has been calculated, various rounding rules must be used, such as rounding to the nearest gram or half-gram, etc.
• “Recipe Databases”
Although FDA has stated that it would not accept label values generated by computer from ingredient or component composition values or “recipe databases,” it was stated that FDA would work with various trade organizations and companies to develop a model which could be used to calculate label values based on ingredient composition and determination of nutrient loss (if any) during processing. To date we have worked with several organizations but have not yet accepted any models. In the comments received after the manual was announced in the Federal Register, a large number stressed the need for recipe databases. FDA still believes that most ingredient composition databases are not useful for calculating the final composition of a mixed product for the following reasons:
such databases are usually not accompanied by indicators of the quality of the data for the components
such databases have no indicators of the methods of analysis and sampling used to obtain the data for the components
such databases have no indicators of the design and execution of quality control procedures used to monitor the sampling and analysis of the components
such databases have no indicators of nutrient loss during the processing and handling of the mixed product.
To successfully validate a model to calculate final composition based on ingredient composition, extensive analyses of ingredients and final product composition would be required. The application of this model would be limited by the products for which it was developed.
FDA believes that in the future ingredient composition databases may have the necessary validation to be used in calculating the final composition of mixed products for an acceptable range of food products. At this time, however, the agency believes that the data making up ingredient databases are of mixed quality and, therefore, of limited value. Companies that wish to use ingredient databases must evaluate the individual analytical values for each ingredient to assure themselves that the data are representative, valid from an analytical perspective, and sufficient to account for any variation in the ingredient.
During the comment period several principles were given by companies and trade associations that would be useful and instructive for developing recipe databases. Keeping in mind the general requirements for databases, these proposed principles are given as general guides:
Confidence in the quality of data, supported by documentation of data sources. Companies maintaining or using ingredient composition databases must be able to demonstrate the data source used for each type of product and each nutrient for which ingredient composition databases are utilized.
Proper maintenance of the database. Companies developing or using ingredient composition databases must have procedures in place to ensure that the values in the ingredient composition databases are reviewed and updated as needed and on a regular basis.
Specificity with respect to ingredients, product formulations, and processes. Companies using ingredient composition databases must have procedures in place to ensure that the nutrient values are used only for specific applications. For example, a company should have a procedure to ensure that nutrient data specific for one product formulation or process are not used to prepare nutrient declarations for similar product formulations or processes, when there is no assurance that the data are applicable to those products or processes.
Validation of the database. Companies developing or using ingredient composition databases must have procedures in place to ensure that nutrient values receive reviews, audits, and confirmation through nutrient analyses as often as necessary.
Several other companies offered equivalent guidelines and one other guide that is important:
Companies making nutrient content or health claims should substantiate the claim by analysis of the final product. This applies to products labeled with a health message or those making claims such as low or reduced in certain nutrients.
• Confidentiality of Databases
During the comment period many companies/trade associations objected to the lack of confidentiality of submitted databases. They did not want the information gained through analyses of products and ingredients to be released through freedom of information requests or used in unacceptable ways or for inappropriate products. In addition, in developing databases, costs are shared among the participating companies; these companies sought assurance that the data would not be available without cost to companies that did not participate in development. Formulations that are used to produce mixed products are also regarded as confidential company information, and there should be some assurance that this information will not be available to anyone who requests it. The agency is aware that the development of a database is costly, and that it may contain confidential information. FDA agrees that release of a database could reveal substantial proprietary interests in documents that have been submitted to the agency. Furthermore, it has never been the agency's intent, nor does it have the resources, to maintain and manage databases that are developed by manufacturers or associations. The agency believes that the availability of a database is, therefore, the primary responsibility of the developer.
FDA will continue with the policy of assisting the developers of databases, providing guidance to those who ask for it, and evaluating databases for the products submitted for review. Confidentiality of such data will be determined and maintained in accordance with regulations.
Those database developers who choose to do so are encouraged to make their information available through such compilations as the USDA Handbook No. 8 (9), so that all may benefit from the additional analytical information. In the long run, recipe databases will be useful after extensive information is gathered and placed in these compilations of public information.
The information and procedures given in the manual are generic, and only the parts that pertain to the food or product under study should be used. Because preparation of a different manual for each food type was not practical, all the information is contained in one manual. FDA is always available to help companies with problems or those needing assistance in determining how to proceed in attaining the best label possible while continuing to satisfy the regulations that the agency must enforce.
