Appendix IV
Discussion Paper on the Management of the Work of the Committee
Prepared by
Australia, New Zealand, and United States of America
The Codex Committee on Food Hygiene (CCFH) is moving towards a broad risk management-based approach to developing recommendations on ensuring consumer protection and facilitating fair practices in food trade. This broad risk-management approach may employ microbiological risk assessment and may utilize a spectrum of risk management or risk communication work products including guidance documents, codes of hygienic practice, food safety objectives and microbiological criteria.
The Codex Alimentarius Commission recognized this change in the Committee’s operation by adopting, at its 24th Session, two additional Terms of Reference for the Committee. Specifically, these are:
• To suggest and prioritize areas where there is a need for microbiological risk assessment at the international level and to develop questions to be addressed by the risk assessor.
• To consider microbiological risk management matters in relation to food hygiene and in relation to the microbiological risk assessment activities of FAO and WHO.
The Committee recognized that the process of initiating work, preparing a microbiological risk assessment and developing a risk management strategy is a complex process, involving CCFH, the FAO/WHO Joint Expert Group on Microbiological Risk Assessment (JEMRA) and specific member countries. The Committee also recognized that a structured, yet flexible process was needed to initiate and carry out this work in a timely, orderly and complete fashion. The Committee, at its 34th Session, considered a Document (CX/FH 01/5 – Add.2) on a “Proposal for a Process by which the Codex Committee on Food Hygiene Could Undertake Its’ Work in Microbiological Risk Assessment/Risk Management originally submitted by the United States as a Conference Room Document at the 33rd Session of CCFH.
The Committee agreed that establishing a process in regards to undertaking its work on microbiological risk management was beneficial and invited the United States to prepare a Discussion Paper on the subject for the Committee’s consideration at its 35th Session.
During the 35th Session, CCFH requested the United States to revise the described process concerning work related to risk management so that it was simple, short, and flexible as possible. It was agreed that the revised document should be circulated for further consideration and pending the outcome of the discussions be considered for inclusion in the Codex Alimentarius Procedural Manual. A drafting group consisting of the United States of America in collaboration with Australia, Canada, India, Ireland, France, Germany, Japan, New Zealand, the Netherlands, Sweden, the United Kingdom, the Commission of the European Community, Food and Agriculture Organization, and World Health Organization was established to assist in the revision of the document.
During the 35th Session, CCFH also concluded that because of increasing work load there was a need to develop a transparent procedure with established criteria for prioritizing its work. Furthermore, since much of the work undertaken by CCFH impacts other Codex committees, the Committee also recognized the efficiency at which it achieves its work could be enhanced by fostering improved communications with other Codex committees. Two working groups were established to develop discussion papers to address these needs: “Discussion Paper on the Development of Process, Procedures, and Criteria to Establish Priorities for the Work of the Codex Committee on Food Hygiene” which was led by New Zealand with the assistance of Australia, Austria, Brazil, Canada, Denmark, Finland, France, Japan, Malaysia, Norway, UK and the United States, and “Discussion Paper on the Development of Options for Cross-Committee Communication Process” led by Australia with the assistance of France, Norway, New Zealand, the United States and the European Commission.
During discussions among the three working groups, it was evident that there is substantial overlap in areas being addressed, and that it would be more effective to consolidate the three documents. During the opening session of the 36th Session of the CCFH, the leads of the three working groups recommended to the Committee the consolidation of the work items.
• Proposed Draft Process by which the Committee on Food Hygiene Could Undertake Its Work in Microbiological Risk Assessment/Risk Management. (Agenda Item 5(a), CX/FH 04/5).
