Low levels of As are naturally present in the soil (Matschullat, 2000). The background levels are around 5 mg/kg worldwide with substantial variation depending on the origin of the soil (Mandal and Suzuki, 2002). The behaviour of As is distinctly different under flooded (anaerobic) and non-flooded (aerobic) soil conditions, with flooded conditions being likely the most hazardous in terms of uptake by plants and toxicity, as will be explained in this chapter. Taking into consideration that rice is the staple crop in Asia, that its cultivation largely takes place under flooded conditions, and that its high demand for irrigation water, often from groundwater resources, understanding the behaviour of As under flooded soil conditions is of particular importance.
As exists in the environment in various organic and inorganic forms (species). The most important inorganic species are arsenate (AsV) and arsenite (AsIII). Monomethylarsenic acid (MMA) and dimethylarsenic acid (DMA) are the most common organic species in the soil, but their natural presence is low compared to inorganic As (Abedin et al., 2002c; Fitz and Wenzel, 2002).
Speciation of inorganic As in the soil is largely controlled by reduction and oxidation processes (redox). Under aerobic (oxidizing) conditions AsV predominates, whereas AsIII predominates under anaerobic (reducing) conditions (Fitz and Wenzel, 2002; Takahashi et al., 2004). For example, in an experimental paddy field 30 percent of the As was present as AsIII under non-flooded conditions and up to 70 percent was present as AsIII under flooded conditions (Takahashi et al., 2004). Masscheleyn et al., (1991) reported that under oxidizing conditions, As was mainly present as AsV (>95 percent of the total soluble As) with a relatively low solubility. Under more reducing conditions, AsIII became by far the predominant species and the solubility of As increased sharply. Microbial activity can influence As speciation via various mechanisms such as redox reactions with Fe and As and via (de)methylation of As species (Fitz and Wenzel, 2002; Mahimairaja et al., 2005).
AsV and AsIII adsorb mainly to iron(hydr)oxides (FeOOH) present in the soil and AsV is bound strongest. The behaviour of FeOOH is highly dependent on redox conditions, making Fe redox chemistry the most important factor in regulating As behaviour (Fitz and Wenzel, 2002; Takahashi et al., 2004). Under anaerobic conditions, FeOOH readily dissolves and As is released into the soil solution, where As will be present mainly as AsIII (Takahashi et al., 2004; Masscheleyn et al., 1991). Microbial activity is closely involved in this process (Islam et al., 2004a; Zobrist et al., 2000; Masscheleyn et al., 1991). Under aerobic conditions FeOOH is relatively insoluble and serves as a sink for As. Fe and As behaviour is therefore dynamic and closely related in lowland paddy fields.
The As concentrations in the irrigation water usually differ from those in the soil water. For example, Takahashi et al. (2004) reported that As concentrations in irrigation water were higher compared to the soil water concentrations during the non-flooded period because of sorption to FeOOH. Under flooded conditions, soil water concentrations increased because of remobilization and, important to note, became higher than the irrigation water concentrations. Under flooded conditions, plants can therefore be exposed to much higher concentrations in the soil water than would be expected based on the concentrations in the applied irrigation water.
FeOOH is mainly present in the clay size soil fraction (<2 μm) and clayey soils therefore generally have a higher As content compared to more sandy soils (Fitz and Wenzel, 2002; Mahimairaja et al., 2005). At the same total soil concentration, clayey soils are less toxic compared to sandy soils because As is more strongly bound in the clayey soils. Under specific soil conditions, other sorption substrates such as carbonate minerals and manganese oxides (MnO) can also be relevant (Mahimairaja et al., 2005).
Phosphate (PO4) is an analogue of AsV, making it an important factor in the behaviour of As in aerobic soils (Lambkin and Alloway, 2003; Mahimairaja et al., 2005; Williams et al., 2003). Both ions compete for sorption sites on FeOOH and for uptake by plants. The effect of PO4 additions to aerobic soils on the uptake of As will therefore depend on the balance between competition for sorption sites and competition for uptake.
AsIII is not an analogue of PO4, making the presence of PO4 probably less relevant to As behaviour under flooded soil conditions (Takahashi et al., 2004). It is not known if PO4 plays a role in the rhizosphere (the microenvironment around the roots), where aerobic conditions can occur under flooded conditions. Other ions may also influence As behaviour, but the impact seems to be less compared to PO4 (Cornu et al., 2003; Mahimairaja et al., 2005; Williams et al., 2003).
