M. Murray, J.D. Barry, W.I. Morrison, R.O. Williams, H. Hirumi and L. Rovis
This review of the prospects for developing an immunization procedure against trypanosomiasis explores a number of promising avenues of research. Part I covers variable antigen types (VATs); metacyclic antigens; In vivo and in vitro attenuation; and molecular and genetic engineering.
Part II, to be included in the next issue of this journal, will cover immunogenicity of subcellular fractions; immunological intervention against the tsetse fly; induction of increased resistance by immunostimulants; trypanotolerance; and infection and treatment. This will be followed by a brief statement of the conclusions reached.
The present methods available for the control of African trypanosomiasis, namely, systematic case detection and treatment, and tsetse control, do no more than limit the disease although both these approaches have been shown to be effective where they have been vigorously applied. The disadvantages attending the use of trypanocidal drugs include lack of availability of effective drugs, drug resistance and, in heavy tsetse fly challenge areas, the frequency with which treatment has to be applied, often to economically unacceptable levels. In the same way, while tsetse flies may be completely eradicated in certain areas by insecticide control, few regions of tsetse infestation have circumscribed boundaries and, unless cleared areas are defended (a costly exercise), reinvasion by the tsetse fly inevitably occurs. Thus there is little doubt that the introduction of an effective vaccine, if used strategically along with established control methods, would make an enormous contribution to the control of African trypanosomiasis, not only by increasing productivity in endemic trypanosome areas but also by opening up for exploitation the vast areas of the African continent largely devoid of livestock because of trypanosomiasis.
The major constraint to developing a trypanosome vaccine is the ability of the parasite to undergo antigenic variation. Murray and Urquhart (1977) reviewed the various attempts made to vaccinate both domestic livestock and laboratory animals and it was obvious from the reported studies that complete protection was readily achieved only if the same variable antigen type (vat) was used for immunization and challenge. When a distinct vat was used for challenge no protection occurred. Therefore, it would appear that an effective vaccine would have to contain all vats, possibly an insurmountable task as the number of vats, although as yet undetermined, is likely to be large. The result is that many workers in trypanosomiasis research consider the possibility of vaccination to be remote. It should be borne in mind, however, that many of these conclusions have been drawn from work on laboratory animals, which invariably succumb to massive parasitaemia. There is evidence to show that under certain circumstances cattle can control parasitaemia and then clinically recover. While this is particularly true for trypanotolerant breeds such as the N'Dama, it can also occur in the more susceptible zebu (Stewart, 1951; Chandler, 1958; Desowitz, 1959; Wilson, 1971; Wilson and Cunningham, 1971 and 1972; Murray et al, 1979). The greater capability of the bovine to control parasitaemia creates a new perspective on the question of vaccination. Furthermore, advances in scientific knowledge and technology have opened up several different avenues of research and the present article attempts to explore these.
Antigenic variation, the major obstacle to developing a trypanosome vaccine, is the process whereby trypanosomes sequentially express a series of surface antigens; it is these antigens that are capable of inducing protective immunity. The immune response against each variant, although rapid and highly effective in destroying any trypanosomes that possess that particular antigen, is invariably too late to affect that proportion of the population that has altered its antigenic identity. Thus, parasitaemia rises and falls in waves with each parasite population carrying different surface antigens (reviewed by Cross, 1978; Vickerman, 1978). This picture of successive waves of a specific antibody chasing variant trypanosomes has been likened by Goodwin (1970) to a "Tom and Jerry cartoon with a monstrously inept cat pulling the place down in its efforts to pulverize a diminutive and highly resourceful mouse".
What would appear to be required is as complete as possible an understanding of antigenic variation in order that, eventually, it might be possible to produce an effective vaccine by the strategic use of certain trypanosomes or their components. At the population level, the authors' knowledge has been increasing over the past few years, thanks mainly to the concept of multiple cloning in which bloodstream populations are divided into their component parts, namely single trypanosomes, each of which gives rise, in a fresh host, to a defined population that can be frozen as reference material. It is essential that as large a number of clones as possible be isolated, since only then will it be possible to detect some of the subtle immunological and biological differences within and between populations.
This approach has begun to reveal what occurs within a parasitaemic peak, to the level of the individual parasite. It appears that a peak is usually a mixture of vats (Van Meirvenne, Janssens and Magnus, 1975a) with the switch to expression of another type, probably occurring before the appearance of antibody, which is thought to act merely as a selective agent (Van Meirvenne, Janssens and Magnus, 1975a; Le Ray et al., 1977). Examination of sequence of appearance of vats arising within cloned infections has confirmed and extended the observation of Gray, 1965) that there is a tendency for certain types to occur preferentially in the early parasitaemic peaks. Thus, it would appear that vats can be divided into these early "predominant" types and other groups of vats that occur later (Van Meirvenne, Janssens and Magnus, 1975a; Capbern et al. 1977).
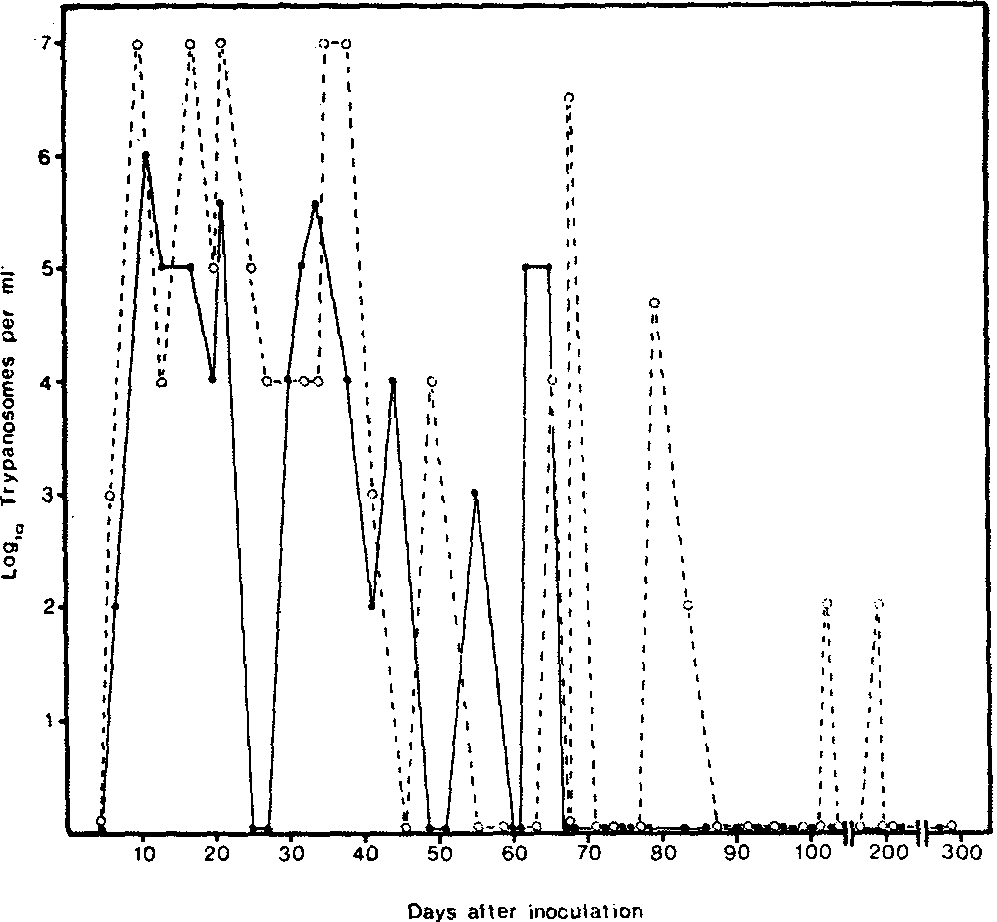
Figure 1 Parasitaemia profile in an individual four-year-old N'Dama (•) and a four-year-old zebu (o) inoculated with Trypanosoma congolense. Note that the level of parasitaemia is lower in the N'Dama as is the duration of parasitaemia. Both animals were negative for detectable parasites for several months prior to the termination of the experiment and both made a clinical recovery.
