ALINORM 04/27/18
APPENDIX II
DRAFT STANDARD FOR SALTED ATLANTIC HERRING
AND SALTED SPRAT
(At Step 8 of the Procedure)
The standard applies to salted Atlantic herring (Clupea harengus) and sprat (Sprattus sprattus)1. Fish products produced by use of added natural or artificial enzymic preparations, acids and/or artificial enzymes are not covered by this standard.
The product is prepared from fresh or frozen fish. The fish is salted as whole fish or as headed or nobbed or headed and gutted or gibbed or filleted (skin-on or skin-off) fish. Spices, sugar and other optional ingredients may be added. Countries where the product are to be consumed may allow this product in an uneviserated state or may require evisceration , either before or after processing, since the margin of error in the control of Clostridium botulinum is small even when good practices are followed and the consequences are severe. The product is either intended for direct human consumption or for further processing.
The fish after any suitable preparation shall be subjected to a salting process and shall comply with the conditions laid down hereafter. The salting process including the temperature and time should be sufficiently controlled to prevent the development of Clostridium botulinum or fish should be eviscerated prior to brining.
Salting is the process of mixing fish with the appropriate amount of food grade salt, sugar spices and all optional ingredients and/or of adding the appropriate amount of salt-solution of the appropriate concentration. Salting is performed in watertight containers (barrels etc.).
2.2.2.1 Very lightly salted fish
The salt content in the fish muscle is above 1 g/100 g in water phase and below or equal to 4 g/100 g or less in water phase.
2.2.2.2 Lightly salted fish
The salt content in the fish muscle is above 4 g/100 g in water phase and below or equal to 10 g salt/100 g in water phase.
2.2.2.3 Medium salted fish
The salt content in the fish muscle is above 10 g salt/100 g water phase and below or equal to 20 g salt/100 g in water phase.
2.2.2.4 Heavily salted fish
The salt content of the fish muscle is above 20 g salt /100 g in water phase.
The products shall be kept frozen or refrigerated at a time/temperature combination which ensures their safety and quality in conformity with Sections 3 and 5. Very lightly salted fish must be kept frozen after processing.
Any presentation of the product shall be permitted provided that it:
2.3.1 meets all requirements of this standard, and
2.3.2 is adequately described on the label to avoid confusing or misleading the consumer.
Salted Atlantic herring and salted sprats shall be prepared from sound and wholesome fish which are of a quality fit to be sold fresh for human consumption after appropriate preparation. Fish flesh shall not be obviously infested by parasites.
Salt and all other ingredients used shall be of food grade quality and conform to all applicable Codex standards.
Products shall meet the requirements of this standard when lots examined in accordance with Section 9 comply with the provisions set out in Section 8. Products shall be examined by the methods given in Section 7.
The products shall not contain more than 10 mg of histamine per 100 g fish flesh based on the average of the sample unit tested
Only the use of the following additives is permitted.
Maximum level in the final product
300 Ascorbic acid GMP
330 Citric acid GMP
Antioxidants
200 – 203 Sorbates 200 mg/kg (expressed as sorbic acid)
210 – 213 Benzoates 200 mg/kg (expressed as benzoic acid)
5.1 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1985, Rev.3, 1997) and other relevant Codex texts such as codes of practice and codes of hygienic practice, as follows;
(i) the Recommended International Code of Practice for Salted Fish (CAC/RCP 26-1979);
(ii) the Recommended International Code of Practice for Fresh Fish (CAC/RCP 9-1976);
(iv) the Recommended International Code of Practice for Frozen Fish (CAC/RCP 16-1978)
5.2 The products should comply with any microbiological criteria established in accordance with the Principles for the Establishment and Application of Microbiological Criteria to Foods (CAC/GL 21-1997)
5.3 The product shall not contain any other substance in amounts which may present a hazard to health in accordance with standards established by the Codex Alimentarius Commission.
Fish flesh shall not contain living larvae of nematodes. Viability of nematodes shall be examined according to Annex I. If living nematodes are confirmed, products must not be placed on the market for human consumption before they are treated in conformity with the methods laid down in Annex II.
The final product shall be free from any foreign material that poses a threat to human health
In addition to the provisions of the Codex General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991) the following specific provisions apply:
6.1.1 The name of the product shall be ...-salted herring or ...- salted sprat in accordance with the law and custom of the country in which the product is sold, in a manner not to mislead the consumer.
6.1.2 In addition the label shall include other descriptive terms that will avoid misleading or confusing the consumer.
Information specified above should be given either on the container or in accompanying documents, except that the name of the food, lot identification, and the name of and address of the manufacturer or packer or importer as well as storage instructions shall always appear on the container.
However lot identification, and the name and address may be replaced by an identification mark, provided that such a mark is clearly identifiable with accompanying documents.
(i) Sampling of lots for examination of the product for quality shall be in accordance with the FAO/WHO Codex Alimentarius Sampling Plan for Prepackaged Foods (AQL-6.5) (CODEX STAN 233-1969).
A sample unit is the individual fish or the primary container.
(ii) Sampling of lots for examination of net weight shall be carried out in accordance with an appropriate sampling plan meeting the criteria established by the Codex Alimentarius Commission.
(iii) Sampling of lots for pathogenic microorganisms and parasites will be in accordance with the Principles for the Establishment and Application of Microbiological Criteria to Foods (CAC/GL 21-1997)
(iv) Sampling of lots for histamine will be in accordance with the Draft General Guidelines on Sampling (under development by the Committee on Methods of Analysis and Sampling)
Samples taken for sensory and physical examination shall be assessed by persons trained in such examination and in accordance with procedures elaborated in Section 7.3 through 7.8 and Annexes and in accordance with the Guidelines for the Sensory Evaluation of Fish and Shellfish in Laboratories (CAC/GL 31-1999).
Determination of salt content is performed according to the method in the Codex Standard for Salted Fish and Dried Salted Fish of Gadidae Family of Fishes – CODEX STAN 167 –1989, Rev.1-1995
Determination of water content is performed according to AOAC 950.46B (air drying)
AOAC 977.13
The net weight (excluding packaging material) of each sample unit in the sample lot shall be determined.
Remove the herring from the container (barrel) and put it on an appropriate sieve. Allow to drain for 5 min and remove adhering salt crystals. Weigh the herring and calculate net weigh.
8.1 The sample unit shall be considered as defective when it exhibits any of the properties defined below.
8.1.1. Foreign matter
The presence in the sample unit of any matter which has not been derived from fish, does not pose a threat to human health, and is readily recognized without magnification or is present at a level determined by any method including magnification that indicates non-compliance with good manufacturing and sanitation practices.
The presence of readily visible parasites in a sample of the edible portion of the sample unit detected by normal visual inspection of the fish flesh (see Annex III).
Fish affected by persistent and distinct objectionable odours or flavours indicative of decomposition (such as sour, putrid, fishy, rancid, burning sensation, etc.) or contamination by foreign substances (such as fuel oil, cleaning compounds, etc.).
A lot shall be considered as meeting the requirements of this standard when:
(i) the total number of defectives as classified according to Section 8 does not exceed the acceptance number ( c ) of the appropriate sampling plan in Section 7; and
(ii) the average net weight of all sample units is not less than the declared weight, provided no individual container is less than 95% of the declared weight; and
iii) the Food Additives, Hygiene and Handling and Labelling requirements of Sections 4, 5 and 6 are met.
ANNEX I
VIABILITY TEST FOR NEMATODES (modified method according to Reference 1)
Principle:
Nematodes are isolated from fish fillets by digestion, transferred into 0.5 % Pepsin digestion solution and inspected visually for viability. Digestion conditions correspond to conditions found in the digestive tracts of mammals and guarantee the survival of nematodes.
Equipment:
- Stacked sieves (diameter: 14 cm or larger, mesh size: 0.5 mm)
- Magnetic stirrer with thermostated heating plate
- normal laboratory equipment
Chemicals:
- Pepsin 2000 FIP-U / g
- Hydrochloric acid
Solution:
A: 0.5 % (w/v) Pepsin in 0.063 M HCl
Procedure:
Fillets of approximately 200 g are manually shredded and placed in a 2 l beaker containing 1 l Pepsin solution A. The mixture is heated on a magnet stirrer to 37 °C for 1- 2 h under continuous slow stirring. If the flesh is not dissolved, the solution is poured through a sieve, washed with water and the remaining flesh is quantitatively replaced in the beaker. 700 ml digestion solution A is added and the mixture stirred again under gentle heating (max. 37°C) until there are no large pieces of flesh left.
The digestion solution is decanted through a sieve and the content of the sieve rinsed with water.
Nematodes are carefully transferred by means of small forceps into Petri dishes containing fresh Pepsin solution A. The dishes are placed on a candling dish, and care has to be taken not to exceed 37 °C.
Viable nematodes show visible movements or spontaneous reactions when gently probed with dissecting needles. A single relaxation of coiled nematodes, which sometimes occurs, is not a clear sign of viability. Nematodes must show spontaneous movement.
Attention:
When checking for viable nematodes in salted or sugar salted products, reanimation time of nematodes can last up to two hours and more.
Remarks:
Several other methods exist for the determination of viability of nematodes (e.g. ref. 2, 3).
The described method has been chosen because it is easy to perform and combines isolation of nematodes and viability test within one step.
