Alina Kabata-Pendias
ALINA KABATA-PENDIAS is a Professor of Geochemistry at both the Institute of Soil Science and Cultivation of Plants in Pulawy, Poland, and the Geological Institute in Warsaw. She is co-author of a forthcoming book entitled Trace elements in soils and plants.
STUDYING CHEMICAL POLLUTION IN INDIA industrialization is already causing problems
Considerable research is still necessary in order to improve man's understanding of the complex processes involved in pollution damage to the biosphere. Meanwhile, convincing demonstrations are desperately needed to show that environmental stresses play a large part in retarding natural and cultivated trees and plants, in terms of both quantity and quality. An awareness of this problem will lead to the development of a proper relationship between industry and agriculture on the one hand, and to increased concern for the needs of the biosphere on the other.
The chemical and physical characteristics of the biosphere are determined by the properties of such basic elements as climate and soil. Also important are the surface of the lithosphere - the lower part of the atmosphere - and the hydrosphere, which includes all bodies of water plus the total water vapour in the atmosphere.
Trees and plants are extremely susceptible to damage by chemical pollutants, yet the kind of damage that occurs is not easily predictable. Air, water and soil interact in complex ways, depending upon the chemical - or chemicals - involved, the kind of vegetative cover, atmospheric conditions and soil types. The control of chemical pollutants and their effects requires not only more knowledge but more extensive international planning and cooperation.
The chemical components of the biosphere, and especially of biological organisms, have been adapted through evolution over a long period of geological time. The concentrations of elements in the biosphere are generally related to their abundance in the earth's crust.
Man, as an integral component of the biosphere, does not adapt to his environment biologically, like other organisms, but through technological and cultural changes. Man's impact on the biosphere has been very broad and complex, and has led to irreversible changes in most cases.
While geological and biological alterations of the earth's surface have been very slow, changes introduced and/or stimulated by man have accumulated extremely quickly in recent years. All man-made changes disturb the natural balance of each ecosystem formed by evolutionary change over a period of time. Consequently, these changes nearly always lead to a degradation of the natural environment. The development of agricultural activities, for example, has resulted in several ecosystems being changed into artificial agro-ecosystems. Although man's impact on the biosphere dates from the neolithic age, the problems of the deterioration of ecosystems due to pollution have increased at an astonishing rate during the last two decades.
Environmental pollution, especially by chemicals, is one of the most potent factors in the destruction of the biosphere components. Among all chemical contaminants, sulphur dioxide (SO2), hydrocarbons, trace elements and oxidant complexes (O3 NO2 and peroxyacetyl nitrates or PAN) are believed to have the most ecological, biological and health significance. However, any chemical compound produced as industrial or urban waste may create environmental stresses on the biosphere.
Rapid industrialization and population shifts from rural areas to urban centres have created unnatural concentrations of chemical pollutants, largely because energy requirements have become highly localized. These concentrations of chemical pollutants, however, do not normally remain localized, but often are widely dispersed, even across national or continental boundaries.
Energy and mineral consumption by man is the main cause of chemical pollution in the biosphere. An estimate of the total global release of chemical elements as contaminants into the environment may be based on established world mineral and energy consumption and demand, which are known to have increased rapidly in recent years. The transport, residence time, fate and long-term effects of the contaminants in a particular ecosystem are still not well understood, although investigations of properties and environmental interactions of chemicals have increased significantly in number and in scope in these last two decades.
It is possible, however, at this stage of progress in the understanding of chemical stress on the biosphere, to indicate some general characteristics of chemical pollutants. These are:
· wide dispersion and long-distance transport in air;
· rapid fixation by organic and mineral fractions in waters;
· long persistence of most contaminants in surface soil;
· deposition on plant surface, blocking the entrance of sunlight;
· bio-accumulation, which generally alters the chemical composition of plants without causing visible injury;
· disturbance of metabolic processes in living tissues;
· resistance to metabolic detoxication, with subsequent entrance into the food-chain;
· interaction with soil components, causing changes of soil pH, and weakening organic and mineral sorption complexes
"The deposition of acid with precipitation was up to three times higher under pine as compared to open terrain; under spruce it was about 1.5 times as high; and under birch it was lower than in the open."
