by
R.Q. Gacutan2 and A.T. Vizcarra3
1. INTRODUCTION
Developments in the technology for shrimp larval hatchery has been greatly aided by contributions from the engineering field. These are by way of the applications of engineering principles and practices which led to more efficient and more economical designs of facilities.
The first tanks utilized for Penaeus monodon larval rearing were of the 50–100 ton type, rectangular or square, wide and deep, roofed or open. This was adapted from the design known to be best for P. japonicus, the “kuruma shrimp”, which had been worked out to perfection in Japan since the early 1930's.
The operation calls for spawning and larval rearing (from nauplius to zoea, mysis and postlarvae) to be done in the same tank up to harvest at PL5 stage. Even the diatom/phytoplankton feed is added to the tank in this form, culture medium and all, and made to bloom right there in the tank. The rotifers and zooplankton are, however, cultured separately. One sees, therefore, that basically a “straight run” a minimum of larval transfer from one tank to another is made. In consequence, large volume of seawater are used up for water exchange especially when a “flow-through” system is called for. Such is instituted during situations when the rearing tank is “suspect”, meaning, there > are indications of a disease.
This type of operation did not suit P. monodon larvae very well. Apparently, it appeared to be much more sensitive to metabolite build-up and subject to a wider spectrum of diseases in the tank than does P. japonicus.
The Taiwanese system scaled down the tank sizes by 1/5 to 1/2, so now one sees the proliferation of 10 to 20 ton sizes, still rectangular or square and of medium depth. In addition, the tanks were roofed, with a structure that made the environs warm, humid and dark. These are definite improvements of this type over the Japanese tanks. For one, there is already a separation of the process into component stages so that there are now the so-called gonadal maturation tanks, spawning tanks, and many larval rearing tanks of almost uniform sizes to be used during subsequent transfers, apparently ad infinitum in response to the state of health of the fry.
The phytoplankton/diatom culture, the most important food for the zoeal stages is grown separately, inside another tank or a separate airconditioned laboratory until a dense population is attained. Once the desired density is attained, the culture is harvested, washed and offered at a “semi-controlled” density or at predetermined numbers.
Almost parallel and simultaneous to the developments in Taiwan were those occurring in the western hemispere, specifically, in Galveston, Texas by Cornelius Mock and co-workers. The tanks, now down to 1–2 tons in size, are compact. The tanks are obviously economical, for the reduction of water volumes needed; practical, for the ease in effecting transfers of larvae at any stage of the rearing cycle; clean and sanitary, for in the endless operations one can keep disease situations in check including even with the use of salts, approximating the composition of seawater as in the “instant ocean” or “artificial seawater”.
Diatoms/phytoplankton are intensively cultured in demijohns, plastic sheets and even bags and, prior to addition to the culture facility, are filtered to remove the original salts of the culture medium which for a time are being eyed as probable causes of mortality. Artemia, the best food known for mysis and later stages is also offered in its cleanest form possible, what with the concerted attempts and works of Sorgeloos and co-workers in Belgium initially, and around the world consequently.
The hatchery designs one sees today in the Philippines is a combination of the abovementioned culture facilities. It goes without saying that the best of each system are adapted for economy and ease in instituting sanitation measures. The circular tanks are good - siphoning of excess unconsumed microencapsulated feeds, now in vogue, is made easy. Furthermore, there is the possibility of aeration coming about evenly in all parts of the tank.
Much of the developments today are engineering in nature. And many more ideas are continually packaged and added as the hatchery operator sees 3 fi
The engineering of ponds followed a separate and late course and was aided by very little back-up research. In the Philippines, much of the pond area devoted to shrimp culture were old milk-fish ponds redesigned to meet the perceived specifications for penaeids,. Shrimps are, of course, known previously among bangos culturists as usual “by-products” in bangos ponds - they invariably turn up in low volumes at harvest, just enough to be noticed.
In the absence of a ready technology, the first culturists were “extensive” or “low density”, in reponse to the resources available to the pond. When feeds were introduced and may come to know of the wonders they do by way of faster growth and greater survival, higher densities (semi-intensive) were tried. The importance of water, in disease control, i.e., exchange volume needed, etc., soon dawned upon the culturist and suddenly, the shift is toward intensification. With this, the culturist tried to cramp several animals within a square meter of the pond bottom 10 first, then 20, 30 and then shooting for 60 and 80.
But the environment can take only so much number of animals, nothing more. Excessively, high stocking densities unduly cause stress to the animals, load the water with hazardous metabolites and, therefore, cause DISEASE.
Late in 1986, many diseases cropped up in Negros, dimming visions of many culturists of huge profits. Before long, they realized the folly of trying to crowd the animals. Back to earth they descended and figures now point out that 15 per square meter or 150 000 per hectare is about the carrying capacity of ponds.
Nothing short of amazing, however, is the determination of many a grower to succeed in the endeavor. To avoid diseases, they tried every option available to them. Thus, we now see innovations that include concrete flumes, much more efficient aerators, central drains of various specifications, and now the “vacuum cleaner”.
There are aspects neglected though. Close to a hundred percent of existing Negros ponds failed to reckon with the innumerable benefits that could be drawn from such components as a combination reservoir - treatment “kitchen” pond. Then, there was very little that was done to site the inlet canals and their corresponding drain canals separately so much so that they now operate on the basis of “what comes out must, at some future time, come in”.
Likewise, they failed to reckon with Baliao's and SEAFDEC co-workers' experience on the modular pond. This system is promising for its vision on disease control. Before diseases could manifest and throw the pond into chaos, the animals are transferred into a next compartment where new conditions, better than the previous one, prevail. Thus, if the course of a disease calls for a 60-day development, it never surfaces as the population is moved to another pond within 30 to 45 days.
This paper attempts to present the areas in which these engineering concepts are applied, and speculates on some others that might be worth looking into. Obviously, this does not present all the possible improvements but it will be just a starter, to tell the engineer that he is very much needed. One has to bear in mind, though, that the engineer must proceed with the advice of the biologist, for obvious reasons.
2technical Staff, Dole Philippines Aquaculture Project, General Santos City, Philippines.
2. HATCHERY
2.1 Laying of water distribution pipes in sloping fashion
At present, water lines are laid on the ground flat or level. With this set up, water is trapped within the pipes for long hours or even a day or two after use. Seawater thus confined in these pipes is a good medium for microbial growth, especially the bacteria. When water is allowed into the tanks and through the pipes subsequently, the pathogen-rich waters get into the tanks and may then become the vector of diseases.
Sloping instead of level or horizontal lines, provided with drain plugs at strategic or lowest points, will allow total draining of water after pumping. In this manner, introduction of pathogen-rich water is prevented. The pipes are also dried in the process.
It is good piping practice to provide a pitch of 0.05 to 0.10 cm for every meter of water line. Steeper slopes may be adopted for faster drainage. The vertical rise should not, however, exceed 2 cm over any one meter horizontal distance to prevent settling of solids along the pipeline.