• References
(1) Anon. (1993) Federal Register 38, 20702
(2) White House Conference on Food, Nutrition, and Health (1969) U.S. Government Printing Office, Washington, DC
(3) Anon. (1979) Federal Register 44, 75990
(4) Nutrition Labeling and Education Act (1990), P.L. 101–535
(5) FDA (1993) Nutrition Labeling Manual—A Guide for Developing and Using Databases; available by sending a self-addressed mailing label to: James T. Tanner, HFS451, Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration, Washington, DC 20204
(6) Official Methods of Analysis (1990) 15th Ed., AOAC, Arlington, VA
(7) Federal Food, Drug, and Cosmetic Act (1938), P.L. 75–717
(8) Horwitz, W., Kamps, L.R., & Boyer, K.W. (1980) J. Assoc. Off. Anal. Chem. 63, 1344–1358
(9) US Department of Agriculture (1976- ) Composition of Foods: Raw, Processed, Prepared, Agric. Handbook No. 8 series, USDA, Washington, DC
Kazuki Shinohara
National Food Research Institute, MAFF, Tsukuba, Ibaraki, Japan
National projects on the physiological functions of foods, which have been carried out by university groups and the Ministry of Agriculture, Forestry and Fisheries in Japan, revealed that foods have functions controlling homeostasis in the body, as well as nutritional and sensory functions, leading to the introduction of the terminology of functional foods, and in 1991, a system for licensing “Foods for Specified Health Use”. To obtain a license for a “Food for Specified Health Use”, the following evidence is required: a) potential to contribute to the improvement of dietary habits and the maintenance and enhancement of health; b) medical and nutritional data on specific health aspects; c) data on intakes determined medically and nutritionally; d) safety data; e) physicochemical data; f) data on nutritional composition, and others. As such foods proliferate there will be a need to collect comprehensive data on them and to make these data systematically available to doctors, dietitians and others.
At present, one can obtain nutritious and appetizing foods whenever necessary in industrialized countries and the daily diet is becoming satisfactory in terms of quantity. Longevity has increased considerably due to improved nutrition and medical treatment. However, with a change in food habits, food-related diseases such as allergy, obesity and geriatric diseases are increasing in Japan. Because of the costs of treatment of geriatric diseases, total medical expenditure is expected to increase. Furthermore, the aged population is expected to reach a maximum in the next century. From these points of view, new health aspects of foods are a public concern. In order to respond to the need for improved foods, it is important to elucidate the physiological functions of food components.
• Research on the Physiological Functions of Food Components
National projects on the physiological functions of food components have been carried out by university groups in Japan under the following headings:
systematic evaluation of the physiological functions of foods (1984– 1986)
evaluation of bioregulatory functions of foods (1988–1990)
analysis of functional foods and molecular design (1992–1994).
These projects have revealed that foods have biomodulating functions (tertiary) which control homeostasis in the body, as well as nutritional (primary) and sensory (secondary) functions. The biomodulating functions include regulation of immunological functions, regulation of biorhythms, prevention of ageing, prevention of food-related disease, and facilitation of recovery from food-related disease. In the Ministry of Agriculture, Forestry and Fisheries, the following projects have been carried out:
the Japanese R&D Association for a new separation system in the food industry (1989–1991)
the Japanese R&D Association for new food materials (1990–1993)
elucidation of the functions and molecular structures of food components (1989–1992)
integrated research program for effective use of biological activities to create new demand (1991–2000)
development of technology for evaluation and utilization of functional properties of agricultural products in relation to health (1993–1999).
From these projects, the physiological functions of components of vegetables, soybeans, milk and other foods or their metabolites have been discovered, such as antimutagenicity, anticarcinogenicity, antioxidant activity, immunostimulation, modifying endocrine function, hypotensive effect, cholesterol control, intestinal control, and others.