References:
1. Anon.: Vorläufiger Probenahmeplan, Untersuchungsgang und Beurteilungsvorschlag für die amtliche Überprüfung der Erfüllung der Vorschriften des § 2 Abs. 5 der Fisch-VO. Bundesgesundheitsblatt 12, 486-487 (1988).
2. Leinemann, M. and Karl, H.: Untersuchungen zur Differenzierung lebender und toter Nematodenlarven (Anisakis sp.) in Heringen und Heringserzeugnissen. Archiv Lebensmittelhygiene 39, 147 – 150 (1988).
3. Priebe, K., Jendrusch, H. and Haustedt, U.: Problematik und Experimentaluntersuchungen zum Erlöschen der Einbohrpotenz von Anisakis Larven des Herings bei der Herstellung von Kaltmarinaden. Archiv Lebensmittelhygiene 24, 217 – 222 (1973).
Treatment procedures sufficient to kill living nematodes
- e.g. freezing to - 20º C for not less than 24 h in all parts of the product
- the adequate combination of salt content and storage time (To be elaborated)
- or by other processes with the equivalent effect (To be elaborated)
Determination of the presence of visible parasites
1. The presence of readily visible parasites in a sample unit that is broken into normal bite-size pieces 20-30 mm of flesh by the thickness of the fillet. Only the normal edible portion is considered even if other material is included with the fillet. Examination should be done in an adequately lighted room (where a newspaper may be read easily), without magnification, for evidence of parasites.
2. Notwithstanding paragraph 1, the verification of the presence of parasites in intermediate entire fishery products in bulk intended for further processing could be carried out at a later stage.
ALINORM 04/27/18
APPENDIX III
(At Step 8 of the Procedure)
INTRODUCTION
Certification is one method that can be utilized by regulatory agencies of importing and exporting countries to compliment the control of their inspection system for fish and fishery products. To help facilitate international trade, the numbers and types of certificates should be limited and could be promoted through international (Codex) model certificates. Notwithstanding, alternatives to the use of official and officially recognized certificates2 should be considered wherever possible, in particular where the inspection system and requirements of an exporting country are assessed as being equivalent to those of the importing country. The establishment of bilateral or multilateral agreements, such as mutual recognition agreements may provide the logical basis for discontinuing with the issuance of certificates.
SCOPE
The model certificates apply to fish and fishery products presented for international trade that meet food safety, wholesomeness and conformity to food production requirements of the importing country. Animal and plant health matters are not covered. Where administratively and economically feasible, certificates may be issued in an electronic format provided that the relevant authorities of both the importing and exporting country are satisfied with the security of the certification system.
Certificates should adequately describe one or several lots or batches of product’s compliance with regulatory requirements based on regular inspections by the inspection service. Additional examinations, analytical results, evaluation of quality assurance procedures or product specifications may also be attested to.
DEFINITIONS
Certification3 is the procedure by which official certification bodies or officially recognized certification bodies provide written or equivalent assurance that fish and fishery products or their control systems conform to requirements. Certification of fish and fishery products may be, as appropriate, based on a range of inspection activities which may include continuous on-line inspection, auditing of quality assurance systems, and examination of finished products.
Certifying Bodies are official certification bodies and officially recognized bodies by the competent authority
Certifying officers4: employees of certifying bodies authorized to complete and issue certificates
.
Inspection2 is the examination of fish and fishery products or systems for control of fish and fishery products, raw materials, processing, and distribution including in-process and finished product testing, in order to verify that they conform to requirements.
Inspection system5 means official and officially recognized inspection systems.
Official inspection systems and official certification systems2 are systems administered by a government agency having jurisdiction empowered to perform a regulatory or enforcement function or both.
Officially recognized inspection systems and officially recognized certification systems 2 are systems which have been formally approved or recognized by an government agency having jurisdiction.
Official Certificates3 are certificates issued by an official certification body of an exporting country, in accordance with the requirements of the importing or exporting country.
Officially Recognized Certificates3 are certificates issued by an officially recognized certification body of an exporting country, in accordance with the conditions of that recognition and in accordance with the requirements of the importing or exporting country.
Requirements are the criteria set down by the competent authorities relating to trade in fish and fishery products covering the protection of public health, the protection of consumers and conditions of fair trading.
GENERAL CONSIDERATIONS CONCERNING THE PRODUCTION AND ISSUANCE OF CERTIFICATES
|
4.1 |
It is recommended that the production and issuance of the certificates for fish and fishery products should be carried out in accordance with the principles and appropriate sections of the: v Guidelines for Generic Official Certificate Formats and the Production and Issuance of Certificates (CAC/GL 38-2001); v Principles for Food Import and Export Inspection And Certification (CAC/GL 20-1995); v Guidelines for the Design, Operation, Assessment and Accreditation of Food Import and Export Inspection and Certification Systems (CAC/GL 26-1997); v Guidelines for the Development of Equivalence Agreements Regarding Food Import and Export Inspection and Certification Systems (CAC/GL 34-1999); v Proposed Draft Revised Code of Ethics for International Trade in Foods (under revision by the CCGP). |
|
4.2 |
The selection of the appropriate language(s) of certificates should be based on adequacy for the importing country’s purpose, comprehension by the certifying officer and minimizing unnecessary burden on the exporting country. |
•
THE FORMAT AND USE OF MODEL CERTIFICATES
5.1 FORMAT
|
5.1.1 |
Model Sanitary Certificate (ANNEX I) - The format of the model sanitary certificate should be considered when developing a certificate to attest that fish and fishery products contained in a consignment were produced in establishments that are under the control of and produced to the laws and requirements of the exporting country, or under conditions cited in equivalence or compliance agreements. |
5.2 USE
Each field of the Model Sanitary Certificate must be filled in or else, marked in a manner that would prevent alteration of the certificate. The Model Certificate should contain and be completed as follows:
|
5.2.1 |
Identification Number should be unique for each certificate and should be authorized by the competent authority of the exporting country. Should additional information be required on temporary basis, this may be incorporated as an addendum or an attestation. If there is an addendum, it must have the same identification number as the primary certificate and the signature of the same certifying officer signing the sanitary certificate. |
|
5.2.2 |
Country of Dispatch for the purposes of the model certificate, designates the name of the country of the competent authority which has the competence to verify and certify the conformity of the production establishments. |
|
5.2.3 |
Competent authority is the competent official organisation empowered to execute various functions. Its responsibility may include the management of official systems of inspection or certification at the regional or local level. |
|
5.2.4 |
Certifying Bodies are official certification bodies and bodies officially recognized by the competent authority. . |
|
5.2.5 |
State or type of processing describes the state in which the fish and fishery product is presented (i.e. fresh, frozen, canned , etc.) and/or the processing methods used (i.e. smoked, breaded, etc.). |
|
5.2.6 |
Type of packaging could be cartons, boxes, bags, cases, drums, barrels, pallets, etc. |
|
5.2.7 |
Lot identifier / Date code is the lot identification system developed by a processor to account for their production of fish and fishery product thereby facilitating traceability of the product in the event of public health investigations and recalls. |
|
5.2.8 |
Means of transport should describe the flight/train/truck/container number, as appropriate and the name of the air carrier, vessel, etc. |
|
5.2.9 |
Attestation is a statement confirming the product or batches of products originate from an establishment that is essentially in good regulatory standing with the Competent Authority in that country and that the products were processed and otherwise handled under a competent HACCP and sanitary programme.. |
|
5.2.10 |
Original Certificate should be identifiable and this status should be displayed appropriately with the mark “ORIGINAL” or if a copy is necessary, this certificate should be marked as “COPY” or terms of this effect. The term “REPLACEMENT” is reserved for use on certificates where, for any good and sufficient reason (such as damage to the certificate in transit), a replacement certificate is issued by the certifying officer. |
|
5.2.11 |
Page numbering should be used where the certificate occupies more than one sheet of paper. |
|
5.2.12 |
Seal and signature should be applied in a manner that minimizes the risk of fraud |
COVERING FISH AND FISHERY PRODUCTS
(At Step 8 of the Procedure)
(LETTERHEAD or LOGO) |
Identification number: |
|
Country of Dispatch: |
||
Competent Authority: |
||
Certifying Body: |
||
I. Details identifying the fishery products
Description of product |
Species (scientific name) |
State or type of processing |
Type of packaging |
Lot Identifier/ date code |
Number of packages |
Net weight |
Sum : |
||||||
Temperature required during storage and transport: |
°C | |
II. Provenance of the fishery products
Address(es) and/or the Registration number(s) of production establishment(s) authorized for exports by competent authority: | |
|
Name and address of consignor: |
|
III. Destination of the fishery products
|
The fishery products are to be dispatched from: |
||
(Place of dispatch) | ||
|
to: |
||
(Country and place of destination) | ||
by the following means of transport: |
|
Name of consignee and address at place of destination: |
||
IV. Attestation
The undersigned certifying officer hereby certifies that:
1) |
The products described above originate from (an) approved establishment(s) that has been approved by, or otherwise determined to be in good regulatory standing with the competent authority in the exporting country and |
2) |
have been handled, prepared or processed, identified, stored and transported under a competent HACCP and sanitary programme consistently implemented and in accordance with the requirements laid down in Codex Code of Practice for Fish and Fishery Products (CAC/RCP 52-2003) |
Done at |
on |
20 |
|||
(Place) |
(Date) |
||||
(SEAL)
(Signature of certifying officer) |
(Name and official position) | |
Tel: |
Fax: |
E-mail: (optional) |
ALINORM 04/27/18
APPENDIX IV
DRAFT AMENDMENT TO THE STANDARD FOR QUICK FROZEN LOBSTERS6
(At Step 8 of the Procedure)
(CODEX STAN 95 - 1981, Rev 1 - 1995)
This standard applies to quick frozen raw or cooked lobsters, rock lobsters, spiny lobsters and slipper lobsters.7 Furthermore it applies to quick frozen raw or cooked squat lobsters (red and yellow).
