Intensification of shrimp culture production is limited by seed supply. On the other hand, the production of seeds in hatcheries depends on availability of broodstocks and quality of spawners. A healthy spawner is described with illustration in Figure 3.
Shrimp hatcheries still depend on broodstocks obtained from the wild although eye-stalk ablation technique to induce maturation of shrimp has been found to work very well. However, it has been reported to be more expensive to raise breeders in captivity than to buy the wild mature shrimps from the sea. Furthermore, it was found that the ablated shrimps are not good which results to low rate of survival. Fry produced from pond-grown ablated broodstocks are not as hardy as those from spawners obtained from the sea. This may be due to a combination of many factors such as nutrition, genetics and environment (Liao, 1986). More research in these problem areas are necessary.
In Taiwan, this problem is being solved by “sea ranching”. Pond-grown shrimps are released in suitable sites that are protected and recaptured after several months when they are mature. A recovery rate of about 15 percent was considered high enough to be profitable. In the Philippines, perhaps keeping them in enclosures at sea would provide higher recovery rate. This would ensure the supply of shrimp broodstocks all year round, thus, also ensure seed supply of better quality.
HOW TO TELL A HEALTHY P. MONODON
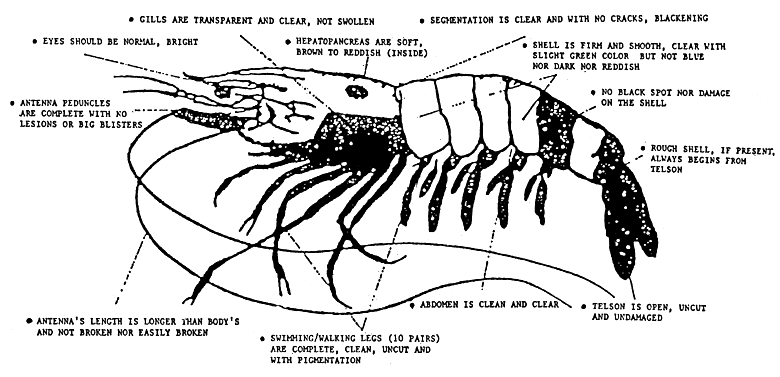
Figure 3. Illustration of a healthy spawner
Source: Tong Kuwan Aqua Development Co., Ltd.
RM 4 5F 218 TA Shun 3 Rd, Kaohsiung, Taiwan, Province of China
Shrimp ranching has been done in Japan, particularly in Hamana-ko Lagoon. Post-larvae of P. japonicus were released in the lagoon in 1978. For a five-year period, a total of 17.6 million postlarvae were released. This programme has become an extensive programme reaching a total of over 300 million postlarvae released. The main sites of releases were inlets, open or semi-open waters (Figure 4). The result of this programme showed that shrimp production in the area was 2.4 times greater than natural catches in open waters.
The advantages of shrimp ranching are (Uno, 1985):
increased supply of shrimp breeders and increased shrimp production,
stabilized production through adjust ment of the time in releasing the post larvae
additional recruitment to the natural population of shrimp not caught for breeding purposes
Hatchery technique of seed production of P. monodon is known and the application of shrimp ranching under Philippine condition could well be made. The use of sea enclosures like the fishpen would be a good approach to solve shrimp broodstock availability. Juveniles of shrimps produced from hatcheries could be released in coastal waters. However, the government should initiate the programme and set up specific areas for the purpose. Shrimp ranching could be allocated to small fishermen cooperatives in selected areas only. Otherwise, installation of enclosures at sea would also proliferate if not properly controlled. Guidelines for this purpose should be made; releases and recapture of juveniles should also be monitored.