Most air pollution has arisen from the burning of coal and other fossil fuels, and from the smelting of iron and nonferrous metals. However, domestic furnaces, fuelwood and forest fires may also contribute significantly to local pollution. Materials released by man's activities are not the only contribution to global air pollution; some natural sources such as eolithic dusts, volcanic eruptions and evaporation from water surfaces should also be taken into account.
Air pollution is a serious source of water and soil pollution. Water pollution caused by airborne pollutants, waste and drainage waters is known to kill living organisms in small water-basins in many different countries. This problem is also much associated with drinking-water resources. Most chemical elements, especially trace metals, do not exist long in soluble forms in water. They are present mainly as suspended colloids, or are fixed by organic and mineral substances. Thus, in time, they tend to be concentrated in bottom sediments and in plankton. However, easily, volatile elements (iodine, bromine) and elements readily alkylated by micro organisms (mercury, selenium, arsenic, are mobilized and can evaporate from the water surface.
Contaminated bottom sediments can lead to the accumulation of chemicals in the soil. Soil is the most specific component of the biosphere because it acts not only as a geochemical sink for contaminants but also as a natural buffer controlling the transport (cycling) of chemical elements and substances to the atmosphere, hydrosphere and biota.
All chemical pollutants from whatever source they originate, eventually reach the surface soil, where their fate depends on the chemical and physical properties of the host soil. Anionic pollutants such as sulphur dioxide and fluoride are known to damage several soil properties, to be relatively easily leached through the soil and to enter underground waters. However, the persistence of cationic pollutants, especially heavy metals, in soil is much greater than in other components of the biosphere. Metals accumulated in surface soils are depleted slowly by leaching, plant uptake, erosion, or deflation. For soils contaminated with some heavy metals (e.g. zinc, cadmium, lead), it may take decades or even several thousand years to reduce the volume of the metal by 50 percent (Kitagishi and Yamane, 1981).
Trace pollutants in soil are deposited by fertilizers, pesticides, and sewage-derived materials as well as by aerial sources. Thus, industrial and agricultural activities cause concentrations of heavy metals in surface soil several times above the natural level.
Plants are good indicators of the chemical composition and physical state of the soil. If the soil is imbalanced either naturally, because of abuse by cultivation, or by pollution or contamination, various changes in plant appearance, chemical composition and biochemical functions occur.
Contamination of the soil has already become relatively common and is likely to increase. It is a noticeable fact that soils are nearly always contaminated by several metallic pollutants simultaneously (such as sulphur dioxide, nitrogen dioxide, hydrogen fluoride), often accompanied by acidic precipitation. Possible synergistic interaction between these pollutants in soil greatly complicates their impact.
... AND RIO... acid rain doesn't need a passport to cross borders
Sulphur oxides
On a world-wide basis, sulphur oxides SO2 in particular - are the most destructive pollutants for vegetation and soil. They also damage manmade constructions such as buildings.
In fact, only about one-third of all sulphur oxides in the atmosphere are believed to be produced by man's activities (Taylor, 1980). However, SO2, emissions readily oxidize in the atmosphere to form SO3. This reacts immediately with atmospheric water vapour (H2O) to form sulphuric acid (H2SO4) which is responsible for acidic precipitation (acid rain), whose role in causing serious damage to both vegetation and soil is well known.
The regional distribution of sulphur emissions varies greatly. For European countries it has been calculated to range from below one to more than 30 tonnes of SO2/km2/yr. In spite of a great deal of research, our knowledge of the effects of acidic precipitation, the dry fall-out of sulphur, and of gaseous SO2 on vegetation and soil is still inadequate. It can be stated, however, that the complex (direct and indirect) terrestrial effects of sulphur - dioxide pollution depend on climatic factors, buffer capacity of the soil and plant tolerance.