AsV adsorption decreases with increasing pH, in particular above pH 8.5, whereas the opposite occurs for AsIII. The extent to which pH influences As sorption differs between soils. The adsorption maximum for AsV on FeOOH lies around pH 4, whereas for AsIII the maximum is found at approximately pH 7-8.5 (Fitz and Wenzel, 2002; Mahimairaja et al., 2005; Masscheleyn etal., 1991).
As may be lost from the soil via the formation of volatile As components (Abedin et al., 2002c; Mahimairaja et al., 2005). As summarized by WHO (2001), this can contribute a removal of 12 to 35 percent per year. The extent to which this process is relevant to flooded paddy fields with their distinct soil conditions is however still unknown.
Conditions in the the rhizosphere may deviate substantially from the bulk soil. As summarized by Fitz and Wenzel (2002), plants will influence the pore water composition by uptake and excretion of substances. Micro-organisms in the rhizosphere will also influence its composition (Harvey et al., 2002; Nicolas et al., 2003). Because Fe and As behaviours in the soil are closely related to each other, it can be expected that plant processes related to Fe uptake may also influence As bioavailability and uptake. The same is true for PO4.
When a paddy field is flooded, the rhizosphere can still be aerobic. The main reason is that rice plants can transport oxygen from the leaves to the roots, resulting in the transfer of O2 to the rhizosphere. A number of micro-organisms is also capable of oxidizing the rhizosphere. The oxidized conditions can result in the precipitation of FeOOH around the roots, also known as Fe-plaque. Fe-plaque has been reported frequently on roots of wetland plants including rice (Meharg, 2004). It may influence As speciation, bioavailability and uptake and Fe reducing and oxidizing bacteria are likely to play a major role (Fitz and Wenzel, 2002; Meharg, 2004; Weiss et al., 2003; Weiss et al., 2004). The importance of Fe-plaque on As uptake by wetland plants including rice remains to be resolved (Liu et al., 2004; Chen et al., 2005).
AsIII and AsV are taken up by different mechanisms. AsV is taken up via the high affinity phosphate uptake system (Meharg, 2004). PO4 additions have therefore been suggested to reduce uptake because of competition between PO4 and AsV for uptake.
For rice grown in pots with soil and irrigated with AsV contaminated water, no effect of PO4 on As accumulation in rice plants was observed (Abedin et al., 2002a; Abedin et al., 2002b). Abedin et al. (2002a) suggested that the plants were effectively exposed to AsIII and not to AsV because of the reducing soil conditions. An alternative explanation is that PO4 competes with AsV both for both sorption at Fe-plaque and for uptake, minimizing the overall effect of PO4 (Chen et al., 2005). As summarized in various papers, the addition of PO4 to As-contaminated soils to minimize As uptake is controversial under non-flooded conditions (Abedin et al., 2002c; Fitz and Wenzel, 2002).
AsIII is actively taken up by so-called water channels (aquaporins) in the roots (Meharg and Jardine, 2003). Laboratory experiments have shown that Boro (dry season) rice cultivars take up less AsIII and AsV than Aman (rainy season) rice cultivars. This may be related to physiological or morphological differences between the root systems (Abedin et al., 2002c). However, this does not imply that Boro rice will accumulate less As than Aman rice under field conditions, because Boro rice is irrigated with As-rich groundwater whereas Aman rice is rainfed.
The uptake mechanism of organic As is largely unclear (Meharg, 2004). It seems that monomethylarsenic acid (MMA) and dimethylarsenic acid (DMA) are taken up by rice plants but that the rate of uptake is much lower compared to inorganic As (Abedin et al., 2002c).
To date, it has not been possible to predict As uptake by plants from the soil. Most papers only include total As concentrations in the soil and the As concentration in the irrigation water. It has been suggested that total As can be regarded as potentially bioavailable in paddy fields, because most of it is bound to FeOOH (R. Loeppert, personal communication, 2004). Good correlations between total As in soil and plants are however not always found (see also Section 3.1) (Jahiruddin et al., 2005; Miah et al., 2005).