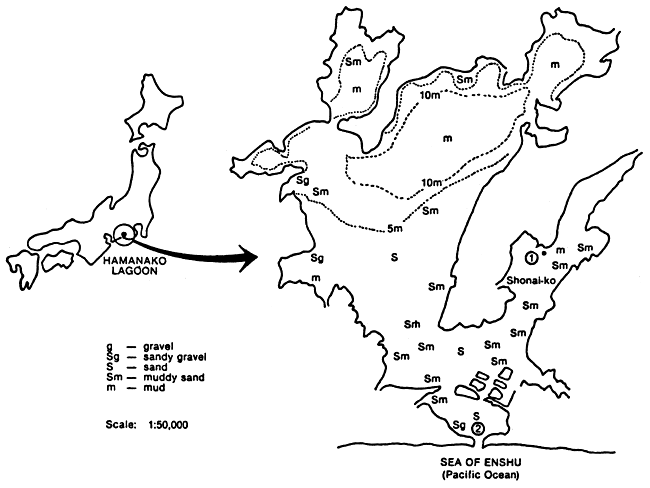
Figure 4. Topography of Hamana-ko Lagoon showing bottom conditions and depth. (1) site of enclosure for nursery culture; (2) mouth of lagoon (Imakiriguchi)
Source: Uno, 1985
The development of shrimp hatcheries in the Philippines is quite haphazard in the sense that many hatchery facilities sprang up without adequate technical guidance. This was a result of private sector interest and enthusiasm in getting the profits quickly and be ahead of the rat race for money-making. As a consequence, several hatcheries, especially the big ones end up losing money for not being able to operate them viably for several reasons.
Further refinement in hatchery management technique for P. monodon should be made as it is not well established compared to that of P. japonicus in Japan. The research programme on hatchery techniques on P. monodon lacks sustained support and coordination of efforts. Thus, the private sector has been left alone to hit-and-miss affair in their operations. The interest of the private sector is a healthy sign of willingness to take risks. However, capital resources are wasted if the operations do not succeed and the blame almost always go to the inefficiency of the government in providing the right technical assistance and guidance.
Hatchery operations require the following conditions to become successful:
Appropriate design of hatchery facility particularly with respect to quality of water and availability
Supply of healthy spawners
Experienced and knowledgeable technicians conversant with the behavior and feed requirement of shrimp larvae at certain stage of its life cycle
Availability of the appropriate type of feeds
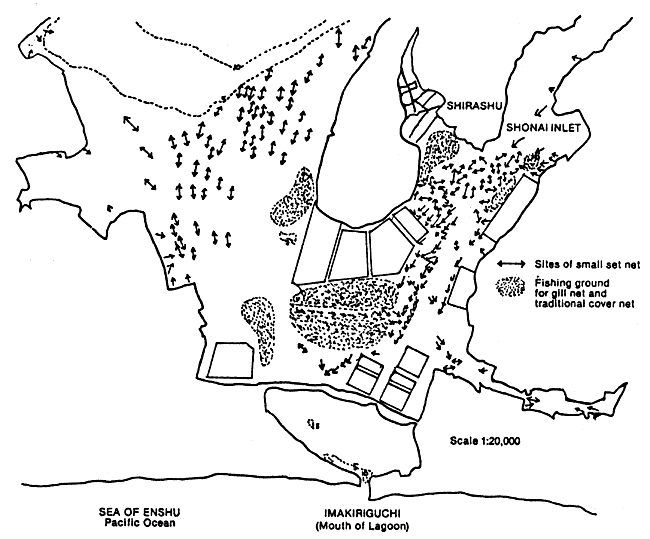
Figure 4a. Fishing grounds of kuruma shrimp, Penaeus japonicus, in Hamana-ko Lagoon. Figures denote depth in m.
Source: Uno, 1985
In view of the different stages of larval development of the shrimp, specialization of production would perhaps be better to maximize the use of available skills and provide more efficiency in the chain of seed production. This situation has developed in Taiwan as well as in Thailand although in a different context. In Taiwan, several specialized sub-businesses related to shrimp seed production include: (a) spawner supplier; (b) nauplius producer; (c) nauplius broker; (d) early stage postlarvae producer (PL10–PL12); (e) late stage postlarvae producer (PL20–PL35); and (f) postlarvae (PL10–PL35) broker. Figure 5 illustrates the specialized sub-businesses of the prawn industry in Taiwan. A firm or family business enterprise raises its crop to a certain stage of maturity and. sell the crop to the customers with the skill and capacity to raise the larvae to the next higher level of maturity (Liao, 1987). This avoids a total failure of operation of a hatchery if the facility cannot completely produce shrimp postlarvae or juveniles for grow-out use. Specialization also strengthens the seed production base of the shrimp culture industry. This is seen in the Philippine milkfish fry industry where there are the fry gatherers, the brokers and the milkfish nursery operators.