• Functional Foods
These projects exerted a great influence on the Japanese food industry and the Ministry of Welfare and Health (MWH) leading to the introduction of the terminology of “functional foods”. A functional food was defined as a food containing compounds which satisfy the following criteria: clear effectiveness for a specific health purpose, defined chemical structure, clear mode of action at cellular level, proven effectiveness by oral administration, safety, stability in foods, acceptability as food, and potential for use in a range of diverse food products. In comparison with most conventional nutritious foods, functional foods have more potential efficacy for health. Functional foods claim to control homeostasis, resulting in the prevention of geriatric diseases. In contrast to conventional healthy foods, these foods have direct scientific evidence about their functionality. The member companies of the Japan Health and Nutritional Food Association collaborate in working groups which are responsible for collecting scientific evidence about 11 identified categories of functional components: dietary fibers; oligosaccharides; sugar alcohols; polyunsaturated fatty acids; peptides and proteins; glycosides, isoprenoids and vitamins; alcohols and phenol; choline; lactobacteria; minerals; and others.
• Physiological Functionality of Food Components
The physiological functions of food components are as follows:
Dietary Fibers
Dietary fibers such as polydextrose, wheat, bran, corn, apple, soybean, and beet fibers have been recognized to have beneficial effects. Fibers are considered to be promising candidates for functional components of foods. The physiological functions of dietary fibers are claimed to be: a) regulation or control of intestinal functions, including prevention of constipation, improvement of bacterial flora in the intestine, inhibition of absorption of harmful substances and promotion of their excretion, prevention of colon cancers, and immuno-stimulation, b) regulation or control of blood sugar content, including inhibition of insulin secretion, inhibition of glucagon secretion, and prevention of diabetes mellitus, and c) regulation or control of cholesterol levels, prevention of gallstone formation, decrease in fat deposition, prevention of obesity, and hypotensive effects. Dietary fibers can be used in foods (insoluble fibers) and beverages (soluble fibers). Polydextrose has been used in beverages as a substitute for sugar.
Oligosaccharides
Oligosaccharides such as lactulose, fructo-, galacto-, isomalto-, and xylo-oligosaccharides, and cyclodextrins are considered to have potential for use as functional ingredients in foods. They can be used as food modifiers that do not affect the texture and physicochemical properties of foods and can be replacements for sugar. The physiological functions are claimed to be: low energy, prevention of tooth decay, intestinal control, and bifidobacterium activation. Products containing oligosaccharides are believed to have great sales potential in Japan.
Sugar Alcohols
Sugar alcohols such as malcitol, erythritol, and reduced palatinose are promising materials which have low energy content and preventative effects against obesity and tooth decay.
Polyunsaturated Fatty Acids
Polyunsaturated fatty acids, especially eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and linolenic acid, have great potential for sales and development into functional foods. EPA and DHA are derived from fish body oils. Their functionalities are claimed to be: decrease in fat deposition, decrease in plasma cholesterol, hypotensive effect, decrease in viscosity of blood, and prevention of cancers of the breast, colon and prostate gland.
Peptides and Proteins
These include casein phosphopeptide (CPP), lactoferrin, and peptides from casein, fish, and soybean. CPP prepared from milk is a promising functional ingredient which enhances the absorption of calcium and iron. Recently, hypotensive peptides have been recovered from milk casein, fish proteins and soybean. Their functional properties are claimed to be: hypotensive effect, control of cholesterol level, detoxification of harmful substances, antiviral effects, and promotion of bone and tooth growth.
Glycosides, Isoprenoids and Vitamins
The functions of this class which includes saponins, carotenoids, flavonoids, and tocopherols are claimed to be: antioxidant activity, intestinal control, improvement of stomach, liver, and kidney metabolism, decrease in blood sugar and cholesterol levels, and hypotensive effects.
Alcohols and Phenols
The functions of this class which includes tea polyphenols, oryzanol, and octacosanol are claimed to be: prevention of tooth decay, deodorant effect, hypotensive effect, and decrease in plasma cholesterol. Among tea polyphenols, catechin, epicatechin, epicatechin gallate, and epigallocatechin gallate have received considerable attention.
Cholines
This class of compounds, including soybean and egg yolk lecithins, is claimed to have functions such as improvement of plasma lipid metabolism, prevention of arteriosclerosis, and prevention and improvement of fatty liver.
Lactobacteria
Fermented foods with lactobacteria and bifidobacteria are popular in Japan, because they have the function of intestinal control, decrease in cholesterol, and immuno-stimulation.
Minerals
The claimed benefits of minerals (e.g., calcium salts, heme-iron, magnesium include: promotion of bone and teeth growth, prevention of osteoporosis, and prevention of anemia.
Others
The last category are classified as health-enhancing foods which cannot be assigned to any of the categories above. It includes fermented vinegar, and Chlorella. The claimed benefits are: improvement of plasma lipid metabolism, immuno-stimulation, and anticarcinogenic effects.