• Development of Process, Procedures and Criteria to Establish Priorities for the Work of the Codex Committee on Food Hygiene (Agenda Item 5(b), CX/FH 04/5-Add.2. (Discussion Paper)
• The Development of Options for a Cross-Committee Interaction Process (Agenda Item 5(c), CX/FH 04/5, Add.3). (Discussion Paper)
In recommending to the Committee the consolidation of the three documents, the three working groups were cognizant of the “Codex Strategic Framework 2003-2007” and the activities of the Codex Committee on General Principles (CCGP) related to their review of the Codex Procedural Manual in response to Codex Evaluation. In particular, the working groups considered the draft changes related to “Criteria for the Establishment of Work Priorities” and the draft text on guidelines for the establishment and functioning of work groups, both physical and electronic. The following document is intended to be consistent with and further elucidate how CCFH will implement and augment the general approach being developed by CCGP.
The Process by which the Codex Committee on Food Hygiene Will Prioritize and Undertake Its Work
The following guidelines are established to assist the CCFH to:
• Identify, prioritize and efficiently carry out its work; and,
• Interact with other Codex committees and task forces as the need arises.
These guidelines apply to all work undertaken by the CCFH and encompass: guidelines and procedures for proposing new work; criteria and procedures for considering the priorities for proposed and existing work; procedures implementing new work; and means for fostering and guiding the interaction of CCFH with other Codex committees and/or task forces on items of mutual interest.
As specified in the Codex Procedural Manual, work undertaken by the Codex Committee on Food Hygiene should fall within its Terms of Reference1 and meet the Codex Criteria for the Establishment of Work Priorities2. Furthermore, new work should be consistent with the strategic plan established by the Commission and the general procedures established by the Codex Committee on General Principles. As a means of assisting it in meeting these goals, the Committee will use the following procedures to consider, accept and prioritize proposals for new work.
Criteria for new work:
• The proposal for new work should address a known or emerging public health issue or problem.
• Public health issues or problems that impact on international trade.
• Public health issues or problems that do not impact international trade but are of interest to a substantial number of member countries.
• There is sufficient scientific knowledge available to provide scientifically sound guidance.
The Codex Committee on Food Hygiene will normally employ the following process for undertaking new work.
1. A proposal for new work shall be developed. New work may be proposed by the Codex Alimentarius Commission, by the Committee on its own initiative, by another Codex subsidiary body upon referral to CCFH, by an individual country or countries, or by a recognized international intergovernmental organization.
The proposal shall be consistent with, and include the specified elements of the project document required for approval of new work by the Codex Alimentarius Commission3 .
The proposal should indicate the specific nature of the new work that is being proposed (e.g., new or revised code of hygienic practice, risk management guidance document).
New or revised codes of hygienic practice should be consistent with the framework of the Recommended International Code of Practice: General Principles of Food Hygiene, (CAC/RCP 1-1969, Rev. 4 (2003).
Risk management guidance documents, other than specific codes of hygienic practice may also be developed to provide risk management guidance. Two possible formats for the development of such risk management guidance documents are presented in Annex I.
The proposal for work should be provided in written form that is consistent with the required Codex “project document,” preferably in sufficient time to be included in the formal agenda of the CCFH meeting at which the nominator wishes to have the proposal considered.
To facilitate decision-making, when undertaking new work in the management of foodborne microbial hazards, the proposal should include a risk profile. A risk profile is an abbreviated discussion paper that lays out the key elements of a microbiological risk management concern in order to facilitate decision-making on the part of the Committee in relation to the need and scope of the newly proposed work. The Risk Profile should provide the Purpose, Scope and Rational, and a Recommendation regarding the microbiological risk management concern and the proposed work and should, to the extent possible, provide information on the following elements:
• Pathogen(s) and food(s) combination(s) of concern;
• Description of the public health problem;
• Food production, processing, distribution and consumption;
• Other risk profile elements (e.g., extent of international trade of the food commodity, potential public health and economic consequence of establishing Codex risk management guidance; public perceptions of the problem and the risk; existence of regional/international trade agreements and how they impact the public health with respect to the hazard/commodity combination);
• Risk assessment needs and questions for the risk assessors;
• Available information and major knowledge gaps.