In Thailand, on the other hand, specialization came about as a consequence of over-production of prawn fry resulting in very low prices. The existing hatchery facilities, particularly the backyard prawn hatcheries went into shrimp larvae rearing and live food organism culture. The prawn hatchery operators learned to diversify the use of their facilities for the production of shrimp and marine fish larvae such as P. monodon, P. merguiensis, seabass and grouper according to season and demand. At present there are over 800 backyard prawn hatcheries which specialize in nauplii, postlarvae and food organisms (Csavas, 1988).
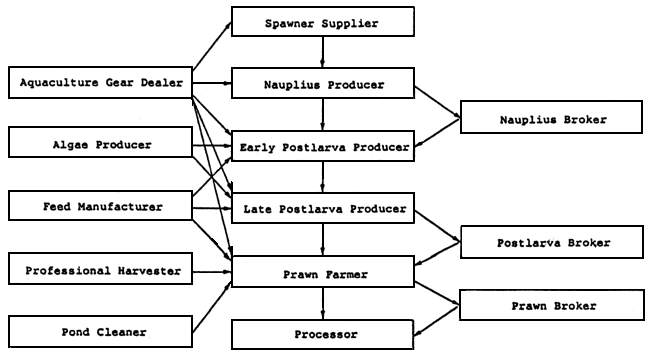
Figure 5. Specialized sub-businesses in the Taiwanese prawn culture industry
Source: Liao, 1988
The same situation has not yet occurred in the Philippines. There are a number of hatchery facilities already lying idle due to failures in hatchery operations not because of overproduction but more on failure in design of hatchery facilities and the lack of skills in hatchery management.
For information on stages of the life history of P. monodon, Table 2 shows the duration in each stage. Figure 6 illustrates the larval stages of the shrimp.
The most difficult stage of the shrimp larvae is the transformation from zoea to mysis stages. Each stage of larval development requires different kind of food. Table 3 summarizes the kinds of food of shrimp larvae from zoea to postlarvae. The feeding regimes during the developmental stages of penaeid shrimp is illustrated in Figure 7.
Table 2. Life history phases of the giant tiger prawn, Penaeus monodon
| Phase | Begins at | Duration | Carapace length (mm) | Mode of life | Habitat | |
| Male | Female | |||||
| Embryo | Fertilization | 12 hours | 0.26 1 | Planktonic | Outer littoral area | |
| Larvae | Hatching | 20 days | 0.5–2.2 | Planktonic | Outer/inner littoral area | |
| Juvenile | Completion of gill system | 15 days | 2.2–11.0 | Benthic | Estuarine area | |
| Adolescent | Stability of body proportion,development of outer genitalia | 4 months | 11–30 2 | 11–37 3 | Benthic | Estuarine area |
| Sub-adult | Commencement of sexual maturity, first copulation | 4 months | 30–37 4 | 37–47 5 | Benthic | Inner/outer littoral area |
| Adult | Completion of sexual maturity | 10 months | 37–71 6 | 47–816 | Benthic | Outer littoral area |
1Egg diameter
2Minimum size with jointed petasma
3Minimum size with adult-like thelycum
4Minimum size with spermatozoa in terminal ampoules