2.1. The product is prepared from lobsters from the genus Homarus of the family Nephropidae and from the families Palinuridae and Scyllaridae. It may also be prepared from Nephrops norvegicus provided it is presented as Norway lobster. For squat lobsters the product is prepared from species of Cervimunida johnii, Pleuroncodes monodon and Pleuroncodes planipes of the family Galatheidae.
2.1.2 The pack shall not contain a mixture of species.
The water used for cooking shall be of potable quality or clean seawater.
The product, after any suitable preparation, shall be subjected to a freezing process and shall comply with the conditions laid down hereafter. The freezing process shall be carried out in appropriate equipment in such a way that the range of temperature of maximum crystallization is passed quickly. The quick freezing process shall not be regarded as complete unless and until the product temperature has reached -18°C or colder at the thermal centre after thermal stabilization. The product shall be kept deep frozen so as to maintain the quality during transportation, storage and distribution.
Quick frozen lobsters shall be processed and packaged so as to minimize dehydration and oxidation.
2.3.1 Any presentation of the product shall be permitted provided that it:
2.3.1.1 meets all requirements of this standard;
2.3.1.2 is adequately described on the label to avoid confusing or misleading the consumer.
2.3.2 The lobster may be packed by count per unit of weight or per package or within a stated weight range.
The product shall be prepared from sound lobsters which are of a quality fit to be sold fresh for human consumption.
If glazed, the water used for glazing or preparing glazing solutions shall be of potable quality or shall be clean sea-water. Potable water is fresh-water fit for human consumption. Standards of potability shall not be less
than those contained in the latest edition of the WHO "International Guidelines for Drinking Water Quality". Clean sea-water is sea-water which meets the same microbiological standards as potable water and is free from objectionable substances.
All other ingredients used shall be of food grade quality and conform to all applicable Codex standards.
Products shall meet the requirements of this standard when lots examined in accordance with Section 9 comply with the provisions set out in Section 8. Products shall be examined by the methods given in Section 7.
Only the use of the following additives is permitted.
5.1 The final product shall be free from any foreign material that poses a threat to human health.
5.2 When tested by appropriate methods of sampling and examination prescribed by the Codex Alimentarius Commission , the product:
(i) shall be free from microorganisms or substances originating from microorganisms in amounts which may present a hazard to health in accordance with standards established by the Codex Alimentarius Commission;
(ii) shall not contain any other substance in amounts which may present a hazard to health in accordance with standards established by the Codex Alimentarius Commission.
5.3 It is recommended that the products covered by the provisions of this standard be prepared and handled in accordance with the appropriate sections of the Recommended International Code of Practice - General Principles of Food Hygiene (CAC/RCP 1-1969, Rev. 3-1997) and the following relevant Codes:
(i) The Recommended International Code of Practice for Lobsters (CAC/RCP 24-1978);
(ii) The Recommended International Code of Practice for the Processing and Handling of Quick Frozen Foods (CAC/RCP 8-1976);
(iii) The sections on the Products of Aquaculture in the Proposed Draft International Code of Practice for Fish and Fishery Products (under elaboration).8
In addition to the provisions of the General Standard for the Labelling of Prepackaged Foods (CODEX STAN 1-1985, Rev. 1-1991) the following specific provisions apply:
The product shall be designated:
(i) Lobster if derived from the genus Homarus;
(ii) Rock Lobster, Spiny Lobster or Crawfish if derived from species of the family Palinuridae;
(iii) Slipper Lobster, Bay Lobster or Sand Lobster if derived from species of the family Scyllaridae;
(iv) Norway Lobster if derived from the species Nephrops norvegicus.
(v) Squat Lobster if derived from the species Cervimunida johnii, Pleuroncodes monodon and Pleuroncodes planipes
6.1.1 There shall appear on the label, reference to the form of presentation in close proximity to the name of the product in such descriptive terms that will adequately and fully describe the nature of the presentation of the product to avoid misleading or confusing the consumer.
6.1.2 In addition to the specified labelling designations above, the usual or common trade names of the variety may be added so long as it is not misleading to the consumer in the country in which the product will be distributed.
6.1.3 Products shall be designated as cooked or raw as appropriate.
6.1.4 If the product has been glazed with sea-water, a statement to this effect shall be made.
6.1.5 The term "quick frozen", shall also appear on the label, except that the term "frozen" may be applied in countries where this term is customarily used for describing the product processed in accordance with subsection 2.2 of this standard.
6.1.6 The label shall state that the product should be maintained under conditions that will maintain the quality during transportation, storage and distribution.
Where the food has been glazed the declaration of net contents of the food shall be exclusive of the glaze.
The label shall include terms to indicate that the product shall be stored at a temperature of -18°C or colder.
Information specified above shall be given either on the container or in accompanying documents, except that the name of the food, lot identification, and the name and address of the manufacturer or packer as well as storage instructions shall always appear on the container.
However, lot identification, and the name and address may be replaced by an identification mark, provided that such a mark is clearly identifiable with the accompanying documents.
(i) Sampling of lots for examination of the product shall be in accordance with the FAO/WHO Codex Alimentarius Sampling Plans for Prepackaged Foods (AQL - 6.5) (CODEX STAN 233-1969). In the case of shell on lobster the sample unit is an individual lobster. In the case of shell-off lobster the sample unit shall be at least a 1 kg portion of lobster from the primary container. In the case of squat lobster the sampling unit shall be at least 1 kg portion.
(ii) Sampling of lots for examination of net weight shall be carried out in accordance with an appropriate sampling plan meeting the criteria established by the Codex Alimentarius Commission.
Samples taken for sensory and physical examination shall be assessed by persons trained in such examination and using procedures elaborated in Sections 7.3 through 7.6, Annex A and the Guidelines for the Sensory Evaluation of Fish and Shellfish in Laboratories (CAC/GL 31 - 1999).
The net weight (exclusive of packaging material) of each sample unit representing a lot shall be determined in the frozen state.
(Alternate Methods)
(1) As soon as the package is removed from frozen temperature storage, open immediately and place the contents under a gentle spray of cold water until all ice glaze that can be seen or felt is removed. Remove adhering water by the use of paper towel and weigh the product.
(2) The pre-weighed glazed sample is immersed into a water bath by hand, until all glaze is removed, which preferably can be felt by the fingers. As soon as the surface becomes rough, the still frozen sample is removed from the water bath and dried by use of a paper towel before estimating the net product content by second weighing. By this procedure thaw drip losses and/or re-freezing of adhering moisture can be avoided.
(3) (i) As soon as the package is removed from frozen temperature storage, place the product in a container containing an amount of fresh potable water of 27°C (80°F) equal to 8 times the declared weight of the product. Leave the product in the water until all ice is melted. If the product is block frozen, turn block over several time during thawing. The point at which thawing is complete can be determined by gently probing the block.
(ii) Weigh a dry clean sieve with woven wire cloth with nominal size of the square aperture 2.8 mm (ISO Recommendation R565) or alternatively 2.38 mm (U.S. No. 8 Standard Screen.)
(a) If the quantity of the total contents of the package is 500 g (1.1 lbs) or less, use a sieve with a diameter of 20 cm (8 inches).
(b) If the quantity of the total contents of the package is more than 500 g (1.1 lbs) use a sieve with a diameter of 30 cm (12 inches).
(iii) After all glaze that can be seen or felt has been removed and the lobsters separate easily, empty the contents of the container on the previously weighed sieve. Incline the sieve at an angle of about 20° and drain for two minutes.
(iv) Weigh the sieve containing the drained product. Subtract the mass of the sieve; the resultant figure shall be considered to be part of the net content of the package.
When declared on the label, the count shall be determined by counting all lobsters or tails in the primary container and dividing the count of lobster by the average deglazed weight to determine the count per unit weight.
The sample unit is thawed by enclosing it in a film type bag and immersing in water at room temperature (not greater than 35°C). The complete thawing of the product is determined by gently squeezing the bag occasionally so as not to damage the texture of the lobster, until no hard core or ice crystals are left.
The following procedures are based on heating the product to an internal temperature of 6570°C. The product must not be overcooked. Cooking times vary according to the size of the product and the temperature used. The exact times and conditions of cooking for the product should be determined by prior experimentation.
Baking Procedure: Wrap the product in aluminum foil and place it evenly on a flat cookie sheet or shallow flat pan.
Steaming Procedure: Wrap the product in aluminum foil and place it on a wire rack suspended over boiling water in a covered container.
Boil-in-Bag Procedure: Place the product into a boilable film-type pouch and seal. Immerse the pouch into boiling water and cook.