2. The proposal, including, as appropriate, the Risk Profile and the scientific issues underlying the new work, will be reviewed by the Committee.
3. The proposal will be accepted, submitted for revision or denied. If accepted: a) the priority of the new work will be established using the criteria and procedures presented below; b) a project document will be developed and submitted to the Codex Alimentarius Commission (CAC) with a request for approval of the proposed new work. The project document should contain the following elements:
• The purposes and scope of the standard;
• Its relevance and timeliness;
• The main aspects to be covered;
• An assessment against the Criteria for the Establishment of Work Priorities;
• Relevance to the Codex strategic objectives
• Information on the relation between the proposal and other existing Codex documents;
• Identification of any requirement for and availability of expert scientific advice;
• Identification of any need for technical input to the standard from external bodies so that this can be planned for;
• The proposed time-line for completion of the new work, including the start date, the proposed date for adoption at Step 5, and the proposed date for adoption by the Commission; the time frame for developing a standard should not normally exceed five years.
The Committee will annually review, evaluate and prioritize its work. This will be carried out by the Committee through a “Working Group for the Establishment of CCFH Work Priorities.” This working group will provide recommendations for review and approval by the Committee. The recommendations will present a proposed prioritization of potential new work.
In establishing priorities for its work, the Committee will use the criteria for new work outlined above. The Committee will also use the following additional criteria.
1. There is a need for urgent action to address an identified public health problem or issue.
2. The work affects the ability of Codex to fulfill its mandate; that is, other work within CCFH or other committees cannot progress until the issue is addressed.
3. New work is needed to facilitate risk analysis activities, e.g., establishment or revision of general principles or guidance.
4. The need to revise existing CCFH texts to reflect current knowledge and/or consistency with the Recommended International Code of Practice: General Principles of Food Hygiene (CAC/RCP 1-1969, Rev. 4-2003).
5. The total resource capacity available to CCFH would dictate the total workload that could be undertaken by CCFH at any one time.
The “Working Group for the Establishment of CCFH Work Priorities” should also assess the need for cross- committee interactions (see below).
If the proposed new work will benefit from the acquisition of additional expert scientific advice such as an international risk assessment to be conducted by the FAO/WHO Joint Expert Committee on Microbiological Risk Assessment (JEMRA), this should also be considered in prioritizing work.
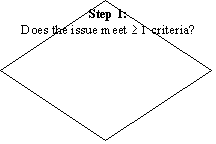
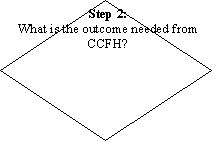
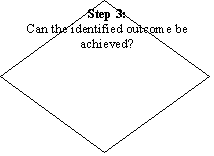
A flow diagram for the categorization/prioritization of new and existing work for CCFH is given in flow chart.
1. Upon approval of the project document by the CAC, the new work will be undertaken through the Codex Step Procedure as provided for in the Codex Procedural Manual “Procedures for the Elaboration of Codex Standards and Related Texts”.
2. An electronic or physical working group may be established to assist the Committee to undertake the new work. Working groups established by the Committee will follow the criteria established by Codex Alimentarius Commission.4
3. In certain instances, CCFH work will require a risk assessment of other expert scientific advice. This will be sought from FAO/WHO using the procedure outlined below.
4. Upon completion of work the Committee will submit the final draft text to the Codex Alimentarius Commission at Step 8 of the Codex Step Procedure.
There are instances where progress on the work of the Committee will require an international risk assessment or other expert scientific advice. This advice will be sought through FAO/WHO Joint Expert Committee on Microbiological Risk Assessment (JEMRA) or through one or more FAO/WHO Joint Expert Consultations or Workshops. In such instances the Committee shall serve as the risk managers and the FAO/WHO Joint Expert Committee on Microbiological Risk Assessment shall serve as the risk assessors. When undertaking such work, the Committee should follow the structured approach given in the Codex Principles and Guidelines for the Conduct of Microbiological Risk Management (under development). The Committee will also keep in mind the Codex Working Principles for Risk Analysis for Application in the Framework of the Codex Alimentarius5.