5Minimum size with spermatozoa with thelycum
6Maximum size ever found
Source: Motoh, 1985
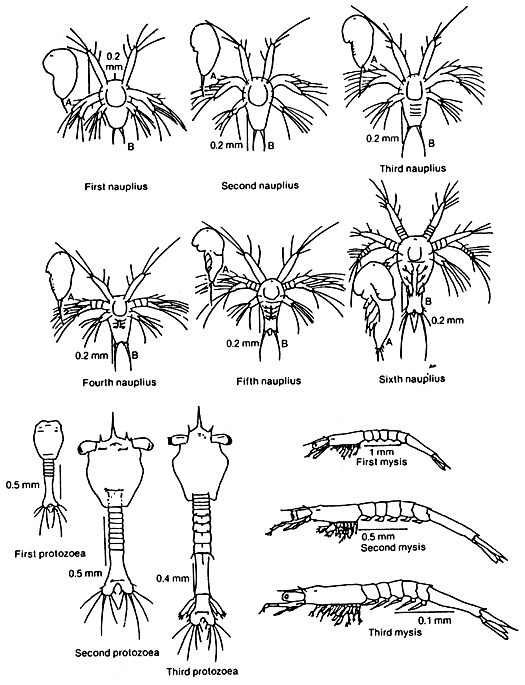
Figure 6. Larval stages of Penaeus monodon A - lateral view; B - ventral view (Source: Motoh, 1985)
Table 3. Feed materials used for shrimp larval stages
| Kinds | Zoea | Mysis | Postlarvae | Postlarvae | References | |
| (Early: P1–P10) | (Later: P11–P25) | |||||
| VEGETABLE SOURCES | ||||||
| Skeletonema sp. | ++ | ++ | Hudinaga, 194 | |||
| Tetraselmis sp. | + | + | Beard, et. al. | |||
| Isochrysis sp. | + | + | Beard and Wicki | |||
| Chaetoceras sp. | + | + | Hirata, et. al. | |||
| Dunaliella sp. | - | - | SEAFDEC, 1981 | |||
| Spirulina sp. - | - | - | Tang, 1977 | |||
| Chlamydomonas sp. | - | - | Hudinaga and Kittaka, 1975 | |||
| Marine chlorella | - | - | Hudinaga and Kittaka, 1975 | |||
| Soy bean residue | + | + | Hirata, et. al., 1975 | |||
| ANIMAL SOURCES | ||||||
Eggs or fertilized eggs of oyster | ++ | ++ | Liao, 1969 | |||
| Eggs of Mytilus | ++ | ++ | Kittaka, 1975 | |||
| Rotifer | ++ | ++ | Liao, 1969 | |||
| Artemia salina | ++ | ++ | Hudinaga, 1969 | |||
| Brine shrimp flakes | ++ | ++ | Unknown * | |||
| Moina sp. | - | Kittaka, 1975 | ||||
| Copepoda | ++ | ++ | Shigueno, 1968 | |||
| Gammarus sp. | - | ++ | Kittaka, 1975 | |||
| Balanus sp. | ++ | ++ | Kittaka, 1975 | |||
| Nematoda | - | - | Liao, 1969 | |||
| Annelida | ++ | Liao, 1969 | ||||
| Clam meat | ++ | Liao, 1969 | ||||
| Shrimp meat | ++ | Liao, 1969 | ||||
| Fish meat | + | Liao, 1969 | ||||
| OTHER SOURCES | ||||||
| Yeast | - | Furukawa, et. al., 1973 | ||||
| Milled feed | + | + | + | Shigueno, 1975 | ||
| Sprayed dried feed | + | + | + | Shigueno, 1975 | ||
| Microencapsulated diet | + | + | + | Johnes, et. al., 1979 a, b | ||
NOTE: ++ Good;
+ Available;
- Poor
* Unknown - Origins presently being verified but cannot be substantiated at this time
Source: Liao, 1984
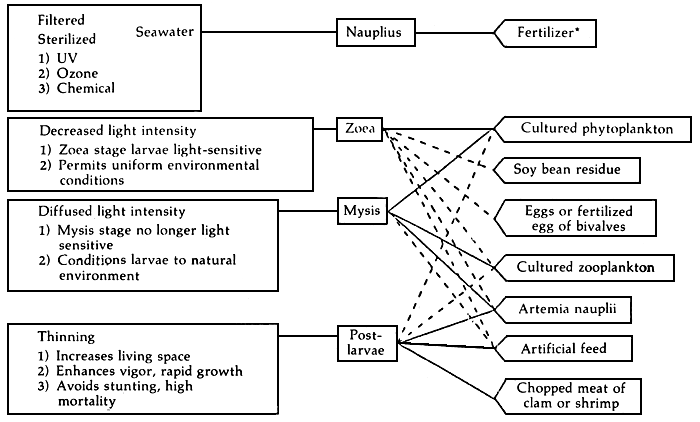
*Community culture method only
Solid lines (—) for most prominent feeding regime
Dashed lines (. . . . .) for occasional, less widely used regime
Figure 7. Schematic representation of feeding regimes for developmental stages of penaeid shrimp and related culture parameters
Source: Liao, 1984
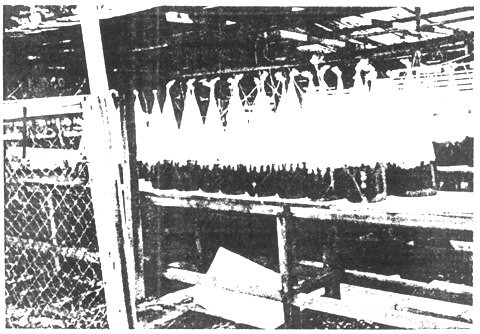
Figure 8. Algae culture using natural light
For larval rearing of P. monodon, some of the essential aspects of postlarvae production based on Taiwan experience and conditions are summarized in Table 4.