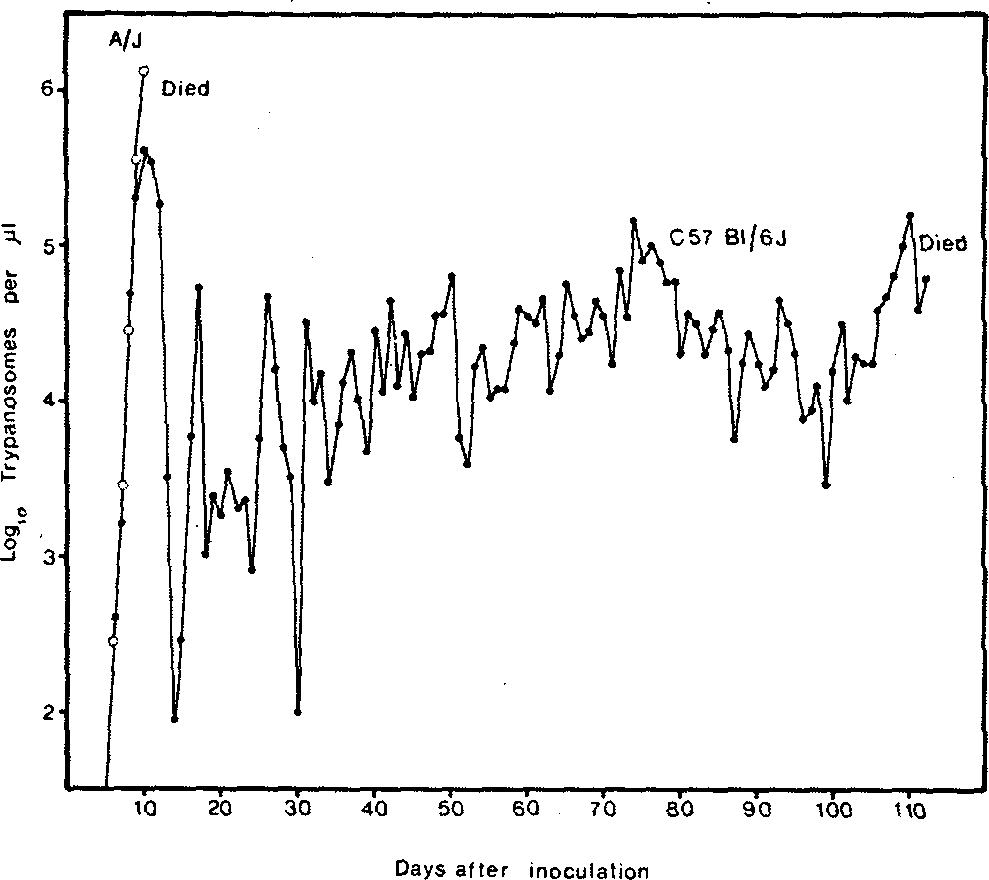
Figure 2 Parasitaemia profile in an individual C57BI/6J mouse (•) and A/J mouse (o) inoculated with Trypanosoma congolense. The CS7BI/6J was able to control and reduce parasitaemia levels to a significantly greater extent than the A/J and as a result was able to survive for over 100 days. Irrespective of breed or strain, cattle were able to control and reduce parasitaemia to a much greater extent than mice. Following infection in mice, death was inevitable, whereas in cattle recovery may occur, particularly in N'Dama animals.
The total number of vats that a trypanosome can express is known as its "vat repertoire," the full extent of which is as yet unknown although Capbern et al. (1977) have been able to isolate 101 vats from one clone of Trypanosoma equiperdum. Comparison of vat repertoires from different clones has been initiated (Van Meirvenne et al., 1975b; Van Meirvenne, Magnus and Vervoort, 1977) and has revealed a surprisingly high degree of similarity; in fact, some vats have been found in every repertoire examined. In addition, there is now indirect evidence from serological studies that during an infection certain vats may recur, in some cases within a few weeks of one another. This has been described in cattle infected with Trypanosoma congolense (Wilson and Cunningham, 1971) and with T. brucei (Nantulya, Musoke, Barbet and Roelants — unpublished results).
As regards vaccination, a rational approach may be successful. Immunization against individual vats is highly effective using such regimes as infection and treatment; irradiated organisms; killed organisms; crude emulsions containing released soluble antigens; formalized whole infected blood or plasma and purified variable antigen glycoprotein (reviewed by Murray and Urquhart, 1977). As little as 3 μg of variable antigen can give protection in mice (Baltz et al, 1977). A cocktail vaccine based on predominant vats is likely to be effective against with that repertoire. Investigation of the feasibility or such an approach requires complete analyses of the number of vats, both predominant and otherwise, within a repertoire, of the extent of crossreaction between repertoires and, eventually, of the number of vats that exist within and without given geographical areas.
A word of warning regarding studies on antigenic variation: it is necessary to define not only the parasite but also the host. The parasitaemic patterns produced by a trypanosome will vary with species of host, breed or strain, age, sex, etc. (Figures 1 and 2). In this regard, there is little doubt that exploitation of the in vitro culture system, which supports the growth of animalinfective forms of trypanosomes (Hirumi, Doyle and Hirumi, 1977) by eliminating the variable effects of the host, must yield new information on the basis and mechanisms of antigenic variation. Since much of the above work has been carried out with T. brucei the authors believe that it is essential that similar efforts be made with T. congolense and T. vivax, which are regarded as the major pathogens of bovine African trypanosomiasis.