2.2 Separation of eggs from debris and traces of chemicals after spawning and treatment (Figure 1)
The termination of spawning at dawn is often indicated by the deposition of a thick orange scum on the tank side just above the water line. This material roughly equivalent to the placental material is a good medium for bacterial growth, which more often than not, proves detrimental to the egg and its hatching or even to the nauplius, when left unattended to. Many culturists remove the deposited orange material by wiping the tank sides with a clean piece of cloth.
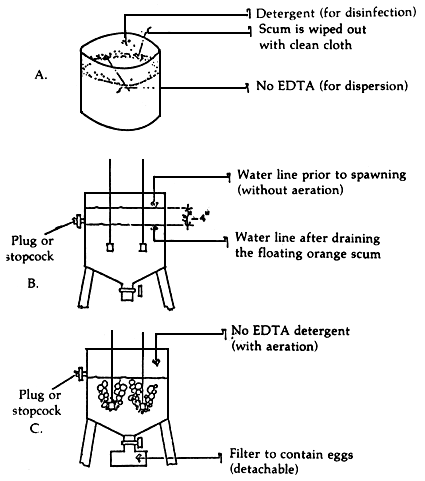
Figure 1. Improvements on spawning tank
Oftentimes also, a solution of sodium EDTA1 is added to disperse the eggs, or prevent them from clumping into a mass. This solution is added to give a final concentration of 10 ppm.
It is also necessary to wash the egg in a weak solution of ordinary detergent to disinfect the surface of the eggs. Oftentimes, a 20 ppm final concentration of the detergent is used.
It is possible to construct a spawning tank that may be operated to prevent the reintroduction of the placental material into the spawning water, and at the same time allow the introduction of NaEDTA and the detergent solution almost at the same time.
The operation calls for the lowering of the water level by a stopcock in the spawning water to about 3 or 4 inches below the water level line without aeration so as not to allow the placental material to mix back into the water. The dispersant NaEDTA is next added, the water is aerated to achieve dispersion. After a specified period of time, the detergent is added. Aeration is again resumed later. The eggs are finally separated very rapidly from the water containing the two chemicals by passing the same through a filter-catchment which may be engineered to fit the spawning tank bottom. The filter should allow the solution to pass readily but should retain the eggs. A 150 micron net is suggested (Figure 1). The eggs are then quickly transferred to a clean tank of filtered seawater for hatching.
2.3 Selection and collection of starters for a diatom/phytoplankton/algal culture
The system used in the culture of diatoms/phytoplankton for larval shrimp stipulates the addition of a high density starter culture to a tank or any other container with the necessary salts before aeration and exposure to the correct light intensity and quality.
The high density starter culture used is oftentimes obtained from an active culture about to reach the highest point in the logarithmic phase (asymptote). Active, senescent, dead cells and even bacterized cells are included. Thus, the quality of the culture is not as high as required. Selection of active cells only may be done by exposing the culture to bright flourescent light (or to sunlight for a few minutes) until a wide intensely-coloured band of the diatom/phytoplankton is concentrated in the upper layers, or a few centimeters below the surface. It is then possible to design tanks for selective collection purposes.
By prior experimentation, one will know the exact spot or depth the active cells will occupy after a specified duration of exposure to light after aeration is stopped. The tank may then be engineered by putting a water spout within the area where the active cells suitable for starter materials would congregate.
2.4 Separation of debris, ungerminated cysts and chorionic membranes from Artemia nauplii after germination
The Artemia nauplius is still the best food for larvae beyond the zoeal stages. Many culturists, however, are not so particular about whether what is being offered are the nauplii, healthy cysts, or ungerminated and, therefore, unhealthy cysts.
One must realize that cysts have chorionic membranes to which many different kinds of micro-organisms are trapped or encased. When offered in the form of cysts, disease can result. The ungerminated cysts should be treated with more concern than ordinarily.
Presently, the most common Artemia hatching facility is made of a conical tank with a tapered bottom from where aeration is directed upward. After hatching (11–14 hours), the aeration is stopped, and light is shone on a portion of the tank which allows light to permeate. The nauplii, being positively phototactic, congregate within the area and these may then be siphoned off simply by directing a rubber hose within the area.
A scheme for an Artemia hatching tank that makes use of the attraction of nauplii to light, the floating of chorionic debris and the sinking of ungerminated cysts has been devised.
2.5 Fitting of a spewing tube (plastic, PVC, or rubber) that washes down postlarvae adhering on tank sides(Figure 2)
It is commonly observed that postlarvae beyond PL10 adhere on the sides whether the tank inner surface is of plastic, canvas, concrete or wood. This is rampant in shaded and dark tanks suddenly opened and exposed to light during a sudden noise or approaching distraction. A few are brought to the sides by turbulence due to aeration. Many of these larvae may not be able to get back to the water and thus, die.
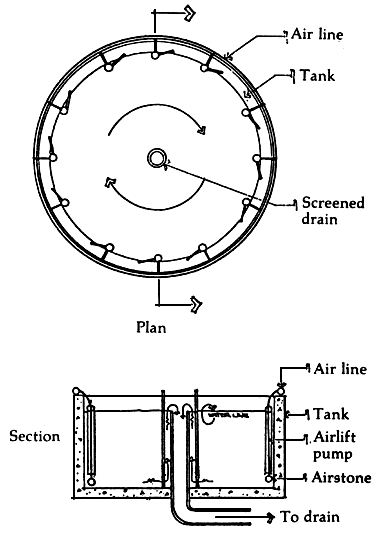
Figure 2. Airlift pumps to wash down post-larvae adhering to tank sides
The tanks may be fitted with circumferential or peripheral ring just above the water line, made of plastic, PVC or even rubber, with perforations from which water flows out and splashes against the tank walls. The water will gently wash down the animals back to the water. The design should see to it that the water spewed out comes from the tank and is brought into the circuit by a recirculating pump. Further-more, no water should be lost during the process.
Alternatively, the tanks may be equipped with airlift pumps at the sides which aside from washing the wayward postlarvae back to the water, will aerate and recirculate the water in the tank (Figure 2).
2.6 Sinking of airstones at different depths in a hatchery tank; closely-spaced aerators (Figure 3)
Many hatcheries especially those operated by Taiwanese employ an unusually large numbers of airstones spaced between 20 to 30 cm. The aeration rate in each air-stone is controlled, however, so that the turbulence created is not violent. These air-stones do not rest on the tank bottom but are elevated 7.5 to 10 cm above. Some technicians elevate these airstones alternately or in a pattern so that some are 7.5, 15, 22.5 and 30 cm above the bottom (Figure 3).
These arrangements assure that no specific spot of the tank becomes stagnant or anoxic. Furthermore, food due to the large number of aerators and their elevation at different heights is suspended for a long time before sinking.