Table 4. Some aspects concerning larvae rearing of Penaeus monodon in Taiwan
| Sources of spawner | It is difficult to guarantee source of spawner. Fortunately with unilateral eyestalk ablation technique some 5–10 percent additional spawners can be provided; 20–40 percent depend on. importation and 55–70 percent on wild spawner. Season to capture spawners is from March to November. In the other months spawners supplemented from Southeast Asia. |
| Number of eggs from one spawner | Around 20–60 × 104 |
| Size of rearing tank for spawning and hatching | Large tanks are usually not suitable for spawning due to difficulty in collecting large numbers of spawners at any one time; 0.5–1.0 ton small round tanks adequate to hold 1–3 spawners are used. Spawning and hatching in the same tank take place where larvae are reared to mysis stage. Before transfer to larger pond, 30–50 thousand larvae are stocked in each small tank. Larger concrete tanks of 20 tons are used in case more spawners are available. |
| Sensitivity to light | Zoea stage should be kept in comparatively dark shaded area because of high sensitivity to light. After mysis III, larvae become adaptable to light. |
| Optimal temperature and salinity | Suggested water temperature 28–31°C. Salinity for nauplius, zoea stage, 31–33 o/oo after mysis stage, below 30 ‰. |
| Recommended method for rearing | "Monoculture method" recommended. Zoea feed mainly on Skeletonema; mysis on rotifera and Artemia nauplii and postlarvae on Artemia nauplii, minced trash fish or shrimp. |
| Diseases | Mass mortality in zoea and mysis stages resulting from unknown causes, while in postlarval stage may be infected by Epistylis sp. Recently the phenomena of regional and seasonal mass mortality have been reported. |
| Survival rate | Usually P12–15 sold to nursery farmers who raise them to P30–40 averaging 0.02 g before delivery to farmers, from nauplius stage to P12–15, survival rates average 40 percent. |
| Prices of juvenile | NT$300–900/l 000 juveniles (US$7.9–23.7/1 000 juveniles) |
Source: Liao, 1984
Significant contributions of research to the shrimp hatchery industry has been done elsewhere, mostly in developed countries. These include the identification of nutritionally good species of algae and strains of Artemia as food organisms of shrimp larvae and the means by which the bio-chemical composition of algae, rotifers and Artemia may be enhanced by altering their nutrient source and rearing conditions. The development of a technique of disinfection and decapsulation of Artemia cyst during hatching has reduced fouling and contamination prior to hatching. The development of microencapsulated feeds for shrimp larvae is a breakthrough in hatchery technology (Wickins, 1986). The use of these imported feed materials are however, costly and small fish farmers are not able to procure them. Techniques of food organism culture have also been developed. Algae or zooplankton culture need not be indoors with sophisticated facilities. The use of plastic bags containing the culture media under a shed outdoors is not difficult to set up as shown in Figure 8. Asia is blessed with adequate sunlight the year round to promote algae culture.
A hatchery design should provide efficiency of water use and conveyance such as a ladder type of hatchery taking advantage of gravity system as shown in Figure 9. Engineering considerations for disease and stress prevention in shrimp hatcheries and grow-out ponds were studied by Gacutan and Vizcarra (1988) which are now being adapted in recent construction of shrimp hatcheries and pond renovations in the Philippines (Appendix 1).
There are three major sizes of hatcheries, namely; large, medium and small. The characteristics of such hatcheries are summarized in Table 5.
Most of the complaints of hatchery operators concern about low survival and disease infections. The low rate of survival may be due to eyestalk ablation. It has been reported that a maximum of 2–3 or 4–5 spawnings are obtained after each process of ablation. Consequently, the health conditions of larvae obtained from the later spawnings are claimed to be poorer than those obtained in earlier spawnings; shrimp larvae obtained from spawners by eyestalk ablation are weaker, hence, lower rates of survival than those obtained from spawners caught from the wild or non-ablated individuals. These observations, however, need confirmation through scientific research to determine the causes of low survival of larvae from ablated spawners (Liao, 1984).