Microwave Procedure: Enclose the product in a container suitable for microwave cooking. If plastic bags are used check to ensure that no odour is imparted from the plastic bags. Cook according to equipment specifications.
The sample unit shall be considered as defective when it exhibits any of the properties defined below.
Greater than 10% of the weight of the lobster in the sample unit or greater than 10% of the surface area of the block exhibits excessive loss of moisture clearly shown as white or yellow abnormality on the surface which masks the colour of the flesh and penetrates below the surface, and cannot be easily removed by scraping with a knife or other sharp instrument without unduly affecting the appearance of the lobster .
The presence in the sample unit of any matter which has not been derived from lobster, does not pose a threat to human health, and is readily recognized without magnification or is present at a level determined by any method including magnification that indicates non-compliance with good manufacturing and sanitation practices.
Lobster affected by persistent and distinct objectionable odours or flavours indicative of decomposition or rancidity, or feed.
Distinct blackening of more than 10% of the surface area of the shell of individual whole or half lobster, or in the case of tail meat and meat presentations distinct black, brown, green or yellow discolourations singly or in combination, of the meat affecting more than 10% of the declared weight.
A lot shall be considered as meeting the requirements of this standard when:
(i) the total number of defectives as classified according to section 8 does not exceed the acceptance number (c) of the appropriate sampling plan in the Sampling Plans for Prepackaged Foods (AQL-6.5) (CODEX STAN 233-1969);
(ii) the total number of sample units not meeting the count or weight range designation as defined in Section 2.3 does not exceed the acceptance number (c) of the appropriate sampling plan in the Sampling Plans for Prepackaged Foods (AQL - 6.5) (CODEX STAN 233-1969);
(iii) the average net weight of all sample units is not less than the declared weight, provided there is no unreasonable shortage in any individual container;
(iv) the Food Additives, Hygiene and Labelling requirements of Sections 4, 5 and 6 are met.
1. Complete net weight determination, according to defined procedures in Section 7.3 (de-glaze as required).
2. Examine the frozen lobster for the presence of deep dehydration. Determine the percentage of lobster affected.
4. Examine product count and weight declarations in accordance with procedures in Section 7.4.
5. Assess the lobster for odour and discolouration as required.
6. In cases where a final decision regarding the odour/flavour cannot be made in the thawed state, a small portion of the sample unit (100 to 200 g) is prepared without delay for cooking and the odour/flavour confirmed by using one of the cooking methods defined in Section 7.6.
PROPOSED DRAFT CODE OF PRACTICE FOR FISH AND FISHERY PRODUCTS
(Sections at Step 5/8)
SECTION 2. DEFINITIONS | |
2.2 AQUACULTURE | |
Aquaculture |
means the farming during part or the whole of their life cycle of all aquatic animals, except mammalian species, aquatic reptiles and amphibians intended for human consumption, but excluding species covered in section 7 of this code. These aquatic animals are hereafter referred to as “fish” for ease of reference in section 2.2 and section 6; |
Aquaculture Establishment |
is any premises for the production of fish intended for human consumption, including the supporting inner infrastructure and surroundings under the control of the same management; |
Chemicals |
includes any substance either natural or synthetic which can affect the live fish, its pathogens, the water, equipment used for production or the land within the aquaculture establishment; |
Colouring |
means obtaining specifically coloured fish flesh by incorporating into the fish food a natural or artificial substance or additive approved for this purpose by the agency having jurisdiction; |
Diseased Fish |
means a fish on or in which pathological changes or other abnormalities are apparent; |
Extensive farming |
means raising fish under conditions or little or incomplete control over such factors as water flow, number and weight of species raised, and low quality and quantity of nutrient inputs; |
Feed Additives |
means chemicals other than nutrients for fish which are approved for addition to their feed; |
Fish farm |
is an aquaculture production unit (either land-or water based); usually consisting of holding facilities (tanks, ponds, raceways, cages), plant (buildings, storage, processing), service equipment and stock; |
Fish Feed |
means fodder intended for fish in aquaculture establishments, in any form and of any composition; |
Good Aquaculture (or Good Fish Farming ) Practices |
are defined as those practices of the aquaculture sector that are necessary to produce quality food products conforming to food laws and regulations |
Harvesting |
Operations involving taking the fish from the water |
|
Intensive farming |
| |
Official Agency Having Jurisdiction |
means the official authority or authorities charged by the government with the control of food hygiene (sometimes referred to as the competent authority) as well as/or with sanitation in aquaculture; | |
Pesticide |
means any substance intended for preventing, destroying, attracting, repelling or controlling any pest including unwanted species of plants or animals during the production, storage, transport, distribution and processing of food, agricultural commodities, or animal feeds or which may be administered to animals for the control of ectoparasites. The term normally excludes fertilisers, plant and animal nutrients, food additives, and veterinary drugs; | |
Pesticide Residue |
means any specified substance in food, agricultural commodities, or animal feed resulting from the use of a pesticide. The term includes any derivatives of a pesticide, such as conversion products, metabolites, reaction products, and impurities; | |
Residues |
means any foreign substances including their metabolites, which remain in fish prior to harvesting as a result of either application or accidental exposure. | |
Semi-intensive farming |
means raising fish under conditions of partial control over dietary nutrient inputs by including external fertilizer and/or supplementary diet nutrient inputs, whereby fish growth is dependent upon the consumption of endogenously supplied live food organism and externally supplied feed as supplementary source of dietary nutrients. | |
Stocking density |
is the amount of fish stocked per unit of area or volume | |
Veterinary Drug |
means any substance applied or administered to any food-producing animal, such as meat or milk-producing animals, poultry, fish or bees, whether used for therapeutic, prophylactic or diagnostic purposes or for modification of physiological functions or behaviour; | |
Withdrawal Time |
is the period of time necessary between the last administration of a veterinary drug to fish, or exposure of these animals to a veterinary drug, and harvesting of them to ensure that the concentration of the veterinary drug in their edible flesh intended for human consumption, complies with the maximum permitted residue limits.. | |
2.6 QUICK-FROZEN COATED FISH PRODUCTS |
||
Batter |
liquid preparation from ground cereals, spices, salt, sugar and other ingredients and/or additives for coating. Typical batter types are: non-leavened batter and leavened batter. | |
Breading |
dry breadcrumbs or other dry preparations mainly from cereals with colorants and other ingredients used for the final coating of fishery products. Typical breading types are: free-flowing breading, coarse breading, flour-type breading. | |
Coating |
covering the surface of a fishery product with batter and/or breading. | |
Pre-frying |
frying of breaded and battered fishery products in an oil bath in a way so that the core remains frozen. | |
Sawing |
cutting (by hand or fully mechanised) of regular shapes QF fish blocks into pieces suitable for later coating. | |
SECTION 6 - AQUACULTURE PRODUCTION
Preamble
Aquaculture establishments should operate in a responsible way such that they comply with the recommendations of the Code of Conduct for Responsible Fisheries (FAO. Rome. 1995) in order to minimize any adverse impact on human health and environment including any potential ecological changes.
Fish farms should operate effective fish health and welfare management. Fry and fingerlings should be disease free and should comply with the OIE Codes of Practice (International Aquatic Animal Health Code, 6th Edition , 2003). Growing fish should be monitored for disease. When using chemicals at fish farms, special care should be exercised so that these substances are not released into the surrounding environment.
Whilst the fish health, environment, and ecological aspects are important considerations in aquaculture activities, this section focuses on food safety and quality aspects.
This Section of the Code applies to industrialised and commercial aquaculture production, producing all aquatic animals, except mammalian species, aquatic reptiles and amphibians for direct human consumption, but excluding bivalve molluscs covered in section 7 of the code, hereafter referred to as “fish”] (1) that are intended for direct human consumption. Such intensive or semi-intensive aquaculture systems use higher stocking densities, stock from hatcheries, use mainly formulated feeds and may utilise medication and vaccines. This Code is not intended to cover extensive fish farming systems that prevail in many developing countries or integrated livestock and fish culture systems. This section of the code covers the feeding, growing, harvesting and transport stages of aquaculture production. Further handling and processing of fish are covered elsewhere in the code.
In the context of recognising controls at individual processing steps, this section provides examples of potential hazards and defects and describes technological guidelines, which can be used to develop control measures and corrective action. At a particular step only the hazards and defects, which are likely to be introduced or controlled at that step, are listed. It should be recognised that in preparing a HACCP and/or DAP plan it is essential to consult Section 5 which provides guidance for the application of the principles of HACCP and DAP analysis. However, within the scope of this Code of Practice it is not possible to give details of critical limits, monitoring, record keeping and verification for each of the steps since these are specific to particular hazards and defects.
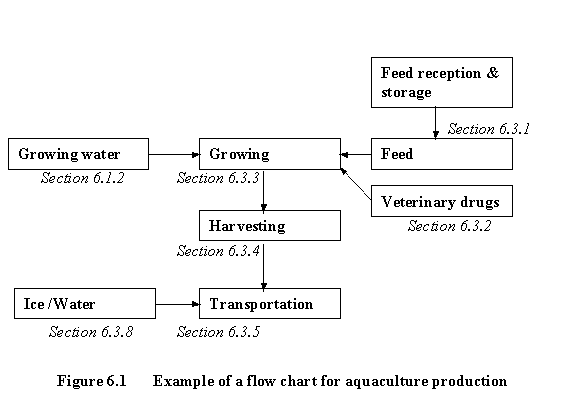
The Example flow diagram will provide guidance to some of the common steps in aquaculture production.