Following ingestion by the tsetse fly, T. brucei loses its surface coat, which contains the variable antigen. It eventually regains the coat in the fly's salivary gland in becoming the mammalianinfective metacyclic stage (Vickerman, 1969). It has been suggested that all trypanosomes of a particular clone revert to a common "basic" antigen type in the salivary gland (Jenni, 1977, for T. brucei; Nantulya, Doyle and Jenni, 1979, for T. congolense) akin to the "basic" type arising in the bloodstream after cyclical transmission (Gray, 1965). Vaccination against such types would obviously be of importance. However, there is now evidence to suggest that this is not the case and that T. brucei metacyclics arising from the passage of a clone through the tsetse are antigenically heterogeneous (Figure. 3) (Le Ray, Barry and Vickerman, 1978; Barry and Hajduk, 1979; Barry et al, 1979b), although it is still the case that there may be only a limited number. A drawback to the potential use of metacyclic populations for vaccination is that they are antigenically unstable (Le Ray et al, 1977; Le Ray, Barry and Vickerman, 1978), preventing mass production of antigen and mRNA (see later, molecular and genetic engineering) for potential vaccine preparation. However, these dfficulties may be overcome by a recently devised protocol (Barry et al, 1979b) whereby antigenically more stable mammalian bloodstream forms with the same vat as metacyclics can be identified and cloned giving rise to populations suitable for bulk preparative procedures. This approach could be pursued to define the vat complement of metacyclic populations with a view to vaccination against trypanosomes of that vat repertoire. Furthermore, it is essential to determine the degree of crossreaction between metacyclics of different repertoires.
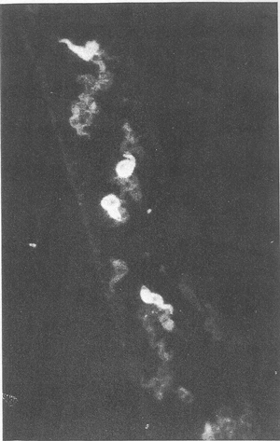
Figure 3 Antigenic heterogeneity among mammalianinfective metacyclic forms in the saliva of a tsetse fly. The fly was allowed to salivate onto a heated glass slide, to which immunofluorescence was applied using specific antiserum against a characterized bloodstream form trypanosome vat. Metacyclics with trypanosome vat fluoresce strongly, while those of other vat display the weak fluorescence of the counterstrain.
The in vitro culture system would also appear to have potential in this area. It has now been shown that "bloodstream forms" of T. brucei in culture ( Figure 4) can be induced to undergo morphological changes similar to those that occur in the fly, including the eventual production of metacyclic types, by appropriate manipulation of the culture conditions (Hirumi, Hirumi and Doyle, 1978a). As it has now become possible to clone parasites in culture (Hirumi, Hirumi and Doyle, 1978b) this approach might offer a source of metacyclic types of defined antigenic identity.
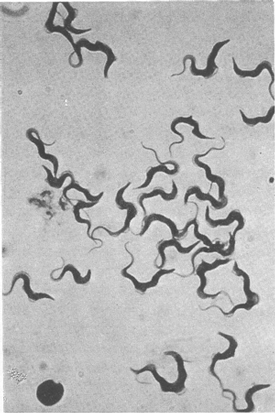
Figure 4 Bloodstream forms of Trypanosoma brucei (ILR-TbC-221) grown in vitro for over 31 months. Giemsa's stain.
In vivo and in vitro attenuation. Another facet of the problem is that, despite the authors' steadily increasing knowledge of antigenic variation, very little is known of how it is linked to the biology of the trypanosome and the hostparasite interaction, apart from the fact that it allows the trypanosome to evade the host's immune response and thus survive. For example, an association between vat and virulence has been proposed (McNeillage and Herbert, 1968; Van Meirvenne, Janssens and Magnus, 1975a) although it is essential that the precise circumstances of such a link are fully investigated (Barry, Le Ray and Herbert, 1979a). It is a common mistake to equate the vat of a clone with all the characteristics displayed by that clone; the vat is just one phenotypic marker. Confirmation of a link between vat and virulence, and the observation that trypanosomes of different vat may interfere with the expression of each other at the population level (Herbert, 1975) conceivably could be exploited to decrease the number of variable antigens required in a vaccine. At a later stage of infection, after expression of predominant vats, it appears that trypanosomes are in some way biologically altered as evidenced by their decreased infectivity and virulence in fresh hosts. The basis of this and whether it is linked to vat or some other characteristic of the parasite remains to be investigated.
Can these changes in behaviour be induced artificially and incorporated into a vaccination protocol ? The possibility now exists of attenuating trypanosomes by continuous passage in culture. In preliminary studies, it has been found that mice infected with parasites maintained in vitro by serial subcultivation over 12 months have shown alteration in pathogenicity when compared with noncultured organisms or organisms that have been maintained in vitro for less than three months (Hirumij unpublished data). The potential protective effect of attenuated protozoa has already been demonstrated in the control of babesiosis in cattle in Australia (Callow, 1977).
There is little doubt that the basis of understanding antigenic variation will come from investigations of the molecular biology of the trypanosome. In vitro cultivation techniques and recently developed tools in biochemistry and genetic engineering have opened up new horizons. Thus studies of the type carried out by Williams et ah (1978) on trypanosomal RNA will provide much essential information on trypanosome biology. Reannealing studies on the nucleic acid coding for the vat repertoire should give an insight into the size of the repertoire, the extent of similarity between different repertoires and the molecular nature of the genes involved. The genetic control of expression of antigenic variation should be studied; artificial restriction of a trypanosome population to expression of only a limited number of its vats might allow effective vaccination.
In any discussion of vaccination, consideration must be given not only to the obvious application of these newly developed techniques to antigen production but also to novel approaches to vaccination. It is possible that in the near future many protein vaccines will be produced from largescale bacterial cultures that contain the gene sequences coding for the appropriate proteins. Recombinant dna technology has already been applied to the largescale production of human somatomammotropin (growth hormone) (Shine et al., 1977), a precious substance that has traditionally been isolated from human placenta.
A further example of the application of recombinant dna technology to vaccine production is the development of a bacterial strain that is capable of producing the native chickalbumin protein at a level of 10 percent by weight of the bacterial cell (Mercereau-Puijalou et al, 1978). Thus bacterial strains can be developed to produce proteins for vaccines that normally would be either too expensive to isolate or impossible to purify because of limited amounts of starting material.
In addition to vaccine production in bacteria recent reports describe new techniques that possibly could find application in vaccination procedures. The transfer of specific genes from one genome to another has now been achieved. An example of such a transfer was reported by Wigler et al. (1978) where a specific viral gene coding for the enzyme thymidine kinase was purified by electrophoresis and introduced into a thymidine-kinasedeficient tissueculture cell line. Many of the tissueculture cells were able not only to incorporate the dna sequence into their genome but also were able to produce the enzyme at apparently normal levels. It may be possible, therefore, to modify certain tissues during a proliferative stage so as to yield a gene product to correct a genetic deficiency or possibly to produce a foreign protein for use in vaccination.
In other novel procedures recently reported by Dimitriadis (1978) and Ostro et al. (1978), differentiated tissue cultures were modified to produce a specific protein for a limited time. In each of these reports, a specific purified messenger RNA (rabbit globin) sequence was encapsulated in a lipid micelle called a liposome. The liposome was introduced to tissueculture cells with the membrane of which the liposome presumably fused. The purified messenger RNA was thereby introduced into the cytoplasm of the cells where it was translated into rabbit globin protein. The messenger RNAS used in such a procedure are degraded at a normal rate and can be modified to delay the cell's normal messenger RNA degradation processes. The normal cell's genome is not permanently modified and would produce the desired protein only for a limited time. In this manner, one could presumably use specific messenger RNAS and specific target tissues to produce the protein required for immunization. The inherent appeal of such a system would be that target tissues could produce sufficient quantities of a specific protein for a limited amount of time, thus allowing immunization to occur. Although such applications of molecular biology to vaccination are presently a dream, there is little doubt of their being a reality in the future. ■
References
Baltz, T., Baltz, D., Pautrizel, R., Richet, C, Lamblin, G. & Degand, P. 1977. Chemical and immunological characterization of specific glycoproteins froir Trypanosoma equiperdum variants. FEBS Letters, 82: 93-96.