In seeking an international risk assessment to be conducted by the FAO/WHO Joint Expert Committee on Microbiological Risk Assessment (JEMRA), CCFH should consider and seek advice on:
1. The availability of sufficient scientific knowledge and data to conduct the needed risk assessment. (An initial evaluation of available knowledge and data will typically be provided within the Risk Profile.)
2. There is a reasonable expectation that a risk assessment will provide results that can assist in reaching risk management decisions related to control of the microbiological hazard without unduly delaying the adoption of the needed microbiological risk management guidance.
3. The availability of risk assessments performed at the regional, national and multinational levels that can facilitate the conduct of an international risk assessment.
If the Committee decides to request that a microbiological risk assessment be developed, the Committee will forward a specific request to FAO/WHO and will provide the JEMRA with the risk profile document, a clear statement of the purpose and scope of the risk assessment, any time constraints facing the Committee that could impact the risk assessment, and the specific risk management questions to be addressed by the risk assessors. The Committee will, as appropriate, also provide the risk assessors information relating to the risk assessment policy for the specific risk assessment work to be undertaken6. FAO/WHO will inform the Committee of its agreement to carry out such work and will provide a scope of work for the Expert Consultation. If a decision is made by FAO/WHO not to perform the requested risk assessment, FAO/WHO will inform the Committee of this fact and the reasons for not undertaking the work (e.g., lack of data, lack of financial resources).
The Committee recognizes that an iterative process between risk managers and risk assessors is essential for the adequate undertaking of any microbiological risk assessment and the development of any microbiological risk management guidance document or other CCFH document(s). The iterative process is described in Annex II.
The FAO/WHO will provide the results of the microbiological risk assessment(s) to the Committee in a format and fashion to be determined jointly by the Committee and the JEMRA. As needed, the FAO/WHO will provide scientific expertise at Committee session or working groups to provide guidance on the appropriate interpretation of the risk assessment.
Unless jointly agreed upon otherwise, microbiological risk assessments carried out by the FAO/WHO JEMRA will operate under the framework contained in the Principles and Guidelines for the Conduct of Microbiological Risk Assessment (CAC/RCP 020-1999).
In many instances, the work of the Codex Committee is interconnected with the work of other Codex committees and task forces. In such instances, it may be appropriate for cross-committee interaction to occur that is more extensive than that which would occur under the standing agenda item relating to “matters referred by the Codex Alimentarius Commission and/or Other Codex Committees to the Food Hygiene.” In such situations, the following approach shall normally be used.
• The Chair of CCFH will annually meet with the Chairs of other appropriate committees for the purpose of identifying (1) potential work that should be undertaken by CCFH and (2) work currently underway or planned by other committees that will require or benefit from consideration by CCFH.
• The “Working Group for the Establishment of CCFH Work Priorities” work should consider as part of their deliberations the potential need for cross-committee interaction. They should include an assessment of specific needs for cross-committee interactions(s) for each work project currently underway or being considered by CCFH. Once a new project has been approved for work by CCFH, the lead country for the projects’ working group should, with the assistance of the CCFH Chair, establish the needed cross-committee interaction(s). This will typically be communicated in writing to the Chair of the appropriate Codex committee(s) with a request for the matter to be considered by the committee and the results communicated back to CCFH.
• As appropriate, when establishing working groups on items for which a cross-committee interaction has been identified, a request will be made to include representatives of that committee on the CCFH working group.
• As appropriate, the Chair of CCFH and the Chair(s) of the other appropriate committee(s), or their designated representatives, will be invited to attend each other Sessions.