2.7 Treatment of larvae using coloured compounds such as trifluralin, malachite green, oxytetracycline, furanace, etc.
Pathogens such as fungi and bacteria can gain foothold anytime during the rearing cycle despite precautions against their introduction. Fungi are treated with trifluralin or malachite green; and bacteria, with oxytetracycline, furanace, furazolidone and other antibiotics. These four compounds have a common characteristic: a chromophore or simply, colour.
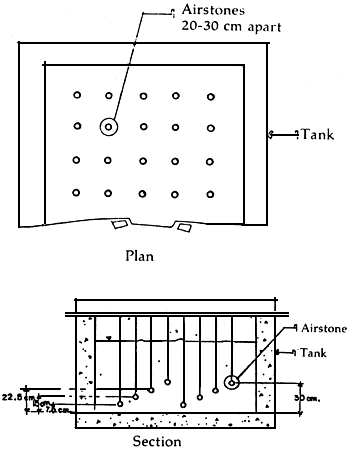
Figure 3. Airstones, closely-spaced and set at different depths
It is emphasized that these are to be used following some rigid requirements. On exposure to light, for example, coloured compounds may undergo changes but the more common reactions are: rapid decay, or a heightening of the killing effect, not only on the target microorganisms but also on the animals which they are supposed to protect.
Thus, these coloured compounds should be used as water additive only at night, or in the almost complete absence of light. It is, therefore, suggested that hatchery tanks are provided with an adjustable shed, the roof of which will cut off light before it strikes the water surface or the larvae.
2.8 Regulation of diatom/phytoplankton blooms by controlling light that filters into the hatchery at certain times of day
The diatom/phytoplankton density needed in larval rearing progressively increases from Zoea-1 up to Mysis-3. Chaetoceros calcitrans density, for example, should be 50–60 × 103 cells/ml by Z1 and up to 100–120 × 103 cells/ml for M1. Since diatoms/phytoplankton are usually offered during the height of their log phases, they can continue their growth and multiplication in the rearing tank such that the density may be more than what is required. Excessive density is not good as more food leads to higher levels of toxic metabolites, and when the diatoms/phytoplankton are senescent they settle on the tank bottom to be acted upon by bacteria which increase in number at the expense of the dying diatoms/phytoplankton.
The larval rearing tanks used for zoea and mysis should, therefore, include provision for movable/detachable roof that will enable one to regulate the amount of light and the duration of exposure of diatoms/phytoplankton to daylight.
Three of the more troublesome contaminations in hatcheries are diatoms/phytoplankton species Licmophora abbreviata, Nitzschia closterium and a blue-green alga, Schizothrix calcicola. Licmophora attaches to the exoskeleton and makes the larvae heavy and unable to swim normally while Nitzschia, considered the “pest” of hatcheries multiplies fast. The cells then clump and attach to the setae, gills and extremities. Oftentimes, covering the tank with a black cloth or plastic will prevent the diatoms/phytoplankton from multiplying rapidly. Schizothrix calcicola, on the other hand, succeeds in a situation where there are plenty of bottom organic deposits. A complete water change, coupled with a black cover and addition of fresh diatom/phytoplankton food usually controls Schizothrix.
2.9 Inhibition of the growth of certain bacteria by exposing tanks of light at certain times of day
At times, bacterial growth at the bottom, characterized by a faint red or pink colour develops. This is especially true in shaded tanks. Presumably, this is due to unconsumed diatoms/phytoplankton or micro-encapsulated feed.
This is easily controlled by exposing the tank to full sunlight in the morning. It is, therefore, a good plan to have the roof detachable or movable by sliding.
2.10 Transfer of larvae from one tank to another without lifting them out of the water (Figure 4)
Transfer of animals from one tank to another especially in the earlier larval stages are invariably stressful. The stress may, however, be reduced or minimized if the larvae are transferred without lifting them out of the water. This means that transfers will be effected purely on the basis of water seeking its own level.
The “ladder” system of setting up tanks offers a means of transferring larvae from one tank to another earlier stage of culture are elevated with respect to a succeeding stage culture tank.
2.11 Percolation pond
It is common knowledge that when one hatchery is struck by a disease, it is purely a matter of time before nearby hatcheries are stricken by the same disease. The obvious explanation is that these neighbouring hatcheries obtain their water supply from the same source. Moreover, they usually discharge their waste waters directly into the same body of water that serves as their source of water. The chances of cross-contamination are naturally high. And then, as experience shows, there is nothing to prevent hatcheries from clustering along and around an area that has been tested to be suitable for hatchery purposes.
Hence, certain measures to prevent or minimize cross-contamination are in order. One inexpensive means is the use of a percolation pond. Its primary purpose is to “treat” waste water, mainly through the unit process of filtration, before this is disposed back to nature. The waste water, in effect undergoes some degree of cleansing before it finds its way back to the sea. Disease-causing or carrying agents originating from one hatchery are, therefore, prevented from spreading to neighbouring hatcheries.
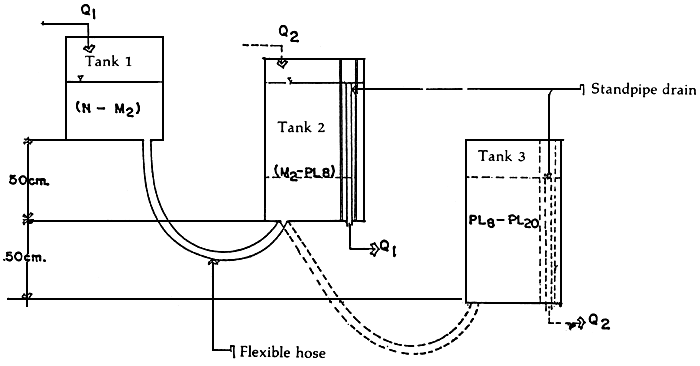
Figure 4. “Ladder” system of setting up tanks
The percolation pond or seepage pit is appropriate where the underlying earth, is absorbent and the water table is low. Where these conditions are not satisfied, a tile drain field may be substituted.
2.12 Intake systems with prefiltration
A large PVC pipe direct intake pipe may be perforated with 1/2” holes and covered with 2–3 layers of a fibrous material like Ecofelt T-21 or T-22 or any similar material that serves to filter out some of the suspended solids in the water. This system will, however, only do in pacific waters.
Fine-textured granular materials such as those found in the seabed near most hatcheries, are effective in filtering out pollutants. Water that passes through these materials, while not bacteriologically pure, is at least relatively free of suspended sediments. Hatchery operators would, therefore, be well-advised to make good use of such natural cleansing ability. Two types of intake systems which avail of the filtering ability of the seabed are the vertical well and the buried perforated pipes. Both systems are good in the sense that the seawater they provide has already been filtered.
At present, the more common water intake is the vertical well which consists of a covered, circular concrete ring or similar structure to which a pipe is connected and through which water is pumped by suction to a filter box, and later to a reservoir. Seawater is allowed to seep only through the well bottom.