Barry, J.D. & Hajduk, S.L. 1979. Antigenic heterogeneity of bloodstream and metacyclic forms of Trypanosoma brucei. In Recent advances in the knowledge of pathogenicity of trypanosomes. Ottawa, Canada, IDRC. (In press)
Barry, J.D., Le Ray, D. & Herbert, W.J. 1979a. Infectivity and virulence of Trypanosoma {Trypanozoon) brucel for mice. IV. Dissociation of virulence and variable antigen type in relation to pleomorphism. J. comp. Path. (In press)
Barry, J.D., Hajduk, S.L., Vickerman, K. & Le Ray, D. 1979b. Detection of multiple variable antigen types in metacyclic populations of Trypanosoma brucei. Transactions of the Royal Society of Tropical Medicine and Hygiene. (In press)
Callow, L.L. 1977. Vaccination against bovine babesiosis. In Immunity to blood parasites of animals and man. Eds. L.H. Miller, J.A. Pino and J.J. McKelvey, Jr. New York and London, Plenum Press. Advances in Experimental Medicine and Biology. Vol. 93. p. 121-149.
Capbern, A., Giroud, C, Baltz, T. & Mattern, P. 1977. Trypanosoma equiperdum: étude des variations antigéniques au cours de la trypanosomose expérimentale du lapin. Experimental Parasitology, 42: 6-13.
Chandler, R.L. 1958. Studies on the tolerance of N'Dama cattle to trypanosomiasis. J. comp. Path., 68: 253-260.
Cross, G.A.M. 1978. Antigenic variation in trypanosomes. Proceedings of the Royal Society of London, B 202: 55-72.
Desowitz, R.S. 1959. Studies on immunity and hostparasite relationship. I. The immunological response of resistant and susceptible breeds of cattle to trypanosomal challenge. Annals of tropical Medicine and Parasitology, 53: 293-313.
Dimitriadis, G.J. 1978. Translation of rabbit globin mRNA introduced by liposomes into mouse lymphocytes. Nature, 274: 923-924.
Goodwin, L.G. 1970. The pathology of African trypanosomiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 64: 797-817.
Gray, A.R. 1965. Antigenic variation in a strain of Trypanosoma brucei transmitted by Glossina morsitans and G. palpalis. Journal of general Microbiology, 41: 195-214.
Herbert, W.J. 1975. Interference between two strains of Trypanosoma brucei. Transactions of the Royal Society of Tropical Medicine and Hygiene, 69: 272.
Hirumi, H., Doyle, J.J. & Hirumi, K. 1977. African trypanosomes: cultivation of animalinfective Trypanosoma brucei in vitro. Science, 196: 992-994.
Hirumi, H., Hirumi, K. & Doyle, J.J. 1978a. Cultivation, complete cyclic development and cloning of Trypanosoma brucei in vitro. Fourth International Congress of Parasitology, 19-26 August 1978, Warszawa, Poland. Section A: p. 54.
Hirumi, H., Hirumi, K. & Doyle, J.J. 1978b. Cloning of African trypanosomes in the presence of bovine fibroblastlike cells. In vitro, 14: 379.
Jenni, L. 1977. Comparisons of antigenic types of Trypanosoma (T.) brucei strains transmitted by Glossina m. morsitans. Acta tropica, 34: 35-41.
Le Ray, D., Barry, J.D., Easton, C. & Vickerman, K. 1977. First tsetse fly transmission of the "AnTat" serodeme of Trypanosoma brucei. Annales de la Société beige de Médecine tropicale, 57: 369-381.
Le Ray, D., Barry, J.D. & Vickerman, K. 1978. Antigenic heterogeneity of metacyclic forms of Trypanosoma brucei. Nature, 273: 300-302.
McNeillage, G.J.C. & Herbert, W.J. 1968. Infectivity and virulence of Trypanosoma (Trypanozoon) brucei for mice. II. Comparison of closely related trypanosome antigenic types. J. comp. Path., 78: 345-349.
Mercereau-Puijalou, O., Royal, A., Cami, B., Garapin, A., Krust, A., Gannon, F. & Kourilsky, P. 1978. Synthesis of an ovalbuminlike protein by Escherichia coli K12 harbouring a recombinant plasmid. Nature, 275: 505-510.
Murray, M. & Urquhart, G.M. 1977. Immunoprophylaxis against African Trypanosomiasis. In Immunity to blood parasites of animals and man. Eds. L.H. Miller, J.A. Pino and J.J. McKelvey, Jr. New York and London, Plenum Press. Advances in Experimental Medicine and Biology. Vol. 93. p. 209-241.
Murray, M., Morrison, W.I., Murray, P.K., Clifford, J.D. & Trail, J.C.M. 1979. A review: trypanotolerance. Wld Anim. Rev., 31. (In press)
Nantulya, V.N., Doyle, J.J. & Jenni, L. 1979. Studies on Trypanosoma {Nannomonas) congolense. III. Antigenic variation in cyclically transmitted strains. Parasitology. (In press)
Ostro, M.J., Giacomoni, D., Lavelle, D., Paxton, W. & Dray, S. 1978. Evidence for translation of rabbit globin mRNA after liposomemediated insertion into a human cell line. Nature, 274: 921-923.
Shine, J., Seeburg, P.H., Martial, J.A., Baxter, J.D. & Goodman, H.M. 1977. Construction and analysis of recombinant dna for human chrionic somatomammotropin. Nature, 270: 494-499.
Stewart, J.L. 1951. The West African Shorthorn Cattle. Their value to Africa as trypanosomiasisresistant animals. Veterinary Record, 63: 454-457.
Van Meirvenne, N., Janssens, P.G. & Magnus, E. 1975a. Antigenic variation in syringe passaged populations of Trypanosoma {Trypanozoon) brucei. I. Rationalization of the experimental approach. Annales de la Société beige de Médecine tropicale, 55: 1-23.
Van Meirvenne, N., Janssens, P.G., Magnus, E., Lumsden, W.H.R. & Herbert, W.J. 1975b. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. II. Comparative studies on two antigenictype collections. Annales de la Société bslge de Médecine tropicale, 55: 25-30.
Van Meirvenne, N., Magnus, E. & Vervoort, T. 1977. Comparison of variable antigenic types produced by trypanosome strains of the subgenus Trypanozoon. Annales de la Société beige de Médecine tropicale, 57: 409-423.
Vickerman, K. 1969. On the surface coat and flagellar adhesion in trypanosomes. J. Cell Sci., 5: 163-194.
Vickerman, K. 1978. Antigenic variation in trypanosomes. Nature, 273: 613-617.