Diseases often result to high losses of hatchery operators. Some of the common diseases encountered in shrimp hatcheries, the symptoms and treatment are summarized in Table 6.
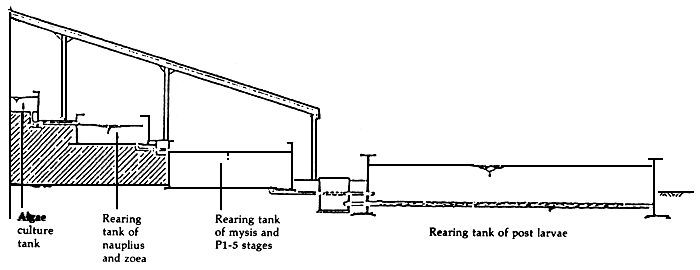
Figure 9. A ladder system hatchery taking advantage of ground slope and water levels
Source: Liao, 1984
Table 5. Distinctive characteristics of three major sizes of hatcheries
| Size | Large | Medium | Small |
| Ownership | Company | Family or partner | Family |
| Personnel | Consultant, super-visor, technicians and workers | Owner and experienced workers | Owner and worker |
| Size (unit of pond) | 600–800 m2 plus excessary tanks(40–60 tons) | (1–20 tons) | (1–5 tons) |
Electricity (generator power) | 100 KW | 50 KW | 5 KW |
| Water storage | 600 tons | 200 tons | 10–50 tons |
| Water treatment | Filtered at the source or UV light treated | Filtered at the source or UV light treated | Filtered at the hatchery |
| Number of spawners/yr | 500 spawners | 200–300 spawners | 40–50 spawners |
| Sources of spawners | Private shrimp trawler or broker | Fishermen or broker | Fishermen or broker |
| Length of active operation (mo/yr) | 11 | 10–11 | 6–8 |
| Maximum capacity(fry/yr) | 10 000 000 | 5 000 000 | 1 000 000 |
| Nursery | Necessary | Necessary | Not necessary |
| Shipping | Airplane, truck or exfarm | Truck or exfarm | Truck or exfarm |
Source: Liao, 1984
Table 6. Diseases found in the developmental stages of penaeid prawn larvae and their control methods
| Diseases | Affected parts | Symptoms | Treatment | Life stages affected * | References | |
| Bacteria: | ||||||
| Bacterial necrosis | Appendages | Appearing as localized necrosis or discoloration on any appendage, causing high mortality of zoea and mysis stages, affects postlarvae to a lesser extent. | Furanace | 1.1 ppm | Z | Tareen, 1982, |
| Erythromycin | 1.5 ppm | M | I.ightner, 1983 | |||
| Achromycin | 1.2 ppm | PL | ||||
| Vibrio infection | Hemolymph, midgut gland | In initial stages of one form, some larvae will show yellow-vermillion and red color permeating entire nervous system. Another form exhibits "white-turbid liver",where the midgut gland of the larvae becomes generally white-turbid. Turbidity becomes more apparent and well-defined as the disease progresses. | Furazolidone | 2.0 ppm | PL | Nickelson and Vanderzant, 1971 |
| Terramycin | 450 mg/kg biomass | Lewis, 1973 | ||||
| Furanace | 1.3 ppm | Shigueno, 1975 | ||||
| Lightner, 1977 | ||||||
| Johnson, 1983 | ||||||
| Cipriani, et. al., 1980 | ||||||
| Tareen, 1982 | ||||||
| Lightner, 1983 | ||||||
| Filamentous bacteria | Gills, pleopods | Commonly found attached to the gill filaments and the pleopods, turning blackish when bacteria mix with dirt. If severely affected, the respiratory function of the gill suffers damage. | Cutrine plus | 0.5 ppm | PL | Delves-Broughton and Poupard, 1976 |
| Malachite green | 10 ppm | Streenbergen and Schapiro, 1976 | ||||
Potassium permanganate | 8.5 ppm | Johnson, 1978 | ||||
| Cuprous chloride | 1.0 ppm | Solangi, et. al., 1979 | ||||
| Lightner, et. al., 198 | ||||||
| Tareen, 1982 | ||||||
| Lightner, 1983 | ||||||
| Shell disease | Exoskeleton, muscles | If infected by chitinoverous bacteria, the exoskeleton will display eroded blackened areas. The edges or tips of the exoskeleton parts are typically attacked.Also,bacteria can rapidly enter the body through surface breaks to cause internal damage. | Malachite green and Formalin combined | 0.9 ppm | PL | Cook, 1973 |