It appears though, that between the two, the perforated pipe system would make the better alternative, for two reasons. Firstly, it is less susceptible to damage caused by high and strong waves because it has no exposed superstructure. Secondly, it can be relied upon to deliver more water. With a given cross-sectional area, the amount of water that a seawell can supply is limited by the difference between the levels of water inside and outside the well. On the other hand, a pump that is directly connected to the perforated pipes can create a negative pressure inside the pipes which effectively multiplies many times over the actual physical difference between the water level inside the pipe and that of the sea. This enables the perforated pipe type of intake to supply much more water than the well.
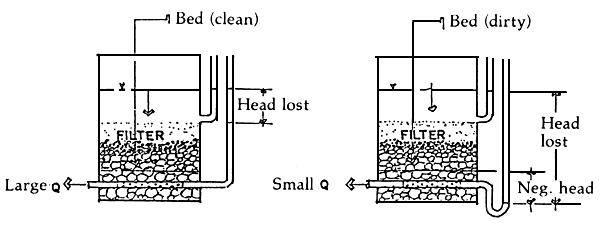
2.13 Filtration and backwashing
Filtration is the removal of suspended solids contained in the water as it passes through the filter bed by a complex process involving one or more removal mechanisms such as straining, sedimentation, interception, adhesion and flocculation. Back-washing is the removal of the suspended solids that have accumulated within the filter bed by washing these away with sufficient water flow usually in reverse direction to that of filtration. Invariably, hatcheries filter their water supply. It is, however, doubtful whether the filters are being backwashed as often as necessary.
Filters in prawn hatcheries must be back-washed when either of two conditions is reached, i.e.,
When the suspended solids in the effluent begin to exceed the level that can be tolerated by the larvae.
When the head loss across the filter bed exceeds the static head, that is the underdrains are under negative head, at which point the filter output capacity drops drastically.
In properly designed filters, both conditions should occur simultaneously (Figure 5).
3. PONDS
3.1 Daily water change of at least 10 percent to replace anoxic/toxic waters with fresh seawater to reduce pathogen load
For human kind, water therapy, or the consumption of extra volumes of drinking water in excess of the usual daily requirement all in a very short duration often leads to well-being at least, or to the extent of curing some diseases and disorders. There is a biological basis in fact to this practice and which is not ordinarily, adequately and precisely explained in scientific and medical circles.
In shrimp culture, a parallel technique, water change, is now emerging as a very powerful tool for disease control. Not only are potentially pathogenic microorganisms washed away from the soil and water when pond water is changed, but toxins and other hazardous metabolites and substances are diluted and are, therefore, made ineffective against the animals.
For a theoretical example: let us assume that a particular toxin (toxin A) is lethal to shrimp when a concentration of 0.1 ppm is built up in the environment. Let us assume further that on a particular day (say, Day 4) toxin A has already reached a concentration of 0.09 ppm and that the 0.1 ppm level is to be attained on the next day (Day 5) if no water change is instituted. If 10 percent of the water is replaced on Day 4, however, the upward trend (to reach 0.1 ppm) is temporarily stopped and the concentration of toxin A is reduced to 0.081 ppm (instead of reaching 0.1 ppm). The level of toxin A falls short of the lethal concentration, and diseases and, therefore, mortalities due to the toxin are averted.
| 1. Start of filtration | 2. Time to backwash |
| A. Backwashing as governed by head loss across the filter bed. | |
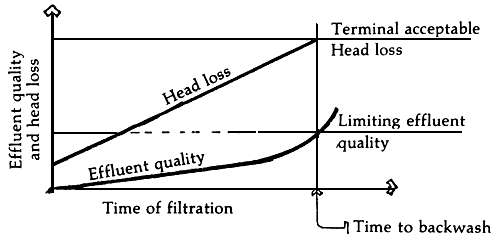
B. Conditions prevailing in properly designed sand filters.
Figure 5. Indicators of when to backwash sand filters
In pond construction, therefore, before even a shovel of soil is taken out, an ample source of CLEAN water, free of pathogens and contaminations should be assured. In the planning process, the installations and other support system should be to supply the minimum daily 10 percent water volume necessary to make this important replacement in pond water.
3.2 Water changes (20–33 percent or more) to induce molt periodically should be resorted to
From the foregoing discussion, water changes do not only lead to reduction of toxins and neutralization of unfavorable factors but may also be employed to induce molting and, therefore, physical growth.
In shrimp, molting is induced when the water change far exceeds the 10 percent level. It is believed that 20–33 percent of water change can induce molt. Each molt does not necessarily reflect growth; it actually leads to the loss of 6 to 10 percent of the body weight, and apparently of lots of energy. The weight and energy lost are, however, more than made up for by nutrition and other consequent activities provided sufficient time is given for the animals to replenish and recover.
The interval for induced molting in shrimp is ideally every 6 days or so for shrimps less than 20 g in average weight; every seven days for 21 g animals, and so on until 15 days for shrimps of average body weight of 33–35 g.
In the planning of a pond system, the design should not only include provisions for 10 percent water replacement per pond daily but also for induced or forced molting at 20–33 percent at specified days. Therefore, the pumps to be specified should be checked and double-checked to avoid under-utilization as well as overutilization. If there are reservoirs, treatment or impoundment ponds, the volumes necessary for the water changes outlined above should be fulfilled.
3.3 Provision for an increased surface area for shrimps by constructing “hotels” in the pond
When stocking density adopted was 150 000 animals per hectare, hardly were there reports on mass mortalities directly attributable to pathogens or unfavorable environment. When higher densities were attempted, many reports of mass mortalities and diseases surfaced.
In the stocking density of 150 000 animals per hectare, 15 individuals are confined within one square meter area. When 30 to 60 are placed, instead of 15, the area intended for an individual is drastically reduced. In other words, there is now over-crowding.
It is well to realize that the square meter area can take only so many shrimps and higher stocking densities unduly stress the animals. The resources have to be stretched a little bit more and the animals become less healthy. There is a maximum density of animals which does not bring about stress. This is the carrying capacity. What is the carrying capacity then, is it 15/m2, or is it 60/m2? We have to research some more to fix this figure.
In shrimp ponds, what is important is the capacity of the bottom and not the volume of the column or depth of water above this area. The shrimps rest on the bottom and do not swim. Therefore, the depth is apparently immaterial in this regard.
The carrying capacity of ponds may be stretched if a series of refuges is added. The best way to do this is to provide multistoreyed “hotels” for the shrimp. In a 1.5 m deep pond, the additional tiers or storeys may be placed at 0.5 m and 1.0 m depths to complement the bottom. The shrimps can then dissipate in space and the density is decreased when they move out to the extra tiers or false bottoms (Figure 6).
3.4 Surface waters versus underground waters as factors for disease
For a quite a time, seawater tapped from the sea surface was the usual source of water. When pumped in at high tide, the water is clean and even needs no mechanical filtration. The seawater can thus, be obtained in its unadulterated raw form. When pumped in during low tide, however, the water is turbid and definitely needs filtration.