Wigler, M., Pellicer, A., Silverstein, S. & Axel, R. 1978. Biochemical transfer of singlecopy eucaryotic genes using total cellular dna as donor. Cell, 14: 725-731.
Williams, R.O., Marcu, K.B., Young, J.R., Rovis, L. & Williams, S.C. 1978. A characterization of mRNA activities and their sequence complexities in Trypanosoma: brucei partial purification and properties of the vssa mRNA. Nucleic Acids Research, 5: 3171-3182.
Wilson, A.J. 1971. Immunological aspects of bovine trypanosomiasis. III. Patterns in the development of immunity. Tropical Animal Health and Production, 3: 14-22.
Wilson, A.J. & Cunningham, M.P. 1971. Immunological aspects of bovine trypanosomiasis. IV. Patterns in the production of common antibodies. Tropical Animal Health and Production, 3: 133-139.
Wilson, A.J. & Cunningham, M.P. 1972. Immunological aspects of bovine trypanosomiasis. I. Immune response of cattle to infection with Trypanosoma congolense and the antigenic variation of the infecting organism. Experimental Parasitology, 32: 165-173.
M Murray, J.D. Berry, W.I. Morrison, R.O. Williams, H. Hirumi and L. Rovis
In Part I of this review of the prospects of vaccination against African trypanosomiasis, contained in the previous issue of World Animal Review, the constraints in developing a vaccine were discussed and current knowledge on the molecular biology of the trypanosome and on antigenic variation with respect to possible future vaccines was evaluated. In this part other possible regimes for immunological intervention, including the immunogenicity and cross reactivity of trypanosome subcellular fractions, reduction of host susceptibility to African trypanosomes by the nonspecific use of immunostimulants or chemotherapy and also the role of trypanotolerant livestock in such approaches, are discussed.
Modern biochemical technology has allowed the isolation, purification and characterization of a whole range of trypanosomal subcellular fractions. Thus, the variable antigen, which is responsible for induction of protective immunity, has been shown to be a glycoprotein with a molecular weight of 60 000 to 65 000 daltons, depending on species (Cross, 1975 and 1977 with T. brucei; Baltz, Baltz and Pautrizel, 1976, and Baltz et al., 1977 with T. equiperdum; Rovis, Barbet and Williams, 1978 with T. congolense). While (at least with T. brucei) the N minerals of different variable antigen types (vats) differ in amino acid sequence (Bridgen, Cross and Bridgen, 1976), there is now evidence that different vats of the same and also of different species of trypanosomes (T. brucei/T. congolense) may have cross-reacting determinants (Barbet and McGuire, 1978). Although it would seem likely that these are hidden from the host's immune response the possibility of vaccination against these determinants must be pursued.
Crossreaction may occur at different levels. For instance, some crossreacting determinants may be found only on vats within one vat repertoire (the total number of vats that a trypanosome can express), while others may be universal. Complete characterization of all determinants, using such techniques as monoclonal antibody production (Kohler and Milstein, 1975), should reveal the structural and functional significance of any crossreacting components. Hyperimmunization against such components may prove effective if manipulated properly.
Most biochemical and immunological studies to date relating to immunogenicity of subcellular fractions have been aimed at analysing the variable antigen of the surface coat It is possible, however, that at some time in the trypanosome's complex lifecycle "weak spots" amenable to immunological control might be exposed. Thus, recent investigations have been made into the purification of a range of subcellular fractions of the trypanosome such as flagellum, membranes and kinetoplast. The biological characteristics and immunogenicity of these fractions have been investigated and compared with those of variable antigen. What the authors have found in studies on T. brucei in the mouse is that flagellum and membrane fractions stimulate protection against homologous vat challenge to the same degree as variable antigen (Table 1). It is likely that this is the result of the presence of variable antigen in these subcellular fractions although it is interesting that, per unit weight protein, flagellum is more effective than the purified variable antigen. No protection was achieved on challenge with a different vat although with the membrane and kinetoplast preparations there was significant prolongation of survival accompanied by an alteration in the parasitaemic profile. This was possibly a result of a nonspecific stimulant effect of these fractions (see below, the section on "Induction of increased resistance by immunostimulants").
Using a subcellular fraction of T. brucei or T. rhodesiense that probably contained a mixture of variable antigen, mitochondrion and kinetoplast to immunize mice, Powell (1976; 1978) found increased survival times and reduced parasitaemias in mice challenged with T. brucei. Using T. brucei in C57BI/6J mice and a similar fraction for immunization, the authors were able to stimulate protection only if trypanosomes of the same vat were used for challenge (Table 1). When another vat was used for challenge protection was not achieved although there was a significant increase in survival time. Of considerable interest is the report of Powell (personal communication) that the use of the above fraction in aluminium hydroxide protected across trypanosome species. Three sheep were immunized in the "feet" with three doses of 1-mg protein fraction of T. rhodesiense in aluminium hydroxide. On subsequent challenge with T. vivax each of the three sheep developed a transient parasitaemia and then made a complete recovery. All three challenge control sheep became infected and died. These observations now await confirmation.
TABLE 1. Immunization with various subcellular fractions of Trypanosoma brucei
| Fraction |
Challenge |
|
|
Same VAT |
Different VAT |
|
|
Variable antigen |
Complete protection |
No effect |
|
Flagella |
Complete protection |
No effect |
|
Membrane |
Complete protection |
Prolonged survival |
|
"Powell" fraction |
Complete protection |
Prolonged survival |
| Kinetoplast | Increased resistance | Prolonged survival |
It should be borne in mind that trypanosomes have a complex lifecycle in which there may be "weak spots" susceptible to immunological intervention. For example, stimulation of the mammalian host's response to the tsetse bite or saliva may be such a method. Also, the trypanosomes in the midgut of the tsetse fly are uncoated (Vickerman, 1969) and possess a common surface antigenic identity (Seed, 1964; Barry and Vickerman, 1979). As ingested antibody can retain specific activity for up to four days in the tsetse midgut (Cunningham et ah, 1962) it would be of interest to study the effect on fly infection of uptake of high levels of antibody against these common surface antigens (Barry and Vickerman, 1979). Once again, the antigen could be supplied by in vitro culture techniques.
The host's immune response to the trypanosome is still poorly understood but there are indications that it is defective. For example, a feature of African trypanosomiasis is the development of a state of immunosuppression (reviewed by Murray et al., 1974) and hypergammaglobulinaemia — involving mainly igM (Mattern et al., 1961; Luckins, 1972), a large proportion of which would appear not to be specific for the trypanosome (Freeman et al., 1970; Corsini et al., 1977). It is possible that the capacity of the trypanosome to survive may be related to the immunologically compromised state of the host. Thus, a complete understanding of the basis of immunosuppression and the relevant immunological effector mechanisms that kill the trypanosomes might allow some form of intervention so that effector mechanisms are stimulated and the host is able to control or eliminate the parasite.