With the exception of hyperaccumulators such as certain ferns, the translocation of inorganic As from the roots to the above ground parts is limited. Organic As is more readily translocated but the uptake is much lower compared to inorganic As (Carbonell et al., 1998; Carbonell-Barrachina et al., 1998). In pot experiments with rice plants exposed to As added via AsV in irrigation water, plant parts were ranked according to the As concentrations as follows: root > straw > husk > grain. Concentrations in all plant parts increased with the exposure concentration (Abedin et al., 2002a; Abedin et al., 2002b). This is a common observation for other plants as well (Bleeker et al., 2003; Carbonell et al., 1998; Carbonell-Barrachina et al., 1998; Carbonell-Barrachina et al., 1997; Hartley-Whitaker et al., 2001; Sneller et al., 1999b).
After uptake, AsV is rapidly reduced to AsIII, causing oxidative stress. This induces the formation of certain antioxidants. This is regarded as a detoxification mechanism that is also activated by heavy metals such as cadmium (Meharg and Hartley-Whitaker, 2002; Sneller et al., 1999a; Sneller et al., 2000). On the contrary, exposure to AsIII does not induce this system. In spite of the rapid reduction of AsV to AsIII, high levels of AsV have been found in plant material. Abedin et al. (2002b) reported that more than 70 percent of the As in the straw of rice was present as AsV. Schmidt et al. (2004) found AsV in plants that were only exposed to AsIII, showing that oxidation of AsIII in plants took place. Many organic As species have been found in plants as well, but only in minor amounts (Dembitsky and Rezanka, 2003). It is unclear whether organic As species found in plants are taken up from the soil or are formed by the plants (Meharg and Hartley-Whitaker, 2002; Sneller et al., 1999b).
AsV can compete with PO4 within the plant cells disturbing the energy flow in the cell. AsIII reacts with a number of enzymes and tissue proteins that can cause inhibition of cellular function and finally death (Meharg and Hartley-Whitaker, 2002). Exposure to As also influences concentrations of other elements in plant tissue (Carbonell et al., 1998).
A specific form of As toxicity to rice known as straighthead disease has been reported in the USA (Meharg and Hartley-Whitaker, 2002). Straighthead disease is a physiological disorder that causes panicle sterility. Visual symptoms are empty panicles standing upright instead of bending downward at maturity. This disease was related to rice production on former cotton fields heavily contaminated with MMA used as a pesticide. It was most frequently observed on sandy loam soils but seldom on clay soils. Affected plants were usually found in spots scattered throughout a field. Besides As, a high organic matter (OM) content seemed to play a role as well. Rice cultivars show a great variation in their tolerance to MMA, which was used to select/develop tolerant cultivars.
A generalized ranking of plant parameters according to sensitivity to metals including As is as follows: root length > root mass > shoot length > total mass (root plus shoot) > shoot mass > germination (Abedin and Meharg, 2002). This is in agreement with e.g. Abedin et al. (2002b) who found that root biomass production of rice plants was most sensitive to As whereas plant height was not very sensitive. Carbonell-Barrachina et al. (1998) reported for coastal marsh grasses that dry matter production of roots was most sensitive. Abedin and Meharg (2002) proposed that shoot height can be used in the field as an indicator. Abedin et al. (2002a) reported that shoot height is however (much) less sensitive than root length. Abedin and Meharg (2002) proposed the next chain of effects: reduced shoot height, reduced leaf area, reduced photosynthesis, reduced yield. It is likely that toxicity on the root system is actually the first step.
The relative toxicity of different As species to plants depends on a range of factors including experimental conditions and plant species and examined plant parameters. Therefore, one should be cautious about using a generalized classification of As species according to toxicity. Taking that into account, inorganic As is generally regarded as being more toxic than organic As, with AsIII being the most toxic form (Dembitsky and Rezanka, 2003; Fitz and Wenzel, 2002; Liu et al., 2004; Mahimairaja et al., 2005; Meharg and Hartley-Whitaker, 2002).
AsV tolerance is related to PO4 metabolism (suppression of the AsV/PO4 uptake mechanism) (Bleeker et al., 2003; Hartley-Whitaker et al., 2001; Meharg, 2004; Meharg and Hartley-Whitaker, 2002; Sneller et al., 1999b). Most AsV tolerant plants accumulate less AsV than non-tolerant plants and this could be used to develop/select rice cultivars with a low accumulation of As.