The latter situation must have convinced growers to tap other sources. This was filled in by waters from underground and collected in wells by percolation. Since this water passes through sand and other subterranean materials, by filtration, the water is made cleaner.
It did not take long, however, to prove otherwise. Based on the Taiwanese experience in 1987 when many crops failed due to diseases, ponds which received waters pumped up from the underground wells had the more devastating diseases. Is it due to the fact that waters supplied thus contained lower levels of dissolved oxygen? Or was it due presumably to higher levels of chemical compounds (such as ferrour sulfide) obtained from down the depths? The evidences are there.
With these, it is a nice idea to institute both systems in a farm the owner can shift to either set up handily depending on the prevailing conditions.
3.5 Selective “shutting off” and “switching on” of paddlewheel aerators as determined by wind directions, etc.
It is observed that paddlewheel aerators are switched on and used without regard to such factors as wind direction, temperature, and dissolved oxygen. Worse, these are switched on while it is raining, or immediately thereafter or at a time when the wind is blowing hard.
The paddlewheel, aside from increasing the dissolved oxygen is used to disrupt stratification, if there is any, of temperature, dissolved oxygen and other factors. It should be used intelligently and under unfavorable environment.
When the wind is blowing hard, it is best to switch on the aerators whose ripples and currents are directed against the direction of the wind. Aerators which run with or parallel to the direction of the wind only cloud the water.
It is also good to stop the aerators during and after a strong rain until after the water clarifies a little. The operation of the paddlewheel at this time will suspend silt, making the water turbid, especially if the water is shallow.
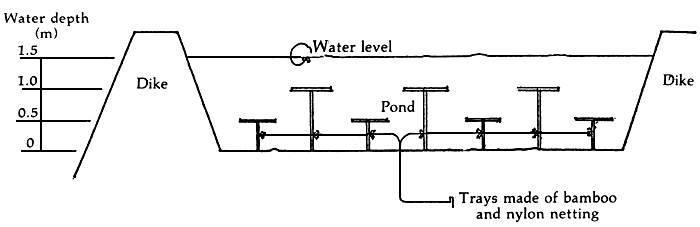
Figure 6. Multi-level refuges for shrimps in ponds
3.6 Central drains to remove anoxic/toxic waters
The old system of pond construction is one characterized by an “out-flow from the top” system. When water is replaced and the pond gates are open, the water from the surface flows out first. What should be the object of a water-replacement scheme are the toxic/anoxic waters at the soil-water interface. This water is laden with ammonia and hydrogen sulfide, the results of feed decomposition.
Knowledge of these phenomena led to the adoption of a bottom draining system that avails of the existing designs of sluice gates. A more recent introduction is what is now known as the “central drain”. In this system, the bottom is sloped toward the center. This center acts as a catchment area for unconsumed feeds. It is connected to the outside of the pond by a canal or per-forated PVC pipes. One or more stand pipes regulate the water level and also determine the rate of water discharge out of the pond.
When the standpipe is put down or opened, anoxic/toxic waters are sucked down to the pipe and discharged out, and if the perforations allow, even bring the unconsumed bits and pieces of food with the outrushing water. Of course, something must cover the perforations (a nylon net or polyfelt 220 or any other suitable material) so as to deter escape of the animals.
The set up may be modified further by laying down a series of pipes radiating out from the central drain, with each pipe complete with perforations and covering net just like the above.
Lately, we have seen the introduction of a “vacuum cleaner” as the Negros fish-farmers call it. It is nothing but a submersible pump fitted with a long plastic tube through which anoxic/toxic mud/substratum empties when the unit is switched on. So that the submersible pump will not sink and get stuck in mud, the unit may be fitted on a floating structure and submerged just enough to bring the toxic/anoxic waters and mud out.
3.7 “Kitchen ponds” and “kitchen tanks” on pond dikes to preferentially culture nutritious phytoplankton to be added to the pond after a day of culture
It is considered among the Taiwanese that brown water is best for rearing shrimps. The brown color is due to the high density and predominance of diatoms. The disadvantage lies on the fact that the growth of diatoms cannot be maintained for long. The bloom “collapses”, the cells senesce fast, sink to the bottom and die. Green water, characterized by pre-dominance and bloom of green phytoplankton is less desirable, but persists for a long time.
In a pond, it is very unlikely to have a brown bloom for a long time. However, it is possible to assure large volumes of actively-growing mono-specific cultures of nutritious diatoms by: (a) culturing the diatom in fiberglass or plastic-lined wooden tanks right on the pond dikes for a day, and pump it into the pond when the culture blooms or reaches its assymptote; and (b) by culturing a mixed population of -diatoms in a “kitchen pond” or “reservoir” and subsequently pumping it into a pond when a suitable population density is reached.
These alternatives may be planned and incorporated into the overall pond designs.
3.8 Sedimentation tanks
Water involved in pond operations is far more voluminous than that in hatcheries. The use of filtration to clean the water for ponds is, thus, impractical. Fortunately, the water quality requirements in ponds are less severe considering the sturdier nature of juveniles and adults compared to larvae and postlarvae. Nonetheless, the fact remains that excessive suspended solids in pond waters can cause gill irritation. In pond where the presence of toxic substances cannot be avoided, the abrasive action of suspended solids is enhanced. Aside from its harmful effects to the animals, too much solids in the incoming water can cause siltation in the supply canals and in the ponds. This leads to reduced capacity of the canal and less effective volume of water in the pond.
To mitigate these undesirable conditions, a sedimentation chamber can be constructed at the initial point of the supply canal. In this chamber, the suspended particles that are heavier than water are separated from the water by gravitational settling. Extracting the sediments from a confined chamber is obviously an option preferable to scraping silt all over the canals and ponds.
| Diet | Postlarvae | Juveniles | |||
| Ingredient (%) | 1 | 2 | 3 | 4 | |
| Giant tiger shrimp (P. monodon) - dry diet, pellet | |||||
| Fish meal | 30 | 30 | 27.5 | 29.3 | |
| Shrimp meal | - | - | 27.5 | 17.4 | |
| Shrimp meal | 15 | 15 | - | - | |
| Soybean meal | 15 | - | - | - | |
| Copra meal | - | - | - | 10 | |
| Ipil-ipil leaf meal (dried | |||||
| soaked leaves) | - | 20 | - | - | |
| Wheat/bread flour | 10 | 10 | 15 | 15 | |
| Sago palm starch/corn starch | - | - | 5 | 5 | |
| Rice hulls (filler) | - | - | - | 5.9 | |
| Rice bran | 14.8 | 9.8 | 20 | 10 | |
| Potato starch | 5 | 5 | - | - | |
| Cod liver oil | 9 | 9 | - | - | |
| Corn oil | - | - | 4 | 2.6 | |
| Vitamin/mineral premix V-22 1 | 0.95 | 0.95 | 0.95 | - | |
| Vitamin premix2 | - | - | - | 1 | |
| Mineral premix3 | - | - | - | 1 | |
| Dicalcium phosphate | - | - | - | 2.8 | |
| Vitamin C | 0.05 | 0.05 | 0.05 | - | |
| Antioxidant (BHT) | 0.2 | 0.2 | - | - | |
| Nutrient content, % dry matter | |||||
| Crude protein | 41.9 | 40.7 | 35.7 | NA | |
| Lipid | 14.1 | 15.9 | 7.4 | NA | |
| Crude fiber | 3.4 | 4.9 | 8.0 | NA | |
| Ash | 10.6 | 10.6 | 16.9 | NA | |
Source: Vogt, Quinitio and Pascual (1986) Diet 1 and 2; Pascual (1983) - Diet 3; Lim and Destajo (1979) - Diet 4.