• Foods for Specified Health Use
In 1991, a system for licensing “Foods for Specified Health Use” was established by MWH. According to this system a food for specified health use is “a food which, based on knowledge concerning the relationship between foods or food components and health, is expected to have certain health benefits, and has been licensed to bear a label claiming that a person who uses it for specified health use may expect to obtain the health use through the consumption thereof” (1).
“Foods for Specified Health Use” are defined as a category of food for special dietary uses within the establishment frame of the Special Nutritive Foods under article 12 of the Nutrition Improvement Act (Figure 1).
To obtain a license for a “Food for Specified Health Use”, evidence that the food meets the following requirements must be produced:
potential to contribute to the improvement of dietary habits and the maintenance and enhancement of health
medical and nutritional data on specific health aspects
appropriate serve size of the food or relevant compound, based on medical and nutritional information
safety data of the food or relevant compounds
physicochemical properties, test methods and methods of qualitative and quantitative determination
data on nutritional composition, since the nutritional composition of the product should not be defective when compared with the composition of the regular food product that it may replace.
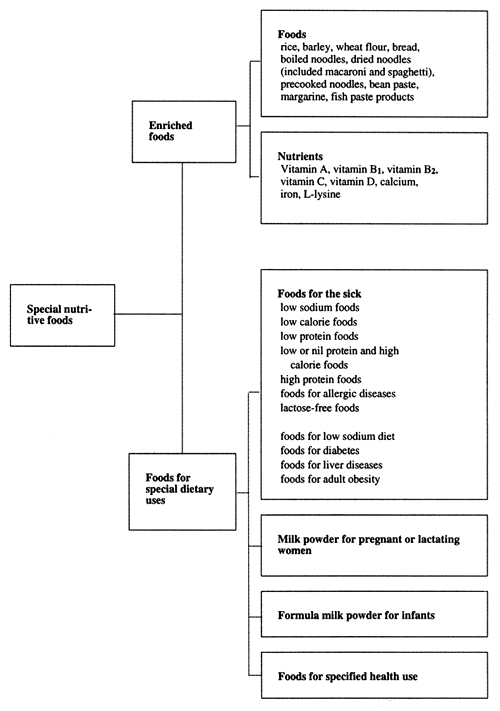
Figure 1. Types of special nutritive foods
In addition to these requirements, the product should preferably be a food that is consumed regularly in the general diet, rather than a food consumed only occasionally. The product should be in the form of ordinary food, rather than pills or capsules. Only functional foods which satisfy these requirements will be approved by MHW as a Food for Specified Health Use.
Official permission for licensing a Food for Specified Health Use is under the control of the MHW. In the system for licensing, overseas applicants need to apply directly to the MHW (Office of Health Policy on Newly Developed Foods, Environmental Health Bureau). The application for licensing by the MHW must be in writing and must be accompanied by a product sample. The MHWs minimum requirements for an application are: brand name of food, list of ingredients by percentage, details of manufacturing procedure, analysis of ingredients, matters for which permission or approval is sought, name, address, and date of birth of applicant, name and address of main office, reason for seeking permission or approval, statement of energy value, list of nutritive elements by amount, instructions for storage, preparation, and administration, and any precautions to be observed in use of such foods.
Future Prospects
Recently, allergen-free rice (fine rice) and low phosphate (LPK) were approved by the HMW as the first Foods for Specified Health Use. Allergen-free rice in which 99 per cent of allergic globulin protein is removed is effective for patients with atopic disease. Low phosphate content milk is effective for patients with chronic renal diseases who are instructed to reduce the intake of phosphate. The phosphate content of the product is 20 per cent of that of regular milk while the protein content remains the same. In addition to these, foods such as beverages containing soybean-, fructo-, xylo-oligosaccharides, or calcium salts, table sugars containing soybean- or fructo-oligosaccharides, and gums containing malcitol and palatinose have also been approved as Foods for Specified Health Use. Foods for Specified Health Use containing dietary fibers, oligosaccharides, lactobacteria and others will be licensed. As such foods proliferate there will be a need to collect data on them and to make this available to doctors, dietitians and others, particularly those concerned with nutritional epidemiology and controlled dietary intervention trials.
• Reference
(1) Nutrition Improvement Act (1991) Article 12, Ministry of Health and Welfare, Tokyo