Annex I
SUGGESTED FORMATS FOR MICROBIOLOGICAL RISK MANGEMENT GUIDANCE DOCUMENTS
Risk management guidance traditionally provided by the Codex Committee on Food Hygiene (CCFH) has primarily been accomplished through the development of the Recommended International Code of Practice: General Principles of Food Hygiene (CAC/RCP 1-1969, Rev. 4 (2003)) and commodity specific codes of hygienic practice. This risk management guidance has focused on the control of hazards generally in a given commodity type, both microbial and chemical (e.g., pesticide residues, heavy metal contaminants).
More recently the need has arisen for the development of microbial pathogen specific risk management guidance; that is, guidance to control a specific microbial pathogen (e.g., Listeria monocytogenes) in one or more foods.
Alternative formats for the presentation of microbial pathogen specific guidance are possible. Two possible formats are the following.
Recommended International Code of Practice: General Principles of Food Hygiene Format
Similar to the development of a commodity code of hygienic practice, a risk management document specific for the control for a microbial pathogen in a commodity(ies) can be developed in which the section and sub-section headings of the Recommended International Code of Practice: General Principles of Food Hygiene are followed. When this format is used, only hygiene provisions needed to control the hazard that are supplemental to those provided the General Principles of Food Hygiene are given. The advantages of using such a format are that it provides a succinct means of providing specific risk management guidance in a format that is well understood by users of CCFH risk management guidance.
An alternative format may be helpful when it desirable to present optional risk mitigation strategies or when it is helpful to present microbiological risk profile and/or risk assessment information or information on monitoring and review programs. In this regard, a risk management document incorporating the following sections, as appropriate, may be appropriate.
Introduction and Background: This section should include an initial statement of the food safety problem. This section should also include the rationale and justification for the work and the Committee’s previous consideration and work on the subject. Included in this section can be summary information on the pathogen/commodity of concern, the effected populations and related information.
Scope: A short statement on the microbiological pathogen(s)/commodity (commodities) to which the risk management guidance applies.
Risk Profile: A description and evaluation of the food safety problem associated with the pathogen(s)/commodity combination(s). This section should summarize the information presented in the risk profile provided to the Committee (see Attachment 2).
Consideration of the Risk Assessment: Consideration and interpretation of the results of a risk assessment carried out by FAO/WHO JEMRA at the request the Committee. Additional risk assessments conducted at a national level may also be considered in this section.
Risk Management Options: This section should present “best practices” options available for managing the risk from the pathogen/commodity combinations(s) over the entire food chain, including good hygienic practices and HACCP. Member countries can then use this guidance to develop specific risk management options that consist of one or more control measures (e.g., guidance for primary production, processing requirements, handling requirements during distribution and marketing, consumer education programs), instituted to control the microbiological food safety hazard, that are consistent with their specific conditions and requirements. Broad information on the types of risk management options can be found in the Codex Principles and Guidelines for the Conduct of Microbiological Risk Management (under development). Information should be presented in sufficient detail to enable development and implementation of food safety programs that adequately control the risk arising from the microbiological pathogen/commodity. Whenever possible, multiple risk management options that achieve the desired level of risk mitigation should be identified as a means of providing flexibility in foods safety control strategies. The use of annexes is recommended to present detailed commodity related risk management control options. If appropriate, Food Safety Objectives, performance criteria and microbiological criteria may be included in this section. The evaluation of risk management options should consider the specific needs and capabilities of developing countries.
Implementation: Implementation of microbiological risk management options is the responsibility of national governments and industry. As appropriate, specific recommendations for the implementation of risk management options may be provided, particularly in relation to international trade.