| Diet | Postlarvae | Juveniles | Production | ||
| Ingredient (%) | 1 | 2 | 3 | 4 | |
| Giant tiger shrimp (P. monodon) - dry diet, pellets | |||||
| Meat meal | - | - | - | 21.5 | |
| Fish meal | 7 | 10 | 27 | - | |
| Soluble fish protein concentrate | 5 | 5 | - | 6 | |
| Shrimp meal | 12 | 15 | - | 8 | |
| Meat and bone meal | 7 | 7 | 10 | - | |
| Soybean meal | - | - | 15 | - | |
| Soybean cake | 24 | 20 | - | - | |
| Sesame cake meal (expeller) | - | - | 5 | - | |
| Groundnut meal (expeller) | - | - | 5 | 17 | |
| Copra cake | 5 | - | 10 | - | |
| Leaf meal | - | - | 5 | - | |
| Rice bran (solvent extracted) | - | - | 10 | - | |
| Maize | - | - | 4 | - | |
| Rice | - | - | - | 6 | |
| Wheat gluten | 7 | 7 | - | 10 | |
| Tapioca | - | - | |||
| Blood meal | 3 | 2 | - | 11 | |
| Alkane yeast | 10 | - | - | - | |
| Brewers yeast | - | 10 | - | - | |
| Cod liver oil | 6 | - | - | 4 | |
| Fish oil | - | 6 | - | - | |
| Cereals (wheat, corn, rice) | - | 10 | - | - | |
| Spirulina | 2 | - | - | - | |
| Peptonal | 5 | - | - | - | |
| Snail meal (Trocus or Achatina) | 2 | 2 | - | - | |
| Vitamins and salt1 | 5 | 6 | - | 8 | |
| Vitamin and mineral premix2 | - | - | 1 | - | |
| Antioxidant (BHT) | - | - | 0.02 | - | |
| Antioxidant (ethoxyquin) | - | - | 0.015 | - | |
| Methionine | - | - | - | 0.5 | |
| Nutrient content, % | |||||
| Crude protein | 52.2 | 49 | 37.1 | 40 | |
| Lipid | 9.5 | 10 | 7.8 | NA | |
| Crude fiber | NA | NA | 7.0 | NA | |
| Ash | NA | NA | 12.9 | NA | |
3Author cites that wheat gluten would be a better binding agent; Kanazawa (1984).
Source: AQUACOP (1983) - Diet 1 and 2; Kanazawa (1984) - Diet 3 (also fed as a complete diet for P. merguiensis, AQUACOP (1977).
PURIFIED COMPLETE EXPERIMENTAL TEST DIETS - FISH AND SHRIMP
| Ingredients (%) | H-4401 | C1022 | NRC (1983) | |
| Fish standard reference diets | ||||
| Casein, vitamin free | 38 | 40-(45) | 32 | |
| Gelatin | 12 | 4 | 8 | |
| Starch | - | 11-(16) | - | |
| Dextrin, white | 28 | 9 | 30 | |
| D-glucose (cerelose) | - | 5 | - | |
| Cellulose flour | - | 3 | 19 | |
| Soybean oil | - | - | 3 | |
| Corn oil | 6 | - | - | |
| Cod liver oil | 3 | - | - | |
| Fish oil | - | 15-(10)4 | 3 | |
| Amino acid supplement4 | - | 2 | - | |
| Vitamin premix H-4405 | 9 | - | - | |
| Vitamin premix C1026 | - | 3 | - | |
| Vitamin premix NRC (1983)7 | - | - | 1 | |
| Mineral premix H-4408 | 4 | - . | - | |
| Mineral premix C1029 | - | 8 | - | |
| Mineral premix NRC (1983)10 | - | - | 4 | |
3Marine oil with 0.05 percent antioxidant (or other oils as required).
4Supplement includes 0.5 percent methionine, 1 percent arginine and 0.5 percent starch.
Source: Castell and Tiews (1980) - H-440 Standard reference diet which has proven satisfactory for use with salmonids, char, catfish, carp, sea bream, seabass, perch, red fish, pampano, red snapper, black cod and black bass. Cho, Cowey and Watanabe (1985) - C102 Test Diet. NRC (1983) - 36 percent crude protein diet containing 2.9 kcal digestible energy/g; semi-purified test diet for warm water finfish.
| Ingredients (%) Diet: | Kanazawa 1 | Crab protein2 | Bodega Bay 81S3 | |
| Shrimp/crustacean standard reference diets | ||||
| Casein, vitamin free | 50 | - | 31 | |
| Egg white, spray dried | - | 4 | ||
| Crab protein | - | 40 | - | |
| Wheat gluten | - | 5 | 5 | |
| Corn starch | 4 | 15 | 24 | |
| Glucose | 5.5 | - | - | |
| Sucrose | 10 | - | - | |
| Glucosamine HC1 | 0.8 | - | - | |
| Dextrin | - | 5 | - | |
| Alpha cellulose | 9.3 | 17.8 | 12.1 | |
| Residual fish oil (vitamin A free) | 8 | - | - | |
| Cholesterol | 0.5 | 1 | 0.5 | |
| Refined soy lecithin | - | - | 10 | |
| Cod liver oil | 6 | 4 | ||
| Corn oil | - | 3 | 2 | |
| Sodium citrate | 0.3 | - | - | |
| Sodium succinate | 0.3 | - | - | |
| Vitamin premix - Kanazawa4 | 2.7 | - | - | |
| Vitamin premix - crab protein5 | - | 2 | - | |
| Vitamin premix - Bodega Bay6 | - | - | 4 | |
| Mineral premix - Kanazawa7 | 8.6 | - | - | |
| Mineral premix8 | - | 4 | 3 | |
| Choline chloride | - | 1 | - | |
| Dl-alpha-tocopherol | - | 0.2 | 0.2 | |
| Vitamin A (50 000 IU/g) | - | - | 0.1 | |
| Vitamin D3 (400 000 IU/g) | - | - | 0.1 | |
1 Prepared as a moist diet by adding 3 g agar and 130 ml water/100 g dry diet.
8 Modified Bernhart-Tomarelli salt mixture.
Source: Kanazawa, Teshima and Tokiwa (1977) - semi-purified test diet for penaeid shrimp; Castell (1986) - crab protein and Bodega Bay reference diets for crustaceans.