TABLE 2. Effect of Bordetella pertussis on survival of A/J and C57BI/6J mice challenged with Trypanosoma congolense
Percentage survival
| Days after challenge. | A/J 1 | C57BI/6J 1 | ||
| Control | B. pertussis |
Control |
B.pertussis | |
| 10 | 68 | 96 | 100 | 100 |
| 15 | 0 | 43 | 88 | 100 |
| 20 | 43 | 88 | 96 | |
| 30 | 43 | 88 | 96 | |
| 40 | 39 | 80 | 96 | |
| 50 | 35 | 80 | 91 | |
| 100 | 8 | 26 | 64 | |
| 150 | 0 | 0 | 24 | |
| Average time to death, in days |
11.2+1 |
26.4+24.6 2 |
75.4+35.4 |
113.3+47.8 2 |
1 25 mice per group.
2 Significant to controls (arithmetic mean + one standard deviation).
In this regard, the authors attempted to improve the host's immune response, and thus host resistance, by using the immunostimulants Bordetella pertussis, Corynebacterium parvum and Bacillus Calmette-Guérin (BCG) prior to or at the time of challenge (Murray and Morrison, 1979). So far this strategy has been successful, at least in mice. It was possible to increase survival times in both susceptible (A /J) and more resistant (C57BI/6J) strains of mice (Table 2). Thus, following challenge with T. congolense, the treated A/J strain behaved in a manner much more akin to the more resistant C57BI/6J. It should be emphasized, however, that complete protection was never induced by this method. The reduced susceptibility appeared to be ? related to the ability of these immunostimulants, particularly B. pertussis and C. parvum, to delay the onset of parasitaemia or to reduce the level of parasitaemia (Figures 1 and 2). The best results were achieved when both of these parameters were affected. The possibility that these immunostimulants acted by improving the immune response is being investigated at present.
The strategy of increasing host resistance by nonspecifically acting immunostimulants offers an attractive alternative or additional approach to the complex undertaking of a breeding programme for trypanotolerant livestock. However, whether immunostimulants can be employed effectively in this way in domestic livestock remains to be determined.
As trypanotolerance was the subject of an earlier review in this journal (Murray et al., 1979), the authors will limit their remarks.
There is now a substantial body of evidence to indicate that certain breeds of cattle, sheep and goats are able to survive and be productive without the aid of treatment in areas of tsetsefly challenge, where other breeds cannot. This attribute is known as trypanotolerance although, as this state is not absolute, it would be better termed as reduced susceptibility. These trypanotolerant breeds are of considerable interest and importance. Not only is there evidence that they are economically exploitable in their own right but they also provide an excellent experimental system for evaluating the important factors that influence host susceptibility to trypanosomiasis. If it is confirmed, as the results of Desowitz (1959) strongly indicate, that the basis of trypanotolerance is the ability to mount a more effective immune response to the trypanosome, it might well be that any immunotherapeutic strategy that may be developed would be more effectively employed if used in trypanotolerant breeds of animals.
Bevan (1928; 1936), working in Southern Rhodesia, was perhaps the first worker to note that bovines that recovered from clinical trypanosomiasis after treatment frequently remained in good health despite reinfection, suggesting that it might be possible to create a "nonsterile" form of immunity. That infection and treatment regimes can achieve this has been confirmed more recently by Wilson and his colleagues working in East Africa (1957 a and b; 1976). Since the method developed is immediately applicable, the authors would like to describe these studies in some detail.
Wilson attempted to evaluate the use of different drug strategies in the development of immunity in young cattle over a period of two to three years. "Immunity" was assessed by trypanocidal drug requirement, development and duration of parasitaemia, ability to maintain normal blood values in the presence of parasites, calving rates in breeding stock and growth rates in a beef herd.
In one experiment a breeding herd located in a high tsetsefly challenge area was managed under the following drug regime. Animals were treated with Berenil, treatment being determined not by the presence of the parasite but on the basis of the development of clinical signs of disease and of packed red cell volume (pcv) below 20 percent (Wilson, Paris and Dar, 1975a).
During the first and second years requirement for treatment did not change; an average of eight treatments was used and animals became parasitaemic about 30 to 40 days after each treatment. However, during the second year there was indirect evidence of reduced susceptibility to trypanosomiasis: for example, the number of live calves born increased and subsequent mortality decreased; abortions, a not uncommon occurrence in bovine trypanosomiasis, also decreased.
Even more promising results were achieved in a later series of experiments in which steers were introduced into an area of medium trypanosome challenge (Wilson et al., 1975b; Wilson et al.. 1976). As before, a treatment regime based onappearance of clinical signs or pvc below 20 percent was used in a first group of cattle over a period of 29 months. The period between drug treatments, which was initially between 50 and 60 days, increased to around 130 days by the ninth treatment; at the same time the periods when trypanosomes were present in the blood without great adverse effect had increased from a mean of 11.7 days prior to the first drug treatment to 30.9 days by the ninth treatment. When the drugs were withdrawn from a number of steers six months before the end of the experiment, all survived and the growth rate and pvc values were the same as in the steers with access to therapy, showing that the resistance that developed was not drag dependent.
In contrast, a second group of steers, all of which were treated with Berenil whenever blood infection rather than clinical signs was detected, showed no evidence of developing immunity and they required treatment every 26 days throughout the course of the experiment. When treatment was withdrawn from some of the steers six months before termination of the experiment, their mean weight gains were 58 kg less than those steers in which treatment continued and, in addition, one animal died.
With a third group of steers, Samorin (isometamidium) was used in the same way as Berenil in Group 2, and there was some evidence of the development of immunity. While the need for therapy did not decrease throughout the experiment, pvc values and growth rates were maintained in the animals in which drug treatment was withdrawn six months prior to termination of the experiment, despite the more frequent presence of parasites in this group.
In terms of weight gain there was no doubt that the use of drugs prophylactically on a group basis, particularly Samorin, gave by far the best results. Nevertheless, as Wilson et d. (1976) pointed out, the particular advantage in encouraging the development of nonsterile immunity by infection and treatment might lie in the development of lesssusceptible breeding herds over periods of several years, particularly in areas of low to medium trypanosome challenge. This procedure might be even more successful if used with trypanotolerant breeds of livestock. It should be emphasized that drug resistance was not experienced in these studies.
The basis of this form of tolerance or "nonsterile" immunity to the trypanosome awaits investigation. It may be that the host has built up a whole battery of immune responses to the range of metacyclic antigens and vat repertoires that occur in that particular location, or alternatively there might exist a common priming antigen that allows the host to make a series of secondary responses to each vat, thus controlling the infection in the manner of the carrierhapten effect proposed for malaria by Brown (1971). However, it might be related to some nonspecific effect such as expansion and activation of the mononuclear phagocytic system.