While a slight increase of soil acidity may stimulate nutrient availability and thereby also enhance the productive capacity of the soil, the further consequence of acidic precipitation is the mobilization and leaching of several nutrients. Side-effects of highly acidified soil may also be manifested by toxic levels of aluminium, manganese or other metals easily available to plants (Hutchinson and Collins, 1978). The biological activity of the acidified soil is often inhibited since most of the soil micro-organisms favour neutral soil.
Ecological consequences of acidic precipitation are most serious in slightly acid, poorly buffered soils of temperate and boreal regions, where even a little acidification may lead to a striking loss of productivity. The fall- out of sulphur may also be destructive to the soil of arid and semi-arid regions where buildup of salts in surface horizons may be critical constraints on fertility.
The kind of soil is instrumental in determining the susceptibility to acidic precipitation of an ecosystem. This precipitation may be neutralized by alkaline salts; be exchanged for cations (positively charged ions); or be leached through soil and enter aquatic systems.
SEWAGE POURING INTO THE TIBER ever since Tarquinius Priscus built the cloaca maxima
In general, soil in temperate sites is more likely to be devoid of SO4 anions, whereas soil in tropical sites shows a much greater SO4 adsorption capacity due to a higher sesquioxide content than in temperate soils.
Vegetation, and particularly the forest canopy is known to alter incoming precipitation. The results are highly dependent on the dominant tree in the canopy. For example, the deposition of acid with precipitation was up to three times higher under pine as compared to open terrain; under spruce it was about 1.5 times as high; and under birch it was lower than in the open (Bjor, Horntvedt and Joranger, 1974).
Rain-water passing over plant surfaces shows a gain in element concentrations due to wash-off or leaching of ions from the foliage. As a consequence, the acidity of plant surfaces, for example of the tree bark, is known to increase (see Fig. 1). The direct effects of acidic precipitation on vegetation are related to:
· damage to protective surface structure;
· disturbance of gas exchange, and of metabolic and growth processes;
· poisoning of plant cells, resulting in necrotic lesions;
· alteration of leaf and root exudation processes, affecting associated microorganism population;
· synergistic interaction with other environmental stresses.
A CHEMICAL PLANT NEAR MEXICO CITY land and water ultimately absorb airborne chemicals
SPRAYING A WHEAT FIELD IN OUTER MONGOLIA AND ...
... A RICE FIELD IN LIBERIA fertilizers and pesticides deposit trace elements in soil
One of the most frequent terrestrial effects of acidic precipitation is a reduction in productivity of unmanaged natural ecosystems, such as forests. Normal agricultural practices may avoid the destructive effects of the deposition of sulphur if acidic precipitation does not directly affect vegetation or if soils are not deficient in plant nutrients.
In fact, acid rain is blamed for various kinds of damage to the biosphere, such as the destruction of vast areas of evergreen trees, the reduction of red spruce trees in mountains, and dying fish in acidified lakes (Bengley and LaBreoque, 1982). In addition to these short-term effects, however, there will be long-term ecological effects resulting from the growing rate of anthropogenic sulphur emissions - and what these effects will be, no one can predict.
Table 1. Balance of atmospheric input and water-flux output of some trace metals in different soils (g/ha/year)
|
Country |
Soil |
Vegetation |
Cadmium |
Copper |
Lead |
Zinc |
||||
|
In |
Out |
In |
Out |
In |
Out |
In |
Out |
|||
|
Denmark |
Sandy loam |
Crop plants |
3 |
0.3 |
- |
- |
260 |
0.3 |
250 |
120 |
|
Federal Republic of Germany |
Silty loam |
Pine forest |
4.5 |
1.4 |
18 |
7 |
110 |
6 |
210 |
76 |
|
Poland |
Sandy loam |
Crop plants |
5 |
3 |
39 |
25 |
207 |
40 |
547 |
180 |
|
Sweden |
Forest soil |
Spruce forest |
2 |
5 |
20 |
29 |
150 |
81 |
180 |
270 |
|
United States |
Sandy loam |
Deciduous forest |
21 |
7 |
- |
- |
286 |
6 |
538 |
140 |
Source: Kabata-Pendias and Pendias (in press).