Tolerance to AsIII is largely unknown. If there is any relevant variation in AsIII tolerance, this may be found in variation in glutathione-levels and/or the activity of certain transporters that transport As complexes within the plant (H. Schat, personal communication, 2004). Schat also reported for Holcus (Velvet Grass) that AsV tolerant plants did not show any tolerance to AsIII at all.
To date, studies on the genetics behind As tolerance have focused only on AsV. In rice and wild grasses, the tolerance to AsV is under the control of a single gene (Dasgupta et al., 2004). If AsV uptake is relevant in rice grown under flooded conditions, this can be an important finding to develop/select rice cultivars with a high tolerance and a low As uptake. Considering the indications that AsIII predominates the As uptake by rice, it is necessary to quantify the uptake of As species under (semi)field conditions. Based on this information, the research on genetics behind As tolerance and uptake can focus on the environmentally relevant As species.
Most toxicity experiments have been carried out with plants grown in water only (hydroponics). Such a design can be useful to study, for example, uptake mechanisms, internal transport, metabolism, and toxic effects. The design is however not suitable to generate toxicity data to evaluate concentrations in the environment because all interactions with the soil matrix influencing bioavailability are neglected.
Toxicity experiments are also carried out with plants grown in soil to which a certain amount of As is added (spiked soil) shortly before the experiment. This setup has various limitations as well. Adding As to reach a certain soil concentration suggests that the results are representative of the field. However, in the field As is added over a number of years. The prolonged contact time between As and the soil in the field can result in a lower solubility of As and therefore lower uptake by plants in the field. Therefore, experiments with spiked soils often result in an overestimation of the adverse effects compared to the actual field situation (Duxbury and Zavala, 2005).
In other studies, As has been added via irrigation water to the soil during the experiments. This is more in agreement with the field situation in Bangladesh compared to hydroponics and spiked soils. However, this experimental setup neglects that As levels in irrigation water in the field are relatively constant and that As is slowly added to the soils over a period of many years. Ideally, experiments should be performed with naturally contaminated soils and constant As concentrations in irrigation water.
To date, toxic effects have only been related to the irrigation water concentration and/or the total soil concentration. The As concentration in soil water (pore water) will surely deviate from the irrigation water because of interactions with the soil matrix. Total As in the soil is also unlikely to be a good predictor of As uptake and toxicity for different soil types as only part of the As in the soil is likely to be potentially available to the plants. Dose-response relationships based on irrigation water concentrations or on total soil concentrations are only valid for the experiment from which they were derived and cannot be extrapolated to any other system. With all these limitations in mind, various studies of the above mentioned types will be summarized and discussed.
Abedin and Meharg (2002) exposed eight Bangladesh rice varieties to AsIII and AsV and tested for germination and seedling growth. Germination was slightly inhibited at 0.5 and 1 mg/l. At 2 mg/l, inhibition was more than 10 percent. AsIII was more toxic than AsV. No significant difference between Boro and Aman cultivars in terms of germination was observed. Root growth was inhibited by ~20 percent at 0.5 mg/l and AsV was more toxic than AsIII. Boro cultivars were slightly more tolerant than Aman cultivars. Shoot height was also affected. At 0.5 mg/l, the shoot height was reduced by ~30 percent with no significant difference between cultivars and As species.
Marin et al. (1992), cited in Abedin et al. (2002b), found a reduced shoot height at 0.8 mg/l AsIII and MMA but not with AsV. In contrast with the findings of Abedin and Meharg (2002), AsIII was more toxic to root growth (dry weight production) than AsV, with the first inhibition observed at 0.8 mg/l. Dasgupta et al. (2004) reported AsV a root growth inhibition of 90 percent for rice cultivar Azucena and 50 percent inhibition for Bala at 1 mg/l.
Spiked soilOnken and Hossner (1995) spiked soil with 25 mg/kg AsIII or AsV. In the silt loam soil, a reduced dry matter was first observed after 40 days exposure. At the termination of the experiment (60 days exposure), the dry matter was reduced by approximately 50 percent with no significant difference between AsV and AsIII. In the clayey soil, no toxicity was observed, suggesting that a greater part of the added As was strongly bound to the soil. Taking into account the large uncertainties and fluctuations in soil water concentrations, water from the clayey soil contained 10 to15 times less As.