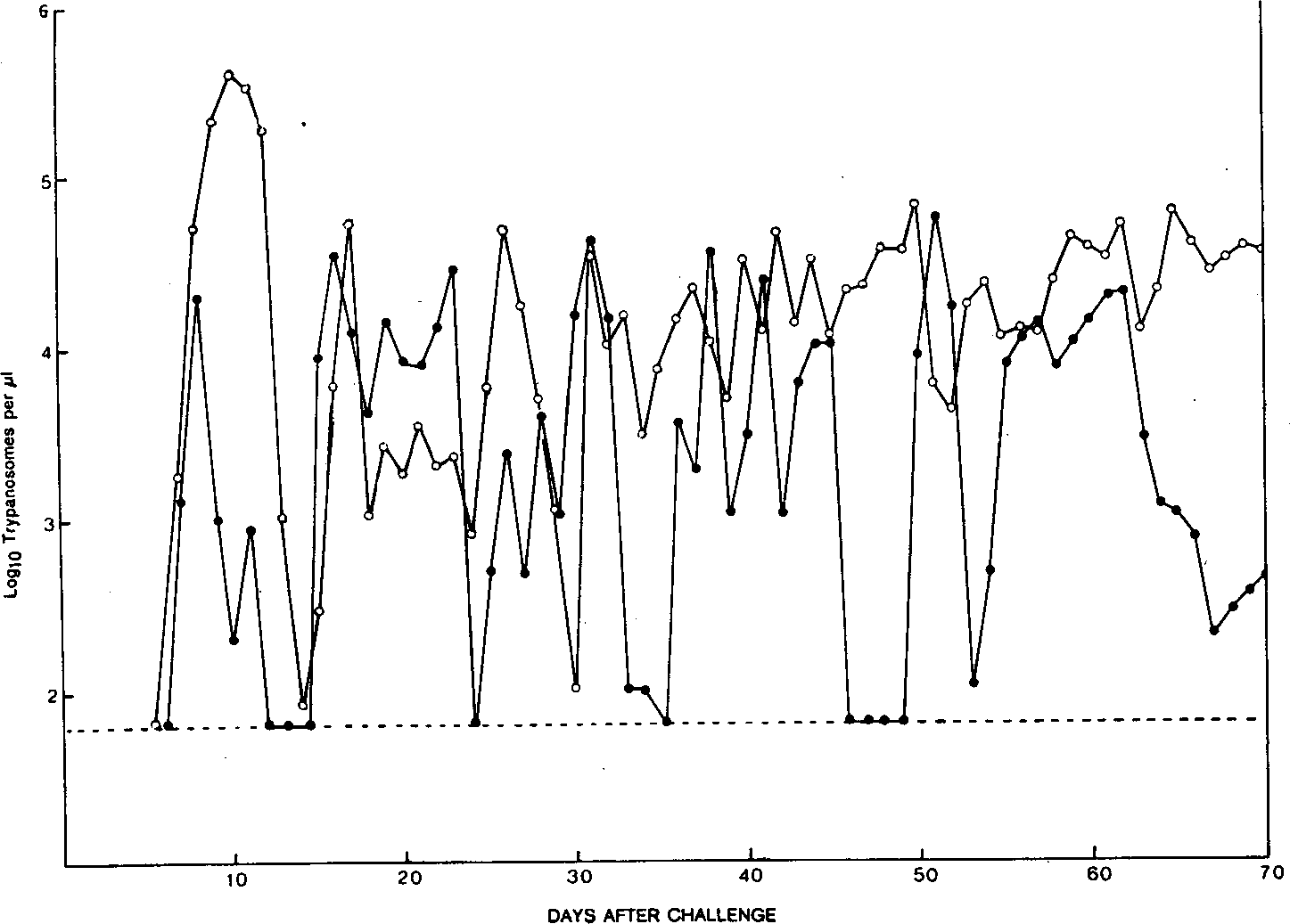
Figure 1.The parasitaemia profile of a Bordetella pertussisfreated C57BI/J61 mouse (•) and a control C57BI/6J mouse (o) challenged with Trypanosoma congolense. The broken line just below 2 logio trypanosomes per \il indicates the level of sensitivity for detection of trypanosomes with the haemocytometer technique.
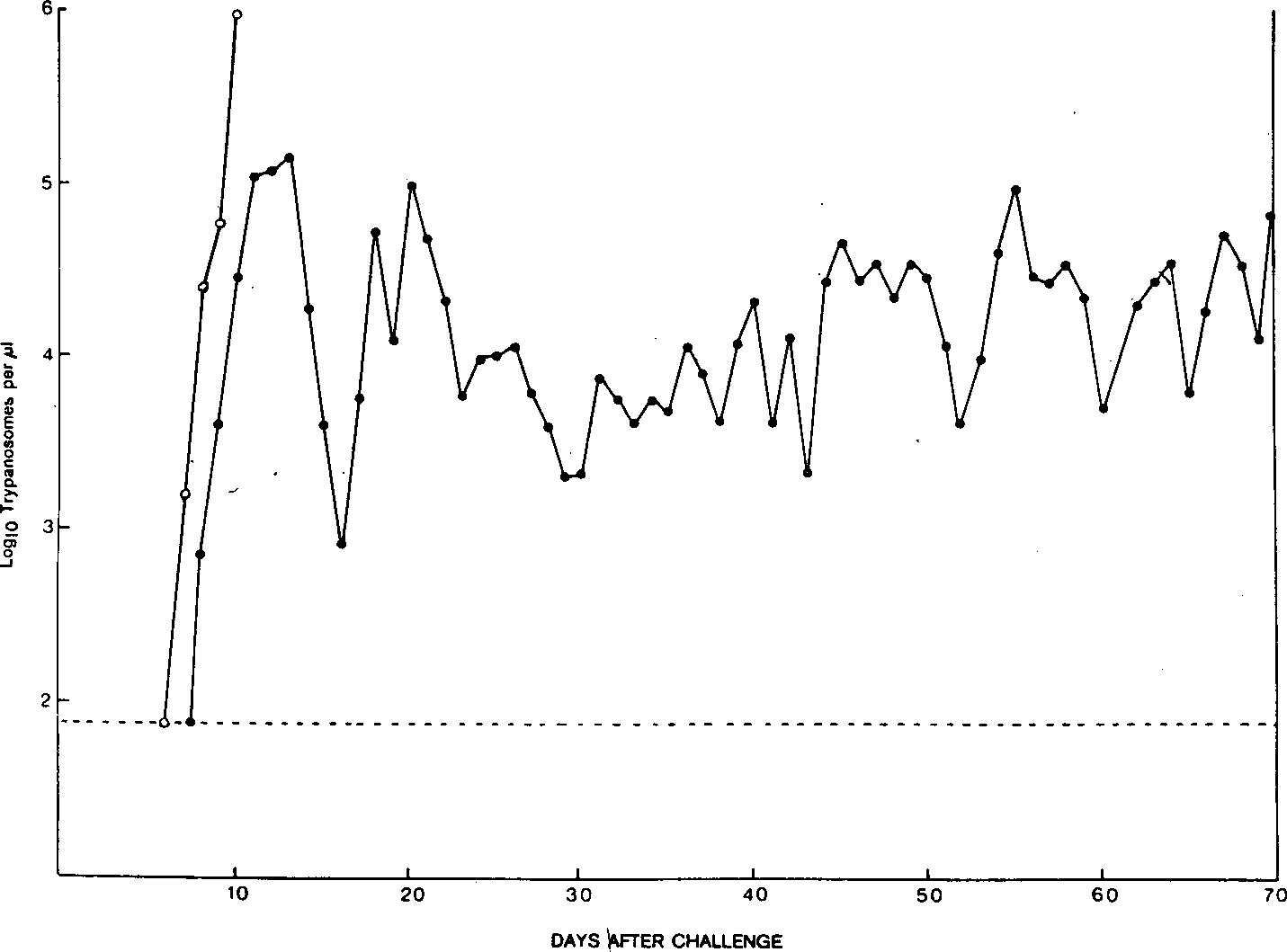
Figure 2.The parasitaemia profile of a Bordetella pertussistreated A/J mouse (•) and a control A\J mouse (o) challenged with Trypanosoma congolense. The broken line just below 2 logio trypanosomes per (i/ indicates the level of sensitivity for detection of trypanosomes with the haemocytometer technique.