This flow chart is for illustrative purpose only. For implementation of HACCP principles, a complete and comprehensive flow chart has to be drawn up for each product. References correspond to relevant Sections of the Code.
This flow chart is for illustrative purpose only. For implementation of HACCP principles, a complete and comprehensive flow chart has to be drawn up for each product. References correspond to relevant Sections of the Code.
![]()
![]() 6.1 GENERAL
6.1 GENERAL
The general principles in Section 3 apply to aquaculture production, in addition to the following:
6.1.1 Site selection
• The siting, design and construction of fish farms should follow principles of good aquaculture practice, appropriate to species.
• The physical environment with regard to temperature, current and depth should also be checked since different species have different environmental requirements.
• Fish farms should be located in areas where the risk of contamination by chemical, physical or microbiological hazards is minimal and where sources of pollution can be controlled.
• Soil for the construction of earthen ponds should not contain such concentrations of chemicals and other substances, which may lead to the presence of unacceptable levels of contamination in fish.
• Ponds should have separated inlets and discharge canals, so that water supplies and effluent are not mixed.
• Water inlets and outlets to ponds should be screened to prevent the entrance of unwanted species.
• Fertilizers, liming materials or other chemicals and biological materials, should be used in accordance with good aquaculture practice.
• All sites should be operated in an environmentally acceptable way as to not impact human health.
6.1.2 Growing Water Quality
• The water in which fish are raised should be suitable for the production of products which are safe for human consumption.
• Fish farms should not be sited where there is a risk of contamination of the water in which fish are reared.
• Appropriate design and construction of fish farms should be adopted to ensure control of hazards and prevention of water contamination.
6.1.3 Source of Fry and Fingerlings
• The source of postlarvae, fries and fingerlings should be such to avoid the carryover of potential hazards into the growing stocks.
6.2 IDENTIFICATION OF HAZARDS AND DEFECTS
Consumption of fish and fishery products is associated with a variety of human health hazards. Broadly the same hazards are present in aquaculture products as in corresponding varieties caught in the wild (Section 4.1). The risk of harm from a particular hazard might be increased, under some circumstances, in aquaculture products compared with fish caught in the wild - for instance the presence of residues of veterinary drugs. High stocking densities, compared with the natural situation, might increase the risk of cross-infection of pathogens within a population of fish. On the other hand, farmed fish can also present a lower risk of harm. In systems where the fish receive artificial feeds, the risks associated with transmission of hazards through the food consumed by the fish could be reduced. For example, infection with nematode parasites is absent from, or very much reduced in, farmed salmon compared with salmon caught in the wild. Raising fish in cages in the marine environment poses few hazards and low risks. In closed recirculation systems hazards are even further reduced. In those systems, the water is constantly refreshed and reused and water quality is controlled within safe measures.
6.2.1 Hazards
Aquaculture products possess broadly the same hazards that are present in corresponding varieties caught in the wild (Section 5.3.3.1). Potential hazards that are specific to aquaculture products include: residues of veterinary drugs in excess of recommended guidelines and other chemicals used in aquaculture production, contamination of faecal origin where the facilities are close to human habitation or animal husbandry.
6.2.2 Defects
The same defects are present in aquaculture products as in corresponding varieties caught in the wild (Section 5.3.3.1). A defect which may occur is objectionable odours/flavours . During transport of live fish, it is important to reduce stress, as stressing fish can lead to deterioration in quality. Also, care should be taken to minimise physicaldamage to fish as this can lead to bruising.
6.3 PRODUCTION OPERATIONS
6.3.1 Feed Supply
Feeds used in aquaculture production should comply with the Codex ‘Draft Code of Practice of Good Animal Feeding’ (under development in the Ad Hoc Intergovernmental Task Force on Animal Feeding).
Potential Hazards: |
Chemical contamination, mycotoxins and microbiological pathogens . |
Potential Defects: |
Decomposed feeds, fungal spoilage |
Technical Guidance: |
|
• Feed and fresh stocks should be purchased and rotated and used prior to the expiry of their shelf life.
• Fish feeds should be stored in cool and dry areas to prevent spoilage, mould growth and contamination.
• Feed ingredients should not contain unsafe levels of pesticides, chemical contaminants, microbial toxins, or other adulterating substances.
• Industrially produced complete feeds and industrially produced feed ingredients should be properly labelled. Their composition must fit the declaration on the label and they should be hygienically acceptable.
• Ingredients should meet acceptable, and if applicable, statutory standards for levels of pathogens, mycotoxins, herbicides, pesticides and other contaminants which may give rise to human health hazards.
• Only approved colours of the correct concentration should be included in the feed.
• Moist feed or feed ingredients should be fresh and of adequate chemical and microbiological quality.
• Fresh or frozen fish, fish silage, offal from fish should reach the fish farm in an adequate state of freshness.
• Rejects from animal slaughterhouses must be processed by an approved procedure, prior to acceptance.
• Feed which is compounded industrially or at the fish farm, should contain only such additives, growth promoting substances, fish flesh colouring agents; anti-oxidising agents, caking agents or veterinary drugs which are permitted for fish by the official agency having jurisdiction.
• Products should be registered with the relevant national authority as appropriate.
• Storage and transport conditions should conform to the specifications on the label.
• Veterinary drug and other chemical treatments should be done in accordance with recommended practices and comply with national regulations.
• Farmers should follow manufacturers’ instructions on the use of veterinary drugs or medicated feeds.
• Product tracing of all feed ingredients should be assured by proper record keeping.
6.3.2 Veterinary Drugs
Potential Hazards: |
Rresidues of veterinary drugs |
Potential Defects: |
Unlikely |
Technical Guidance: |
|
• All veterinary drugs for use in fish farming should comply with national regulations and international guidelines (in accordance with the Recommended International Code of Practice for Control of the Use of Veterinary Drugs (CAC/RCP 38-1993) and the Codex Guidelines for the Establishment of a regulatory programme for control of veterinary drug residues in foods (CAC/GL 16-1993) ).
• Prior to administrating veterinary drugs, a system should be in place to monitor the application of the drug to ensure that the withdrawal time for the batch of treated fish can be verified.
• Veterinary drugs or medicated feeds should be used according to manufacturers’ instructions, with particular attention to withdrawal periods.
• Products should be registered with the appropriate national authority.
• Products should only be prescribed or distributed by personnel authorised under national regulations.
• Storage and transport conditions should conform to the specifications on the label.
• Control of diseases with drugs should be carried out only on the basis of an accurate diagnosis
• Records should be maintained for the use of veterinary drugs in aquaculture production.Pre-slaughter control is a method of controlling drug residues in fish. If the average drug concentration in tested fish is above the MRL, (or in some countries, by an industry imposed lower level), slaughter of the batch has to be postponeduntil the fish complies with the MRLA post slaughter control should reject all fish that do not comply with the requirements set for veterinary drug residues by the Codex Alimentarius.
6.3.3 Growing
Potential Hazards: |
Microbiological pathogens and chemical contamination |
Potential Defects: |
Abnormal colour, muddy flavour, physical damage |
Technical Guidance: |
|
• Source of postlarvae, fries and fingerlings should be controlled to assure healthy stock.
• Stocking densities should be based on culture techniques, fish species, size and age, carrying capacity of the fish farm, anticipated survival and desired size at harvesting.
• Dead or diseased fish should be disposed in a sanitary manner that will discourage the spread of disease and investigate cause of death.
• Good water quality should be maintained by using stocking and feeding rates that do not exceed the carrying capacity of the culture system.
• Growing water quality should be monitored regularly, so as to identify potential hazards and defects.
• The fish farm should have a management plan that includes a sanitation programme, monitoring and corrective actions, defined fallowing periods, appropriate use of agrochemicals, verification procedures for fish farming operations and systematic records should be kept.
• Equipment such as cages and nets should be designed and constructed to ensure minimum damage during the growing stage.
6.3.4 Harvesting
Potential Hazards: |
Unlikely |
Potential Defects: |
Physical damage, physical/biochemical change due to stress of live fish |
Technical Guidance: |
|
• Appropriate harvesting techniques should be applied to minimise physical damage and stress.
• Live fish should not be subjected to extremes of heat or cold or sudden variations in temperature.
• Fish should be free from excessive mud and weed soon after being harvested by washing it with clean seawater or fresh water under suitable pressure.
• Fish should be handled in a sanitary manner according to the guidelines in Section 4 of the Code..
• Harvesting should be rapid so that fish are not exposed unduly to high temperatures.
6.3.5 Holding and Transportation
Potential Hazards: |
microbiological pathogens and chemical contamination |
Potential Defects: |
physical damage, physical/biochemical change due to stress of live fish |
Technical Guidance: |
|
• Quality defects can occur in fish that are subjected to stress.
• Fish should be transported without undue delay.
• Equipment for the transport of live fish should be designed for rapid and efficient handling without causing physical damage or stress.
• Records for transport of fish should be maintained to ensure full product tracing.
• Fish should not be transported with other products which might contaminate them.