Jahiruddin et al. (2004) spiked silt loam soil with As. First, a Boro rice cultivar developed by the Bangladesh Rice Research Institute "BRRI dhan 29" and then an Aman cultivar "BRRI dhan 3" was grown. For Boro rice, the first significant effects occurred at 10 mg/kg soil, causing a grain yield reduction of more than 45 percent (Figure 2.1).
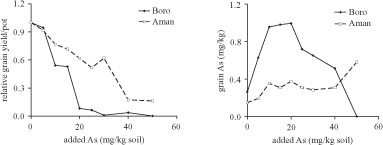
Source: Jahiruddin et al. (2004)
Note: Soil was contaminated just before the experiments.
The As concentration in grains of Boro rice first increased with the exposure level but then decreased. A possible explanation is that the toxic effects became so severe that As was hardly translocated anymore to the few grains that were produced at 25 mg/kg soil and higher (see also Figure 2.1). For Aman rice, the first significant adverse effects were on the number of grains per panicle and straw yield at 10 mg/kg. At 20 mg/kg soil, grain yield became affected whereas the other parameters were not significantly affected below 40 mg/kg soil.
A shortcoming of Jahiruddin et al. (2004) was that no measures to avoid or remove contamination like the dust of samples during sample preparation before digestion were described. This may explain the unlikely high Fe concentrations in grains of ~100 mg/kg whereas concentrations in rice are usually around 5 mg/kg. The chemical analysis did not include a certified reference material (CRM). The reported concentrations can therefore only be regarded as indicative.
Soil culture irrigated with As-contaminated water
Abedin et al. (2002b) exposed rice cultivar BR11 to AsV and studied growth and As uptake. The first observed adverse effect was a reduced root biomass at 0.2 mg/l. Other effects including reduction of plant height, spiklet weight, number of spiklets and grain yield started at 2 mg/l. In an almost similar experimental setup, a reduced root biomass, grain number and grain weight (g/pot; 26 percent reduction) was found at >1 mg/l (Abedin et al., 2002a).
Comparing the two studies suggests that the lowest As concentrations associated with toxic effects deviated substantially despite the similar setup. The main reason is probably the difference in the lowest As concentrations used in the irrigation water, namely 0.2 mg/l in Abedin et al. (2002b) and 1.0 mg/l in Abedin et al. (2002a). In both studies, first effects occurred already at those levels. This indicates that the range of exposure concentrations did not include a concentration so low that it did not cause any effect. It seems that for this particular experimental setup, the lowest concentration causing adverse effects is equal to or below 0.2 mg/l.
Smith et al. (1998), cited in Abedin et al. (2002a), reported that rice, bean, oats can suffer from phytotoxicity at a soil concentration of 20 mg/kg, whereas for maize and radish this is 100 mg/kg. According to Sheppard (1992), also cited in Abedin et al. (2002a), soil type is the most important variable for toxicity of inorganic As to plants, with soil texture one of the most important factors. Inorganic As was five times more toxic in a sandy soil (40 mg/kg) than in a clayey soil (200 mg/kg). Yan-Chu (1994), also cited in Abedin et al. (2002a) found a rice yield reduction often percent at 13 and 23 mg/kg soil. In sandy soil with 47-52 mg/kg, rice growth was reduced by up to 50 percent and completely inhibited at 109-157 mg/kg soil.
Islam et al. (2004b) carried out a similar experiment with the same soil and rice cultivars as Jahiruddin et al. (2004) with the difference being that AsV was added via irrigation water during Boro rice cultivation in the Islam et al. experiment. During the Aman cultivation As-free irrigation water was used, resembling the field situation. With an increase in As concentration in the irrigation water, first an increase in grain yield was observed, both for Boro rice and Aman rice. After that, yields declined (Figure 2.2). As concentrations in grains steadily increased with As levels in irrigation water (Figure 2.2).
Within the tested range of As concentrations in irrigation water, the observed toxic effects and As accumulation in grains reported by Islam et al. (2004b) were far less compared to the observations within the range of soil concentrations used by Jahiruddin et al. (2004). At first, the patterns seem to differ, but a closer look reveals that it is most likely that the range of concentrations used by Islam et al. (2004b) was narrower than that used by Jahiruddin et al. (2004). Comparing the two sets of results for 0-10 mg/kg As in soil shows a similar pattern. In spite of this, it is not known what the true exposure concentrations were and the results cannot be extrapolated to the field. The reports of both sets of authors had the same shortcomings regarding chemical analysis and the overall description of the methodology.