While a vaccine against trypanosomiasis is not an immediate prospect, what the two parts of this article have attempted to show is that there are several promising avenues for immunological exploration, namely vat cocktails, trypanosomes attenuated in in vitro culture systems, genetic engineering, crossreacting subcellular fractions, intervention against the tsetse, nonspecific induction of increased resistance by immunostimulants, and infection and treatment regimes. It is likely, if any one of these areas is rewarding, that the resulting vaccine will be more successfully exploited, at least initially, in trypanotolerant animals.
The authors would like to emphasize that any immunotherapeutic solution for trypanosomiasis control can come only through a thorough knowledge of the lifecycle of the trypanosome and its basic biology coupled with a comprehensive understanding of the immune response of the finite host. ■
References
Baltz, T., Baltz, D. & Pautrizel, R. 1976. Affinité de la concanavaline A pour Trypanosoma equiperdum. Applications a l'isolement de la fraction glycoprot?ique spécifique du type antigénique. Annales d'immunologie (Institut Pasteur), 127: 761-774.
Baltz, T., Baltz, D., Pautrizel, R., Richet, C, Lamblin, G. & Degand, P. 1977. Chemical and immunological characterization of specific glycoproteins from Trypanosoma equiperdum variants. FEBS Letters, 82: 93-96.
Barbet, A.F. & McGoire, T.C. 1978. Crossreacting determinants in variantspecific, surface antigens of African trypanosomes. Proceedings of the National Academy of Sciences, 75: 1989-1993.
Barry, J.D. & Vickerman, K. 1979. Loss of variable antigen from Trypanosoma brucei during development in the midgut of Glossina morsitans. Experimental Parasitology. (In press)
Bevan, L.E.M. 1928. Method of inoculating cattle against trypanosomiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 22: 147-156.
Bevan, L.E.M. 1936. Notes on immunity in trypanosomiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 30: 199-206.
Bridgen, P.J., Cross, G.A.M. & Bridgen, J. 1976. Nterminal amino acid sequences of variant-specific surface antigens from Trypanosoma brucei. Nature, 263: 613-614.
Brown, K.N. 1971. Protective immunity to malaria parasites: a model for the survival of cells in an immunological hostile environment. Nature, 230: 163-167.
Corsini, A.C., Clayton, C, Askonas, B.A. & Ogilvie, B.M. 1977. Suppressor cells and loss of Bcell potential in mice infected with Trypanosoma brucei. Clinical and Experimental Immunology, 29: 122-131.
Cross, G.A.M. 1975. Identification, purification and properties of clonesspecific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology, 71: 393-417.
Cross, G.A.M. 1978. Antigenic variation in trypanosomes. Proceedings of the Royal Society of London, B. 202: 55-72.
Cunningham, M.P., Harley, J.M.B., Southon, H.A.W. & Lumsden, W.H.R. 1962. Detection of antibodies in bloodmeals of haematophagous Diptera. Science, 138: 32-33.
Desowttz, R.S. 1959. Studies on immunity and hostparasite relationship. I. The immunological response of resistant and susceptible breeds of cattle to trypanosomal challenge. Annals of Tropical Medicine and Parasitology, 53: 293-313.
Freeman, T., Smithers, S.R., Targett, G.A.T. & Walker, P.J. 1970. Specificity of immunoglobulin G in rhesus monkeys infected with Schistosoma mansoni, Plasmodium Knowlesi and Trypanosoma brucei. Journal of Infectious Diseases, 121: 401-406.
Kohler, G. & Milstein, C. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature, 256: 495-497.
Luckins, A.G. 1972. Studies on bovine trypanosomiasis. Serum immunoglobulin levels in Zebu cattle exposed to natural infections in East Africa. British Veterinary Journal, 128: 523-528.
Mattern, P., Masseyeff, R., Michel, R. & Peretti, P. 1961. Etude immunochi-mique de la B2-macroglobuline des sérums de malades atteints de trypanosomiase africaine a T. gambiense. Annales d'immunologie (Institut Pasteur), 101: 382-388.
Murray, M. & Morrison, W.I. 1979. Nonspecific induction of increased resistance in mice to Trypanosoma congolense and Trypanosoma brucei by immunostimulants. Parasitology (In press)
Murray, M., Morrison, W.I., Murray, P.K., Clifford, D.J. & Trail, J.C.M. 1979. Trypanotolerance: a review. Wld Anim. Rev., 31: 2-12.
Murray, P.K., Jennings, F.W., Murray, M. & Urquhart, G.M. 1974. Immunosuppression in trypanosomiasis. In Parasitic zoonoses. Clinical and experimental studies. Ed. E.J.L. Soulsby. New York, San Francisco, London, Academic Press, p. 133-150.
Powell, C.N. 1976. Immunoprotective effects of bound particulate subcellular fractions of Trypanosoma brucei and T. rhodesiense. Medical Journal of Zambia, 10: 27-31.
Powell, C.N. 1978. Experimental immunity against trypanosomiasis. Experientia, 34: 1450-1451.
Rovis, L., Barbet, A.F. & Williams, R.O. 1978. Characterization of the surface coat of Trypanosoma congolense. Nature, 271: 654-656.
Seed, J.R. 1964. Antigenic similarity among culture forms of the brucei group of trypanosomes. Parasitology, 54: 593-596.
Vickerman, K. 1969. On the surface coat and flagellar adhesion in trypanosomes. J. Cell Sci., 5: 163-194.
Wilson, A.I., Paris, J. & Dar, F.K. 1975a. Observations on a herd of breeding cattle maintained in an area of high trypanosome challenge. Tropical Animal Health and Production, 7: 63-71.
Wilson, A.J., Le Roux, J.G., Paris, J., Davidson, C.R. & Gray, A.R. 1975b. Observations on a herd of beef cattle maintained in a tsetse area. I. Assessment of chemotherapy as a method for the control of trypanosomiasis. Tropical Animal Health and Production, 7: 87-199.
Wilson, A.J., Paris, J., Luckins, A.G., Dar, F.K. & Gray, A.R. 1976. Observations on a herd of beef cattle maintained in a tsetse area. II. Assessment of the development of immunity in association with trypanocidal drug treatment Tropical Animal Health and Production, 8: 1-12.
The authors are with the International Laboratory for Research on Animal Diseases (ILRAD), PO Box 30709, Nairobi, Kenya.
The authors are with the International Laboratory for Research on Animal Diseases (ILRAD), PO Box 30709, Nairobi, Kenya.