6.3.6 Storage and transport of live fish
This section is designed for the storage and transportation of live fish originating from aquaculture or capture.
Potential Hazards: |
microbiological pathogens, biotoxins, chemical contamination (e.g. oil, cleaning and disinfecting agents) |
Potential Defects: |
Dead fish, physical damage, off flavours, physical/biochemical change due to stress of live fish |
Technical Guidance: |
|
• Only healthy and not damaged fish should be chosen for storage and transport of live fish. Damaged, sick and dead fish should be removed before introduction to the holding or conditioning tanks.
• Holding tanks should be checked regularly during storage and transportation. Damaged, sick and dead fish should be removed immediately when found. (2)
• Clean water utilised to fill holding tanks, or to pump fish between holding tanks, or for conditioning fish, should be similar in properties and composition to the water from where the fish was originally taken to reduce fish stress.
• Water should not be contaminated with either human sewage or industrial pollution. Holding tanks and transportation systems should be designed and operated in a hygienic way to prevent contamination of water and equipment.
• Water in holding and conditioning tanks should be well aerated before fish is transferred into them.
• Where seawater is used in holding or conditioning tanks, for species prone to toxic algae contamination, seawater containing high level of cell concentrations should be avoided or filtered properly.
• No fish feeding should occur during storage and transport of live fish. Feeding will pollute water of holding tanks very quickly.
• Material of holding and conditioning tanks, pumps, filters, piping, temperature control system, intermediate and final packaging or containers should not be harmful to fish or present hazards to humans.
• All equipment and facilities should be cleaned and disinfected regularly and as needed.
6.3.7 Live fish stored and transported at ambient temperature
Potential Hazards: |
microbiological pathogens, biotoxins, chemical contamination (e.g. oil, cleaning and disinfecting agents) |
Potential Defects: |
Dead fish, physical damage, off flavours , physical/biochemical change due to stress of live fish |
Technical Guidance: |
|
• Depending on the source of water, requirements of the species and time of storage and/or transport, it could be necessary to re-circulate the water and filter it through mechanical and/or biofilters.
• Water intake of holding tanks on board of vessels should be located so as to avoid contamination from vessel’s sewage, waste and engine cooling discharge. Pumping of water should be avoided when the vessel comes into harbour or sailing through waters near sewage or industrial discharges. Equivalent precautions should be adopted for water intake on land.
• Facilities for storing and transportation (holding tanks) of live fish should be capable to:
- maintain the oxygenation of water in the holding tanks through either, continuous water flow, direct oxygenation (with oxygen or air bubbling), or regularly and as needed changing of the water of the holding tank;
- maintain the temperature of storage and transport, for species sensitive to temperature fluctuations. It may be necessary to insulate the holding tanks and install a temperature control system;
- keep water in reserve which might be needed in case the holding tank should drain. The volume in fixed facilities (storage) should be at least of the same volume of the total holding tanks in operation. The volume in land transport facilities should be at least capable to compensate water for evaporation, leakage, purges, filter cleaning and eventual mixing of water for control purposes;
• It could be necessary to separate fish in individual tanks or tie them in ways that prevent damage, particularly, in the case of species that exhibit phenomena like cannibalism, strong territoriality or hyperactivity when under stress (an alternative method is reduction of temperature see 6.3.8).
6.3.8 Live fish stored and transported at low temperatures
Potential Hazards: |
microbiological pathogens, biotoxins, chemical contamination (e.g. oil, cleaning and disinfecting agents) |
Potential Defects: |
Dead fish, , physical damage, off flavours, physical/biochemical change due to stress of live fish |
Technical Guidance: |
|
• Conditioning of the fish at low temperatures should be done according to the characteristics of the species (minimum temperature, cooling rate, water/humidity requirements, packaging conditions). Conditioning is a biological operation to reduce the metabolic rate of the fishminimising the stress to them.
• The level of temperature to be reached should be in accordance with the species, transport and packaging conditions. There is a range of temperature in which fish do not exhibit or have reduced physical activity. The limit is attained at the temperature at which the metabolic rate of the fishis minimised without causing adverse effects to them (basal metabolic rate).
• When performing conditioning, only anaesthetics and procedures accepted by the regulations should be utilised.
• Conditioned fish should be packed without delay in proper insulated containers.
• Remaining water or water for use with packaging material for conditioned fish should be clean, of similar composition and pH to the water the fish was taken from, but to the temperature of storage.
• Water absorbent pads, shredded wood, wood shavings or sawdust and tying material that may be utilised for packaging conditioned fish should be clean, first use, free of possible hazards and be wet right at the time of packaging.
• Conditioned and packed fish should be stored or transported under conditions that assure proper temperature control.
SECTION 10 - PROCESSING OF QUICK-FROZEN COATED FISH PRODUCTS
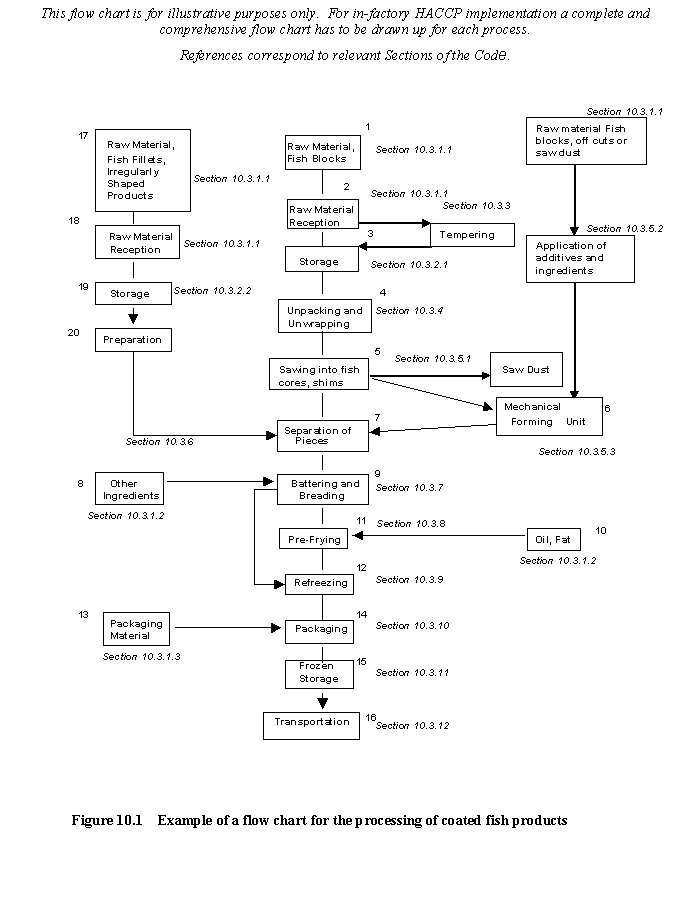
In the context of recognising controls at individual processing steps, this section provides examples of potential hazards and defects and describes technological guidelines, which can be used to develop control measures and corrective action. At a particular step only the hazards and defects, which are likely to be introduced or controlled at that step, are listed. It should be recognised that in preparing a HACCP and/or DAP plan it is essential to consult Section 5 which provides guidance for the application of the principles of HACCP and DAP analysis. However, within the scope of this Code of Practice it is not possible to give details of critical limits, monitoring, record keeping and verification for each of the steps since these are specific to particular hazards and defects.
10.1 GENERAL ADDITION TO PRE-REQUISITE PROGRAMME
• conveyor systems used to transport uncoated and coated fish should be designed and constructed to prevent damaging and contamination of the products;
• shims sawn for formed fish production and held for tempering should be kept at temperatures that will prevent deterioration of the essential quality of the product;
• if the whole process is run continuously an adequate number of processing lines should be available to avoid interruptions and batch-wise processing. If the process has to be interrupted, intermediate products have to be stored under deep-frozen conditions until being further processed;
• pre-frying baths, freezing cabinets used for re-freezing should be equipped with permanent temperature and belt speed control device;
• the proportion of sawdust should be minimised by using appropriate sawing equipment;
• sawdust should be kept well separated from fish cores used for coated products, should be temperature controlled, not stay too long at ambient temperature and should be stored preferably in frozen state prior to further processing into suitable products.
10.2 IDENTIFICATION OF HAZARDS AND DEFECTS
Refer also to Section 5.3.3 and Appendix XI.
This Section describes the main hazards and defects specific to QF coated fish and shellfish.
10.2.1 Hazards
Refer also to Section 5.3.3.1.
The production and storage of batter for application to fish portions, fillets, etc., may involve either rehydratation of a commercial batter mix or preparation from raw ingredients. During the preparation of this batter and its use, the potential hazard for the possible growth and toxins production of Staphylococcus aureus and Bacillus cereus must be controlled.
10.2.2 Defects
Potential defects are outlined in the essential quality, labelling and composition requirements described in the relevant Codex Standard for Quick Frozen Fish Sticks (Fish Fingers), Fish Portions and Fish Fillets – Breaded or in Batter (CODEX STAN. 166-1989).
End product specifications outlined in Appendix XI describe optional requirements specific to QF coated fishery products.
10.3 PROCESSING OPERATIONS
Refer to figure 10.1 for an example of a flow chart for coated fish product processing.