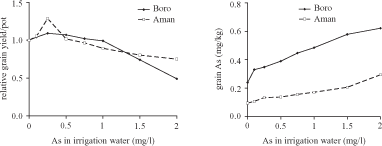
Source: Islam et al. (2004b)
Note: Pots were irrigated with contaminated water only during the Boro cultivation. As-free irrigation water was used during the following Aman cultivation.
In conclusion, none of the existing toxicity data can be regarded as representative of the field situation and extrapolations are not yet possible. A better understanding of As in the soil in relation to uptake and toxicity is therefore urgently needed. Ideally, soil parameters should be identified that correlate with uptake and toxicity. The development of a methodology for toxicity experiments that give results representative of field conditions has to be emphasized.
With the elevated As levels found in various paddy fields because of long-term irrigation with contaminated water, it may be possible to study phytotoxicity at the field level. Results from such studies would by definition be representative of the field situation, but a thorough understanding of the critical parameters involved would still be necessary in order to extrapolate the data to locations with other environmental conditions.
Soil micro-organisms may also be affected by As toxicity (Mahimairaja et al., 2005). Effects of As and on the soil microbial community can be expected with AsIII being more toxic than AsV. Microbes can adapt to As contamination, but this can be accompanied by a change in density and structure of the community. Ghosh et al. (2003) reported that microbial biomass and activity were negatively correlated with total and bioavailable As in soil samples from West Bengal. However, the description of the used soils was limited, making it difficult to assess if there were any other reasons like different soil types and land use that could explain the results.
It is generally recognized that inorganic As is far more toxic to humans than organic As. A well-balanced evaluation of As in foods should thus be based on inorganic As and not on total As to avoid an overestimation of the human health risks.
The methodology to assess As speciation in plant and animal tissue is complicated. To date, the methodology is not yet standardized and certified reference materials (CRMs) for inorganic As are not available. As speciation measurements depend on the pretreatment, extraction technique and storage (Heitkemper et al., 2001; Norra et al., 2005; Pizarro et al., 2003a; Pizarro et al., 2003b). Available values should be regarded as experimentally defined levels of inorganic As species. In Table 2.1, an overview of speciation data on rice has been presented. In summary, rice mainly contained AsV, AsIII and DMA.
Table 2.1 As speciation data for rice
|
Country |
Type |
Total As |
Inorganic As |
Extraction efficiency (%) |
% inorganic |
Reference |
|
Spain |
0.062 |
95 |
1 |
|||
|
Spain |
Paella rice |
0.17 |
0.08 |
78 |
48 |
9 |
|
Spain |
White |
0.149 |
0.126 |
91 |
85 |
|
|
Italy |
0.061-0.069 |
95 |
1 |
|||
|
Italy |
Various |
0.19-0.22 |
0.10-0.14 |
77-103 |
53-65 |
9 |
| USA |
White rice |
<0.025-0.271 |
2 |
|||
| USA |
0.303 |
0.074 |
3 |
|||
| USA |
White rice |
0.21-0.34 |
0.021-0.095 |
86-97 |
11-35 |
4 |
| USA |
Brown rice |
0.160 |
0.098 |
99 |
62 |
4 |
| USA |
Various |
0.11-0.40 |
0.05-0.14 |
59-90 |
20-59 |
9 |
|
India |
Various |
0.03-0.08 |
0.02-0.05 |
62-88 |
36-67 |
9 |
|
Bangladesh |
Aman |
0.03-0.30 |
0.01-0.21 |
51-98 |
34-86 |
9 |
|
Aman |
0.18-0.31 |
0.11-0.22 |
69-81 |
85-94 |
10 |
|
|
Boro |
0.21-0.27 |
0.17-0.22 |
84-90 |
81-83 |
10 |
|
|
China |
Unknown |
0.22 |
0.07 |
85 |
37 |
10 |
|
Unknown |
0.180 |
0.164 |
91 |
11 |
||
|
Thailand |
Various |
0.11-0.20 |
0.06-0.10 |
72-97 |
44-74 |
9 |
|
Unknown |
Unknown |
0.410 |
0.367 |
94 |
90 |
5 |
|
|
NIST 1568a |
0.286 |
0.088 |
99 |
31 |
6 |
|
NIST 1568a |
0.087 |
31 |
6 |
|||
|
NIST 1568a |
0.280 |
0.092 |
94 |
34 |
4 |
|
|
NIST 1568a |
0.283 |
0.085 |
99 |
35 |
7 |
|
|
NIST 1568a |
0.290 |
0.080 |
83 |
33 |
9 |
|
|
NIST 1568a |
0.29 |
0.10 |
84 |
33 |
10 |
a No data.
b Certified reference material for total As in rice.