Monitoring and Review: This section provides guidance on potential strategies for validating and verifying the effectiveness of risk mitigation strategies including the identification of potential metrics that can be used to assess successful, continuing implementation. Information specific and pertinent to the monitoring and review of the specific pathogen/commodity combinations(s) addressed in the guidance document should be provided. Whenever possible, multiple strategies for effective monitoring and review should be provided so that member countries can identify strategies most pertinent to their requirements and conditions. If there are no specific recommendations unique to the pathogen/commodity combination(s), only a reference to the Principles and Guidelines for the Conduct of Microbiological Risk Management (under development) is needed. Where possible, monitoring and review should include guidance on potential metrics that can be used to assess the impact of the food control measures on public health.
PROCESS OF CATEGORIZATION AND PRIORITIZATION OF NEW AND EXISTING WORK FOR CCFH THAT WILL BE USED BY THE “WORKING GROUP FOR THE ESTABLISHMENT OF CCFH WORK PRIORITIES”
![]()
![]()
![]()
![]()
![]()
![]()

![]()
![]()
![]()

![]()
![]()
![]()
![]()
![]()

![]()
![]()
![]()


![]()
Annex II
ITERATIVE PROCESS BETWEEN THE CODEX COMMITTEE ON FOOD HYGIENE AND THE FAO/WHO JOINT EXPERT GROUP ON MICROBIOLGOICAL RISK ASSESMENT (JEMRA) FOR THE CONDUCT OF MICROBIOLOGICAL RISK ASSESSMENT
The Codex Committee on Food Hygiene recognizes that an iterative process between risk managers and risk assessors is essential for the adequate undertaking of any microbiological risk assessment and the development of any microbiological risk management guidance document or other CCFH document(s). In particular, dialogue between the Committee and FAO/WHO is desirable to thoroughly assess the feasibility of the risk assessment, to assure that risk assessment policy are clear, and to ensure that the risk management questions posed by the Committee are understood and addressed appropriately. If FAO/WHO agrees that the requested risk assessment proposed in the Risk Profile is feasible and will be undertaken, a series of planned interactions between the FAO/WHO JEMRA and the Committee or its Working Group established to develop the risk management guidance document should be scheduled to assure effective communication. In certain instances when the subject matter would benefit from additional interaction with other Codex Committees or other FAO/WHO risk assessment bodies, these committees should be included into the iterative process.
It is essential that communications between these entities are timely and effective. Any intermediary (i.e., Working Group) assigned by the Committee to serve as a liaison with the FAO/WHO JEMRA will need to report the progress and facilitate decision making in both a timely and effective manner so that progress in the development of a risk assessment (and the CCFH work products derived from it) is not unduly delayed.
The Committee and/or its liaison (i.e., the Working Group) is likely to receive questions from JEMRA relating to the requested microbiological risk assessment(s). The questions may include those needed to clarify the scope and application of the risk assessment, the nature of the risk management control options to be considered, key assumptions to be made regarding the risk assessment, and the analytical strategy to be employed in the absence of key data needed to perform the risk assessment. Likewise, the Committee and/or its liaison (i.e., the Working Group) may pose questions to the JEMRA to clarify, expand, or adjust the risk assessment to better address the risk management questions posed or to develop and/or understand the risk management control options selected. Timely, appropriate responses are needed for these interactions.
The Committee may elect to discontinue or modify work on a risk assessment if the iterative process demonstrates that:
1) that completion of an adequate risk assessment is not feasible; or
2) it is not possible to provide appropriate risk management options.
1 Codex Procedural Manual, 13th Edition, p. 111.
2 Codex Procedural Manual, 13th Edition, p. 69.
3 Specifications for project document under development by the Codex Committee on General Principles for adoption by the Codex Alimentarius Commission. Specific Codex Procedural Manual reference to be provided.
4 Criteria under development. See ALINORM 04/33, paras. 104-119, and CX/GP 03/19/7.
5 Codex Procedural Manual, 13th edition, pp. 42-48..
6 Codex Procedural Manual, 13th Edition, p. 52 (definition of risk assessment policy) and pp. 43-44) (working principles relating to risk assessment policy).