10.3.1. Reception
10.3.1.1 Fish
Potential Hazards: |
chemical and biochemical contamination,histamine ; |
Potential Defects: |
tainting, block irregularities, water and air pockets, packaging material, foreign matter, parasites, dehydratation, decomposition; |
Technical Guidance: |
|
• Temperatures of all incoming lots should be recorded;
• Packaging material of frozen products should be examined for dirt, tearing and evidence of thawing;
• Cleanliness and suitability of the transport vehicle to carry frozen fish products should be examined;
• Use of temperature recording devices with the shipment is recommended;
• Representative samples should be taken for further examination for possible hazards and defects;
10.3.1.2 Other Ingredients
Potential Hazards: |
chemical, biochemical and microbiological contamination |
Potential Defects: |
mould , colour deviations, filth, sand |
Technical Guidance: |
|
• breading and batter should be inspected for broken packaging material, signs of rodent and insect infestations and other damage such as dirt on packaging materials and wetness;
• cleanliness and suitability of the transport vehicle to carry food products should be examined;
• representative samples of the ingredients should be taken and examined to ensure that the product is not contaminated and meets specifications for use in the end product;
• ingredients should be shipped on transportation vehicles that are suitable for handling food products and ingredients. Vehicles that have previously hauled potentially unsafe or hazardous material should not be used for hauling food products or ingredients.
10.3.1.3 Packaging Materials
Potential Hazards: |
foreign matter |
Potential Defects: |
tainting of products |
Technical Guidance: |
|
• packaging material used should be clean, sound, durable, sufficient for its intended use and of food grade material;
• for pre-fried products it should be impermeable for fat and oil;
• cleanliness and suitability of the transport vehicle to carry food packaging material should be examined.
• pre-printed labelling and packaging material should be examined for accuracy
10.3.2 Storage of Raw Material, Other Ingredients and Packaging Materials
10.3.2.1 Fish (Frozen Storage)
Refer to Section 8.1.3
10.3.2.2 Fish (chilled storage)
For storage of nonfrozen fish, refer to section 8.1.2.
10.3.2.3 Other Ingredients and Packaging Materials
Potential Hazards: |
biological, physical and chemical contamination |
Potential Defects: |
loss of quality and characteristics of ingredients, rancidity |
Technical Guidance: |
|
• all other ingredients and packaging material should be stored in a dry and clean place under hygienic conditions;
• all other ingredients and packaging material should be stored appropriately in terms of temperature and humidity;
• a systematic stock rotation plan should be developed and maintained to avoid out of date materials;
• ingredients should be protected from insects, rodents and other pests;
• defective ingredients and packaging material should not be used.
10.3.3. Frozen Fish Block/Fillet tempering
Potential Hazards: |
Unlikely |
Potential Defects: |
Incorrect dimension due to sawing of over softened fish flesh (applies to fish sticks) |
Technical Guidance: |
|
• Depending on the use of the fish, the tempering of frozen fish blocks/fillets should be carried out in a manner which will allow the temperature of the fish to rise without thawing.
• Tempering block/fillets of frozen fish in chilled storage is a slow process that usually requires at least 12 hours or more
• Over softening of the outer layers is undesirable (poor performance during sawing) and should be avoided. It could be avoided if facilities used for tempering are maintained at a temperature of 0 – 4° C and if fish blocks/fillets are stacked in layers.
• microwave tempering is an alternate method but should also be controlled to prevent softening of outer layers.
10.3.4 Unwrapping, Unpacking
Potential Hazards: |
Microbiological contamination |
Potential Defects: |
remaining undetected packaging material, contamination by filth |
Technical Guidance: |
|
• during unwrapping and unpacking of fish blocks care should be given not to contaminate the fish;
• special attention has to be given to cardboard and/or plastic material partly or fully embedded in the blocks;
• all packaging material should be disposed of properly and promptly.
• Protect wrapped, unwrapped and unpacked fish blocks when cleaning and sanitizing processing lines during breaks and between shifts if the production process is interrupted.
10.3.5 Production of Fish Core
10.3.5.1 Sawing
Potential Hazards: |
foreign material (metal or plastic parts of saws) |
Potential Defects: |
irregularly shaped pieces or portions |
Technical Guidance: |
|
• sawing instruments should be kept in clean and hygienic conditions;
• saw-blades must be inspected regularly, to avoid tearing of the product and breakage;
• saw dust must not collect on the saw-table and must be collected in special containers if used for further processing;
• sawn shims used to form irregularly shaped fish cores by mechanical pressure should be kept in clean, hygienic conditions until further manufacturing.
10.3.5.2. Application of additives and Ingredients
Also refer to Section 8.4.3
Potential Hazards: |
foreign material, microbiological contamination |
Potential Defects: |
Incorrect addition of additives |
Technical Guidance: |
|
• The temperature of the product in the mixing process should be adequately controlled to avoid the growth of pathogenic bacteria.
10.3.5.3 Forming
Potential Hazards: |
foreign material (metal or plastic from machine) and/or microbiological contamination (fish mixture only) |
Potential Defects: |
poorly formed fish cores, cores subject to too much pressure (mushy, rancid) |
Technical Guidance: |
|
Forming of fish cores is a highly mechanised method of producing fish cores for battering and breading. It utilises either hydraulic pressure to force shims (sawn portions of fish blocks) into moulds that are ejected onto the conveyor belt or mechanical forming of fish mixtures.
• forming machines should be kept in hygienic conditions;
• formed fish cores should be examined closely for proper shape, weight and texture.
10.3.6 Separation of Pieces
Potential Hazards: |
Unlikely |
Potential Defects: |
adhering pieces or portions |
Technical Guidance: |
|
• the fish flesh cores cut from the blocks or fish fillets or other irregular shaped QF fish material must be well separated from each other and should not adhere to each other;
• fish cores that are touching each other going through the wet coating step should be removed and placed back on the conveyor in order to get a uniform batter coat and a uniform breading pick-up;
• cored fish should be monitored for foreign material and other hazards and defects before coating.
• Remove from production any broke, misshape or out of specification peaces.
10.3.7 Coating
In industrial practice the order and the number of coating steps may differ and may therefore deviate considerably from this scheme.
10.3.7.1 Wet Coating
Potential Hazards: |
Microbiological contamination |
Potential Defects: |
Insufficient cover or excessive cover of coating |
Technical Guidance: |
|
• fish pieces must be well coated from all sides;
• surplus liquid, which should be reused, must be re-transported under clean and hygienic conditions;
• surplus liquid on fish pieces should be removed by clean air;
• viscosity and temperature of hydrated batter mixes should be monitored and controlled within certain parameters to effect the proper amount of breading pick-up;
• to avoid microbiological contamination of the hydrated batter, appropriate means should be adopted to ensure that significant growth does not take place, such as temperature control, dumping liquid contents and regular or scheduled clean-ups and/or sanitation during the manufacturing shift.
10.3.7.2 Dry Coating
Potential Hazards: |
microbiological contamination |
Potential Defects: |
insufficient coating or excessive coating |
Technical Guidance: |
|
• dry coating must cover the whole products and should stick well on the wet coating;
• surplus coating is removed by blowing away with clean air and/or by vibration of conveyors and must be removed in a clean and hygienic way if further use is intended;
• flow of breading from the application hopper should be free, even and continuous;
• coating defects should be monitored and be in accordance to Codex Standard for Frozen Fish Fingers, Fish Portions and Fish Fillets – Breaded or in Batter (Codex Standard 166-1989);
• the proportion of breading and fish core should be in accordance to Codex Standard for Frozen Fish Fingers, Fish Portions and Fish Fillets – Breaded or in Batter (Codex Standard 166-1989).
10.3.8 Pre-Frying
There are some variations in industrial production for the frying process in so far that QF coated products are completely fried including fish core and re-frozen later. For this case alternative hazards and defects have to be described and not all statements in this section apply. In some regions it is common practice to manufacture raw (not pre-fried) coated fish products.
Potential Hazards: |
Unlikely |
Potential Defects: |
over-oxidised oil, insufficient frying, loosely adhering coating, burnt pieces and portions |
Technical Guidance: |
|
• frying oil should have a temperature between approx. 160°C and 195°C;
• coated fish pieces should remain in frying oil for sufficient time depending on the frying temperature to get a satisfying colour, flavour, and structure to adhere firmly to the fish core, but core should be kept frozen throughout the whole time;
• frying oil has to be exchanged when colour becomes too dark or when concentration of fat degradation products exceeds certain limits;
• remains from coating which concentrate at the bottom of the frying bath have to be removed regularly to avoid partial dark coloration on coated products caused by upwelling of oil;
• excessive oil should be removed from coated products after pre-frying by a suitable device.
10.3.9 Re-freezing- Final Freezing
Potential Hazards: |
foreign material |
Potential Defects: |
Insufficient freezing leads to sticking of units together or to walls of freezing equipment and facilitates mechanical removal of breading/batter |
Technical Guidance: |
|
• re-freezing to -18°C or lower of the whole product should take place immediately after pre-frying;
• products should be allowed to stay sufficient time in freezer cabinet to assure core temperature of products of -18°C or lower;
• cryogenic freezers should have sufficient compressed gas flow to effect proper freezing of the product;
• processors that utilise blast freezers may package the product in the consumer containers before freezing.