From: Tacon, Albert G.J. The nutrition and feeding of farmed fish and shrimp - A training manual 3. Feeding methods. GCP/RLA/075/ITA Field Document No. 7, FAO, Rome, 1988.
Formulated feeds must contain nutrients or substances which are needed to promote growth, maintain life and provide resistance to diseases. These are composed of proteins, fats, carbohydrates, vitamins and minerals. Protein is the principal nutrient which provides growth of the animal; if fats and carbohydrates are not enough in the diet, the protein component is used for heat and energy rather than, for growth. Fats and carbohydrates are sometimes sparers of protein. Vitamins and minerals regulate bodily processes: Vitamin Bs are necessary for proper utilization of proteins, fats and carbohydrates while Vitamin A and C help build up body resistance to diseases and infection; Vitamin D and mineral such as calcium and phosphorus are necessary for shell formation. These nutrients have interrelated functions in the growth and well-being of the cultured animal. Their presence in the diet in the right proportions is, therefore, important.
1. SOURCES OF FEED INGREDIENTS
Protein - fish meal; shrimp meal; shrimp head meal; soybean meal; meat and bone meal.
A combination of animal protein or plant protein is possible; substitution of animal protein with plant protein such as soybean is also possible.
Fat - fish oil; corn oil; coconut oil and beef tallow.
Carbohydrates - bread flour; fine rice bran; cassava flour; sorghum; fine ground corn; corn starch and sago palm starch.
Calcium and phosphorus - available in fish meal; shrimp head meal; meat and bone meal.
Cholesterol - shrimp head meal.
2. EQUIPMENT NEEDED
Sieve
Mixer (5 or 10 kg capacity)
Meat grinder
Coffee grinder
Steamer or a big cauldron and bamboo basket for steaming
Saucepan for gelatinizing corn starch
Drier
Wooden ladle
Covered containers for pellets
3. PROCEDURE FOR FEED PRERATION
Grind dry ingredients finely, separately. Sieve through a No. 40 nylon mesh of 420 microns/sq. cm.
Weigh or measure ingredients.
Mix all dry ingredients thoroughly,
Add the oil and mix for another 5 minutes.
Gelatinize corn starch, bread flour or sago palm starch (the same way corn starch is prepared for starching clothes). One part starch in four parts water, or 50 gms in 200 cc of water for 1 kg of feed. Suspend corn starch in tap water in half the amount for the whole mixture before gelatinizing.
Add gelatinized starch to the dry ingredients mixture and mix well to make a stiff dough.
Pass this dough to a meat grinder with a 1, 2 or 3 mm die depending on the size of the shrimp to be fed. For juveniles of about 0.35 gm body weight, use 1 mm die, 2 gm shrimp - 2 mm die and 10 gm or more - 2.5 to 3 mm die.
Cut the extrusion into 1/2 cm lengths and steam for 5 minutes. This stabilizes the pellets. Unsteamed pellets within 30 minutes but the steamed feed could keep more than 12 hours.
Dry the steamed pellets in an oven overnight at 60°C. In the absence of an oven, an improvised drier might be used.
Store the pellets in plastic bags or buckets after drying and cooling. Keep pellets in dry place to avoid spoilage. If a freezer is available this would keep the feed longer in storage.
4. AMOUNT OF FEEDS DAILY
Eight to 10 percent of total biomass of postlarvae.
Three to 5 percent of total biomass of juveniles.
Suggested feed formulation
| Alternatives | |||
| Ingredients | 1 | 2 | 3 |
| Fish meal | 300 | 175 | 275 |
| Shrimp meal | 150 | 225 | 275 |
| Soybean meal (defatted) 1 | 150 | 200 | - |
| Ipil-ipil leaf meal | - | 100 | - |
| Rice bran | 150 | 80 | 200 |
| Bread flour | 150 | 100 | 150 |
| Sago palm starch or corn starch | 50 | 50 | 50 |
Oil (preferably fish liver oil; soybean oil, 1.1 ratio of cod liver oil: soybean oil) | 40 | 60 | 40 |
| Vitamin-mineral mix1 (V-22) | 9.5 | 9.5 | 9.5 |
| Vitamin C | 0.5 | 0.5 | 0.5 |
| Water | 200 | 200 | 200 |
| Total (with water) | 1 200 | 1 200 | 1 200 |
1If full fat whole soybean is used, roast soybeans at 170°C for 10 minutes.
Source: Pascual, Felicitas, P., 1983.
PUBLICATIONS AND DOCUMENTS OF THE
ASEAN/UNDP/FAO REGIONAL SMALL-SCALE COASTAL FISHERIES DEVELOPMENT
PROJECT
(RAS/84/016)
Working Papers
ASEAN/SF/86/WP/1 Rabanal, H. R. Seafarming as alternative to small-scale fishing in ASEAN region. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 55p.
ASEAN/SF/86/WP/2 Soeyanto, T. The status of Bali Strait fisheries with special reference to Muncar, Kedonganan and Jimbaran coastal villages. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 36p.
ASEAN/SF/86/WP/3 Boongerd, S. and S. Chitrapong. Small-scale fishing for squids and related species in Thailand. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 44p.
ASEAN/SF/89/WP/4 Guerrero, C.V. An evaluation of the socio-economic viability of the introduction of set net on small-scale fishermen in Botolan, Zambales. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1987. 50p.
ASEAN/SF/89/WP/5 Guerrero, C.V. An evaluation of the socio-economic viability of “payaw” on small-scale fishermen using hook and line in Masinloc, Zambales. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 42p.
ASEAN/SF/89/WP/6 Guerrero, C.V. An evaluation of the socio-economic viability of set net operation on small-scale fishermen in Antique and Aklan: Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 47p.
ASEAN/SF/90/WP/7 Chitrapong, S. Demonstration of squid fishing in Indonesia. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. 20p.
ASEAN/SF/90/WP/8 Guerrero, C.V. Assessment of the socio-economic impact of artificial reefs on small-scale fishermen in the Philippines. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. (In preparation).
Workshop Reports/Other General Reports
ASEAN/SF/86/GEN/1 Report of national consultative meeting on aquaculture engineering held in Tigbauan Research Station, SEAFDEC Aquaculture Department, Iloilo City, Philippines, 2–5 October 1985. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 186p.
ASEAN/SF/86/GEN/2 Zabala, P. T. (Comp.) Preliminary annotated bibliography on small-scale fisheries in the ASEAN Region. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 41p.
ASEAN/SF/87/GEN/3 Report of the training course on shrimp culture held in Jepara, Indonesia, 2–19 December 1987. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1987. 63p.