| 22 ppm | Delves-Broughton | |||||
| Black gill disease | Gills | In initial stages, gill color turns dull orange-yellow or light brown. When advanced, the area darkens until it is finally black. | Malachite green | 3.0 ppm | PL | Shigueno, 1975 |
| Methylene blue | 8–10 ppm | Tareen, 1982 | ||||
| Fungi: | ||||||
| Lagenidium infection | Body cavity, appendages | Only thin-cuticled prawn can be infected, thus larval prawn are highly sensitive. The hyphae appear inside the body of zoea and continue into mysis stage,resulting in massive muscle destruction and heavy mortality of zoea and mysis. | TreflanR | 0.1 ppm | Z | Hubschaman and Schmitt, 1969 |
| Malachite green | 0.01 ppm | M | Lightner and Fontaine, 1973 | |||
| Lightner, 1977 | ||||||
| Johnson, 1978 | ||||||
| Gopalan, et. al.,1980 | ||||||
| Tareen, 1982 | ||||||
| Lightner, 1983 | ||||||
| Ectocommensal protozoa: | ||||||
| Ciliate infection(Zoothamnium sp., Epistylis sp.) | Gills, eyes, exoskeleton | Heavy infestation by Zoothamnium sp. on gills and eyes of larval prawn results in high mortality. Epistylis sp. seems to prefer exoskeleton as Attachment site and is less Harmful. When abundant on gill surface, both can cause hypoxia and death. Addiditionally, their abundant presence on general body surface of larvae may interfere with locomotion,feeding, molting, etc.,Parasite burden increases until ecdysis provides relief. | Malachite green and Formalin combined | 1.0 ppm | Z | Johnson, et. al., 1973 |
| 25 ppm | M | Overstreet, 1973 | ||||
| PL | Delves-Broughton and Poupard, 1976 | |||||
| Quinacrine hydrochloride | 0.8 ppm | Lightner, 1977 | ||||
| Chloramine-T Methylene blue Saponin 10% | 5.5 ppm | Liao, et. al., 1977 | ||||
| 8.0 ppm | Johnson, 1978 | |||||
| 5.0 ppm | Lightner, et. al., 1980 | |||||
| Tareen, 1982 | ||||||
| Lightner, 1983 | ||||||
| Viruses: | ||||||
| Penaeid baculoviruses (BP, MBV,BMN) | Hepatopancreas, anterior midgut | Penaeid baculoviruses infect epithelial cells of the hepatopancreas and. less commonly, anterior . midgut, causing high mortality in the postlarval stage. | PL | Johnson, 1978 | ||
| Sano, et. al., 1981 | ||||||
| Lightner, 1983 | ||||||
| Lightner, et. al., 1983 | ||||||
| Couch, 1974 | ||||||
| Infectious hypodermal and hematopoietic necrosis (IHHN) | Hypodermin, hematopoietic organs | Prawn dying from acute IHHN show massive destruction of cuticular hypodermis and often of the hematopoietic organs,of glial cells in the nerve cord, and of loose connective tissues such as the subcutis and gul serosa. Only prawn with in a size range of 0.05–1.0 g have been observed to have these epizootics, resulting in massive mortalities (often 80 to 90'percent within two weeks of onset) | PL | Lightner, 1983 | ||
| Miscellaneous diseases: | ||||||
| Abnormal nauplii | Appendages | Occurs as a result of poor quality of spawner. | N | Tareen, 1982 | ||
| Ameobasis of larvae | Subcutis, muscles | Invasion of muscles and subcuticular tissues located in the abdomen, cephalothorax, antenna and eyestalks, by unclassified amoeba. | Z | Laramore and Barkate, 1979 | ||
| Lightner, 1983 | ||||||
| Larval encrustation | Exoskeleton | Brown to black encrusted deposits which contained iron salts affect larval penaeids. | Z | Lightner, 1983 | ||
| M | ||||||
| PL | ||||||
* N = nauplius; Z = zoea; M = mysis; and PL = post-larva