10.3.10 Packing and Labelling
Refer to Section 8.2.3 "Labelling", Section 8.4.4 "Wrapping and Packing" and Section 8.2.1. “Weighing”.
Potential Hazards: |
Microbiological contamination |
Potential Defects: |
Under- or over-packing, improper sealed containers, wrong or misleading labelling |
Technical Guidance: |
|
• packaging should be made without delay after refreezing under clean and hygienic conditions. If packaging is made later (e.g. batch processing) re-frozen products should be kept under deep frozen conditions until being packed;
• packages should be checked regularly by weight control, end products should be checked by a metal detector and/or other detection methods if applicable;
• packaging of cartons or plastic bags to master shipping containers should be done without delay and under hygienic conditions;
• both consumer packages and shipping containers should be appropriately lot coded for product tracing in the event of a product recall.
10.3.11 Storage of End Products
Also refer to Section 8.1.3.
Potential Hazards: |
Unlikely |
Potential Defects: |
texture and Flavour deviations due to fluctuations in temperature, deep freezer burn, cold store flavour, cardboard flavour |
Technical Guidance: |
|
• all end products should be stored at frozen temperature in a clean, sound and hygienic environment;
• severe fluctuations of storage temperature (greater than 3°C) has to be avoided;
• too long storage time (depending on fat content of species used and type of coating) should be avoided;
• products should be properly protected from dehydration, dirt and other forms of contamination;
• all end products should be stored in the freezer to allow proper air circulation.
10.3.12 Transport of End Product
Also refer to Section 3.6.” Transportation” and Section 17 “ Transport” under elaboration
Potential Hazards: |
Unlikely |
Potential Defects: |
thawing of frozen product |
Technical Guidance: |
|
• during all transportation steps deep-frozen conditions should be maintained -18°C (maximum fluctuation ± 3°C) until final destination of product is reached;
• cleanliness and suitability of the transport vehicle to carry frozen food products should be examined;
• use of temperature recording devices with the shipment is recommended.
ALINORM 04/27/18
APPENDIX VI
PROPOSED DRAFT AMENDMENTS IN THE STANDARD FOR SALTED FISH AND DRIED SALTED FISH OF THE GADIDAE FAMILY OF FISHES
(At Step 5 of the Procedure)
7. SAMPLING, EXAMINATION AND ANALYSES
Section 7.1 Sampling is extended with one paragraph.
New
(iii) Each sampled fish is packed in a plastic bag which is sealed with tape.
The sampled fish(es) must be cooled or refrigerated from the time of sampling to the time of analysis.
The analysis must be performed within 48 hours after the fish has been sampled.
Section 7.4 Determination of Salt Content is moved to Section 7.5, and Section 7.4 is renamed Determination of Water Content in Whole Fish by Cross Section Method.
New
Section 7.4 Determination of Water Content in Whole Fish by Cross Section Method
The fish is cut in sections as described in method. The sections are cut in smaller bits to a collected sample. The water content of the collected sample is determined by drying. Examinations and experience have shown that the water content of this collected sample is closed to the “true” water content of the fish.
- Soft brush
- Basins (steel, glass, porcelain)
- Scissors
- Band saw
- Knife
- Weight, 1 g precision
- Analytical weight (4 decimals)
- Oven. 103-105°C
- Desiccator
Salt particles on the surface of the fish are brushed away.
The weight of the fish is determined to 1 g accuracy.
The length of the fish is measured as the distance between the cleft in the tail and a line drawn between the tips of the earbones.
(i) The sampling of the fish is described in the enclosed figure.
A) Wet salted fish is sliced in sections by knife
B) Salted and dried salted fish is sliced in sections by band saw.
1) A section of 20mm measured from a line drawn between the earbones, dotted line on figure, is cut.
2) The next cut is a 40 mm section.
3) A 2 mm section is cut from the front part of the 40 mm section and collected (See 7. comments).
4) The next cut is a new cut of a 40 mm section.
5) A 2 mm section is cut from the front part of the 40 mm section and collected.
6) The entire fish is cut in 40 mm sections from which are cut 2 mm sections (see enclosed figure).
7) All sections of 2mm, marked II, IV, VI, VIII in the figure, even numbers, are collected to a collected sample.
(ii) The 2mm sections in the collected sample are cut with scissors in smaller pieces directly in tared basins just after the fish is cut.
(iii) The basins containing the sample are weighted.
(iv) The basins containing the samples are put in the oven at 103-105°C for drying to constant weight (18 hours over night).
(v) The basins are taken from the oven to a desiccator.
(vi) The basins are weighted.
5. Calculation of results
In the equation of the calculation of results the following symbols are used:
W1 = Weight of fish and basins before drying, g.
W2 = Weight of fish and basins after drying, g.
Ws = Weight of tared basins, g
The water content in the fish is calculated by using the equation:
Water content, g/100g = 100*(W1-W2)
(W1 – Ws)
The result is reported with 1 decimal, together with the length and the weight of the analysed fish.
6. Control analysis of whole fish
As a reference method should be used which include drying of the whole fish.
7. Comments
The fish must be placed at a temperature +1 - +4 ° C in a store packed in plastic bags before analysis. The analysis must be performed as soon as possible after the fish has been sampled.
It might be difficult to cut sections of 2 mm when the fish has a water content above 50% but the section must be close to 2 mm.
To minimise the loss of water from the 2mm sections it is important to weight the collected sample immediately after the fish is cut in sections.
Amendments in section 7.5:
Delete old 7.4.3, replace it with new 7.5.3.
New
7.5 Determination of Salt Content
3. Preparation of sample
Before preparing a subsample adhering salt crystals should be removed by brushing from the surface of the sample without using water.
If only the salt content is to be determined the entire fish should be subjected to a systematic cutting in slices as described in Section 7.4 Determination of water content part 4. Procedures point (i) to (ii).
If both the water content and the salt content are to be determined in the sample, two subsamples must be collected. The subsample for the water content determination is first collected as described in Section 7.4. The subsample for the salt content determination is collected by cutting 2 mm slices from each of the remaining 38 mm sections given uneven number III, V, VII, etc in the Figure of Section 7.4.
The whole collected subsample of 2 mm slices for the salt content determination should be thoroughly homogenised preferably by using an electric homogeniser.
Determination should be performed at least in duplicate.
ALINORM 04/27/18
APPENDIX VII
DRAFT AMENDMENT TO THE STANDARD FOR QUICK FROZEN FISH STICKS (FISH FINGERS), FISH PORTIONS AND FISH FILLETS – BREADED OR IN BATTER
(At Step 7 of the Procedure)
6. LABELLING
6.1.3 The proportion of fish content should be declared on the label.
7. SAMPLING, EXAMINATION AND ANALYSIS
7.4 Estimation of Fish Content
According to AOAC Method 966.15. In cases where there is some remaining doubts over the composition of the fish core then the method of analysis as outlined below could be used, i.e. as a reference method.
Determination of Fish Content
The fish content of a fish finger (fish stick) is calculated by using the following equation
![]()
For most products therefore, the fish ingredient weight is that of the raw ingredient. Any figure placed or declared on a product label would be a typical quantity reflecting the producer’s normal manufacturing variations, in accordance with good manufacturing practice.
Checking of fish content by chemical analysis
The percentage fish content, corrected for the non-fish flesh nitrogen contributed by the carbohydrate coating, is calculated as follows.
![]()
* appropriate N (nitrogen) factor
The non-fish flesh nitrogen is calculated as follows:
% non-fish flesh nitrogen = % carbohydrate x 0.02
Where the carbohydrate is calculated by difference:
% carbohydrate = 100 – (% water + % fat + % protein + % ash)
References
Determination of nitrogen: ISO 937:1978
Determination of moisture: ISO 1442:1997
Determination of total fat: ISO 1443:1973
Determination of ash: ISO 936: 1978
Table 2: Interim Nitrogen factors to be used for white fish as an ingredient
(i.e. after GMP)
SPECIES |
Nitrogen
|
White fish: |
|
Cod |
2.66 |
Minced Cod |
2.61 |
Coley/Saithe |
2.69 |
European Hake |
2.64 |
Haddock |
2.72 |
Ling |
2.78 |
Plaice |
2.46 |
Alaskan Pollack |
2.59 |
Whiting |
2.68 |
White fish mean |
2.65 |
Additional species important for international trade proposed by Canada, South Africa and the USA but for which there are no nitrogen factors at present:
• Pacific Salmon, Atlantic Salmon, Halibut, Sole, Pacific Cod, Pacific Tomcod, Pacific Whiting, Yellowfin Sole and American Catfish
• South Africa Hake (Merluccins capensis and Merluccius paradoxus)
1 For the purpose of the standard, fish includes herring and sprats
2 For the purpose of this document, “certificates” shall mean “official certificates” and “officially recognized certificates”
3 Principles for Food Import and Export Inspection and Certification (CAC/GL 20-1995)
4 Guidelines for Generic Official Certificates Formats and the Production and Issuance of Certificates (CAC/GL 38-2001)
5 Guidelines for the Development of Equivalence Agreements Regarding Food Import and Export
Inspection and Certification Systems (CAC/GL 34-1999)
6 Amendments are highlighted
7 Hereafter referred to as lobster.
8 The Proposed Draft Code of Practice, when finalized, will replace all current Codes of Practice for Fish and Fishery Products