ASEAN/SF/88/GEN/4 Report of the training course on small-scale fisheries extension held in Semarang, Indonesia, 26 January–14 February 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 130p.
ASEAN/SF/88/GEN/5 Report of the training course on fisheries extension methodology held in Penang, Malaysia, 13–26 March 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 266p.
ASEAN/SF/88/GEN/6 Report of the training course on seaweed farming held in Manila, Philippines, 2–21 May 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 169p.
ASEAN/SF/88/GEN/7 Report of the training/study tour on fishing with “payaw” held in Manila, Philippines, 16 May–4 June 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 87p.
ASEAN/SF/88/GEN/8 Report of the workshop on artificial reefs development and management held in Penang, Malaysia, 13–16 September 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 188p.
ASEAN/SF/89/GEN/9 Report of the training course on seabass breeding and culture, Satul, Thailand, 1–22 August 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. 85p.
ASEAN/SF/89/GEN/10 Report of the training course on marine finfish netcage culture, Singapore, 5–24 September 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. 193p.
ASEAN/SF/89/GEN/11 Report of the workshop on shrimp and finfish feed development, Johore Bahru, Malaysia, 25–29 October 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. 163p.
ASEAN/SF/89/GEN/12 Delmendo, M.N. and P.T. Zabala (Comp.). An annotated bibliography on shrimp feeds and nutrition. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. 34p.
ASEAN/SF/89/GEN/13 Delmendo, M.N. and P.T. Zabala (Comp.). An annotated bibliography on finfish feeds and nutrition. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. (In preparation).
ASEAN/SF/90/GEN/14 Zabala, P.T. (Comp.). Annotated bibliography on women in fisheries in the ASEAN region. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. 24p.
ASEAN/SF/90/GEN/15 A report of the training/study tour on squid fishing, Rayong and Chonburi, Thailand, 17–24 April 1990. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. 43p.
ASEAN/SF/90/GEN/16 Report of the workshop on fishery cooperatives management, Semarang, Central Java, Indonesia, 20–15 August 1990. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. (In preparation).
ASEAN/SF/90/GEN/17 Report of the workshop on assessment of the contribution of women in post harvest processing and marketing of fish and fishery products, Philippines, 15–19 October 1990. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. (In preparation).
ASEAN/SF/90/WP/18 Report of the training course on home-made feeds preparation for small-scale aquaculture use, Philippines, 19–25 November 1990. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. (In preparation).
ASEAN/SF/90/GEN/19 Report of the training course on set net fishing, Philippines, 3–19 December 1990. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. 36p.
ASEAN Fisheries Manuals
ASEAN/SF/86/Manual No. 1 Suprayitno, H. Manual of running water fish culture. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 34p.
ASEAN/SF/88/Manual No. 2 Juanich, G.L. Manual on seaweed farming: 1. Eucheuma spp. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 25p.
ASEAN/SF/88/Manual No. 3 Trono, G.C., Jr. Manual on seaweed culture: 2. Pond culture of Caulerpa. 3. Pond culture of Gracilaria. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 20p.
ASEAN/SF/88/Manual No. 4 Aguilar, E.R. A manual on set net fishing based on Philippine conditions. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 29p.
ASEAN/SF/90/Manual No. 5 Santos, G.A. A manual for the processing of agar from Gracilaria. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. 34p.
ASEAN/SF/90/Manual No. 6 Legaspi, A.M. Fish processing: Tuna ham making and boneless milkfish. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1990. (In preparation).
ASEAN/SF/91/Manual No. 7 Pascual, F.P. A practical guide to the preparation of home-made feeds for aquaculture. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1991. 28p.
Periodic Progress Reports
ASEAN/SF/86/PR-1 Soesanto, V. Project progress report of the ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 5 October 1985–5 April 1986. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 9p.
ASEAN/SF/86/PR-2 Soesanto, V. Project progress report of the ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 6 April–6 October 1986. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 11p.
ASEAN/SF/88/PPER-3 Delmendo, M.N. Project performance evaluation report of the ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 31 July 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 23p.
Technical Reports Contributed to Symposia/Meetings, etc.
ASEAN/SF/85/Tech. 1 Rabanal, H.R. and V. Soesanto. The world fishery and culture of Macrobrahium and related prawn species. Contributed to the National Conference on Prawn Technology, sponsored by the Philippine Fishfarmers Technical Assistance Foundation, Inc., Manila, Philippines, 27–28 November 1985. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1985. 16p.
ASEAN/SF/86/Tech.2 Rabanal, H.R. and V. Soesanto. Commercial species of shrimps and prawns, their sources and export markets. Contributed to the Seminar on Quality Control in the Production, Processing and Marketing of Frozen Shrimps for Export, sponsored by Food Research Department, Food Terminal Incorporated, Taguig, Metro Manila, Philippines, 29–31 July 1986. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 64p.
ASEAN/SF/86/Tech. 3 Rabanal, H.R. Status and prospects of shrimp farming in the Philippines. Contributed to the Monthly Seminar Series on Timely and Related Fisheries Issues, sponsored by the Philippine Council for Agriculture and Resources Research and Development, (PCARRD), Los Baños, .Laguna, Philippines, 5 November 1986. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1986. 24p.
ASEAN/SF/87/Tech. 4 Delmendo, M.N. Fishery administration and policy in the Philippines: Past and present. Contributed to the National Conference on Fisheries Policy and Planning, Baguio City, Philippines, 16–20 March 1987. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1987. 35p.
ASEAN/SF/87/Tech. 5 Delmendo, M.N. Milkfish culture in pens: An assessment of its contribution to overall fishery production of Laguna de Bay. Paper read in the Seminar on the occasion of the Fish Conservation Week, BFAR, October 1987 and lecture material used in the NACA Senior Aquaculture Training Course, SEAFDEC, Tigbauan, Iloilo. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1987. 17p.
ASEAN/SF/87/Tech. 6 Delmendo, M.N. and B.H. Delmendo. Small-scale aquaculture operations in the ASEAN countries. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1987. 49p.
ASEAN/SF/88/Tech. 7 Rabanal, H.R. History of aquaculture. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 13p.
ASEAN/SF/88/Tech. 8 Rabanal, H.R. and M.N. Delmendo. Organization of the aquaculture industry. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 10p.
ASEAN/SF/88/Tech. 9 Rabanal, H.R. Report on the World Aquaculture Society, 19th Annual Conference and Exposition, Honolulu, Hawaii, U.S.A., 4–10 January 1988. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1988. 99p.
ASEAN/SF/89/Tech. 10 Delmendo, M.N. Some advances attained in shrimp farming research and management practices: Insights to future prospects for expansion of production. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. 60p.
ASEAN/SF/89/Tech. 11 Delmendo, M.N. Bivalve farming: An alternative economic activity for small-scale coastal fishermen. Manila, ASEAN/UNDP/FAO Regional Small-Scale Coastal Fisheries Development Project, 1989. 45p.