1 = (Pizarro et al., 2003b); 2 = (Lamont, 2003); 3 = (Schoof et al., 1999); 4 = (Heitkemper et al., 2001); 5 = (Kohlmeyer et al., 2003); 6 = (Pizarro et al., 2003a); 7 = (Pizarro et al., 2003b); 8 = (Cava-Montesinos et al., 2003); 9 = (Williams et al. 2005); 10 = (Williams etal., 2006); 11 = (Zhu andMeharg, 2006).
Total As concentrations ranged from 0.03 to 0.4 mg/kg and inorganic As ranged from 0.01 to 0.363 mg/kg. The percentage of inorganic As was highly variable (11-90 percent). Although the results for As speciation in CRM NIST 1568a (rice) showed consistent results, this CRM is only certified for total As.
Williams et al. (2005) and Williams et al. (2006) presented the first speciation data for rice from Bangladesh and India. Data for Bangladesh indicated inorganic As comprised about 80 percent of the total As present in rice. The results will be discussed in more detail in Section 3.2.
Zhu and Meharg (2006) analysed 600 rice samples from China, mainly Hunan province, for total As, and randomly analysed 17 of those for inorganic As. The average percentage of inorganic As was 91 percent, which was three times higher than that reported by Williams (2006). Assuming a similar percentage of inorganic As in all 600 samples, approximately 50 percent of all samples exceeded the Chinese food safety standard for inorganic As in rice, 0.15 mg/kg.
Kohlmeyer et al. (2003) analysed numerous rice and seafood samples. The percentage of inorganic As was usually at least 50 percent with maximum values of more than 90 percent. Of the 180 rice samples analysed, total concentrations were between 0.08 and 0.5 mg/kg fresh weight. Atypical raw rice sample contained 0.170 mg/kg AsIII, 0.193 mg/kg AsV, and 0.023 mg/kg DMA, whereas MMA was below detection limit. A typical parboiled rice sample contained 0.102 mg/kg AsIII, 0.010 mg/kg AsV, 0.044 mg/kg DMA, whereas MMA was below detection limit. Raw rice and brown rice had higher totals of As and higher percentages of inorganic As compared to white and parboiled rice. This may suggest that parboiling and/or polishing remove As from the rice and that the As is mainly present in the outer husk and bran layer. Marine fish mainly contained arseno-betaine (AsB) (90-100 percent) and no inorganic As was found. Arsenosugars were predominant in marine algae. High concentrations of AsV were found in some brown algae like Hizikia (25.6 mg/kg dw, which is 60 percent of the extractable As). High AsV was also found in a sample of roasted seaweed (12 mg/kg dw, which is 86 percent of the extractable As).
Li et al. (2003) reported that the total As concentrations for seafood were 1.7-19.3 mg/kg dw in red algae, 14.6-38.7 mg/kg dw in brown algae and 0.086-7.54 mg/kg ww in marine fish and shellfish. Fish and shellfish contained less than 2 percent inorganic As, whereas inorganic As was not detected in marine algae (both brown and red algae). Fish mainly contained AsB, whereas As-sugars were predominant in algae.
Huang et al. (2003) studied As speciation in farmed fish (Oreochromis mossambicus) in As-affected areas in Taiwan Province of China. The fish prefer brackish waters and are cultivated in ponds. Although contaminated groundwater is not used anymore for drinking-water, it is still a source of water for aquaculture. Results showed that there was a positive correlation between As in the water and fish. The water mainly contained AsV, whereas AsB predominated in the fish. In fish, total As concentrations were in the range of 18 to 329 mg/kg dw and inorganic As was ~5 mg/kg dw (ranging from 1.7 to 26.1 mg/kg dw).