Inorganic trace pollutants
Recently, there has been a steadily growing awareness of the importance of inorganic trace pollutants. The time has now passed when trace elements were considered only in terms of micro-nutrient problems.
Many trace elements occur in living matter, mainly in concentrations below 0.1 percent. Some trace elements, namely micro-nutrients or microelements (aluminium, boron, cobalt, copper, chromium, fluorine, iron, iodine, manganese, molybdenum, nickel, selenium, silicon, tin, vanadium and zinc) are essential to the growth, development and health of both plant and animal organisms. However, the distinction between harmless and toxic concentrations is very hard to define.
Most trace elements and especially heavy metals, are known to be accumulated in surface soil as a result of both local contamination and long range aerial transport of pollutants. The input output balance of trace metals in soils has been investigated in different countries (see Table 1), and it has been shown that their concentrations in surface soil are likely to increase, on a global scale, with growing industrial and agricultural activities.
It has been estimated that the approximate annual increment of heavy metals in soil caused by dust fall-out in the Tokyo region is 0.05 p.p.m. of cadmium and 0.5 p.p.m. of lead and zinc (Kitagishi and Yamane, 1981). Similar values calculated for European countries, including output flux of the elements, are as follows (in p.p.m.): cadmium, 0.001; nickel, 0.003; chromium, 0.007; copper, 0.02; lead, 0.05: and zinc, 0.08 (Kabata-Pendias and Pendias, in press).
Different kinds of soil plant species and growing conditions contribute to the divergent influences of soil contamination on trace-element status in plants. Although guidelines for safe concentrations of trace elements in soil are still in the stage of experiment and negotiation, some authors have given threshold values for the phytotoxic accumulation of these elements (see Table 2).
In general, the buffer capacity of soil to trace pollutants is closely related to the cation exchange capacity, which controls the critical levels of pollutants that exhibit toxic effects on plants and environments. Usually, the buffer capacity (also called "soil resistance to chemical contamination") of a non-acid heavy soil with a high content of organic matter and a high content of sesquioxides exceeds by several times the buffer capacity of a light sandy acid soil. Thus, loamy neutral soil may accumulate a higher amount of trace elements with less stress on the biosphere. However, in some special cases the mobilization of trace pollutants may also be high in neutral or alkaline soils. For example, in loamy paddy soils several heavy metals are known to be easily mobilized due to the prevalence of reduction processes. In calcareous and ferralitic soils of arid and semiarid regions, some trace elements may exist in easily soluble complex ions.
Figure 1. Tree bark acidity and atmospheric sulphur dioxide (SO2) concentration in a forest as function of downwind distance from a steel mill. The oak tested is Quercus robur; the linden is Tilia cordata; and the pine is Pinus sylvestris. (Approximate regression lines are given in accordance with the study by R. Godzinska, 1979.)
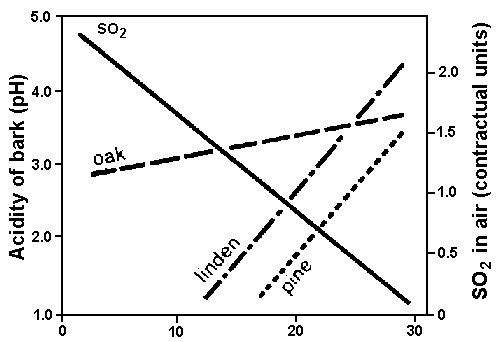
Table 2. Concentrations of some trace elements considered as phytotoxically excessive levels in surface soil (p.p.m. DW)
|
Element |
Range of total |
|
Boron |
25-100 |
|
Cadmium |
3-8 |
|
Chromium |
75-100 |
|
Copper |
60-120 |
|
Lead |
100-400 |
|
Molybdenum |
2-10 |
|
Nickel |
100 |
|
Zinc |
70-400 |
Source: Kabata-Pendias and Pendias (In press).
Generally speaking, plants grown on contaminated soils are likely to absorb more trace elements, and their concentrations in plant tissues are often positively correlated with the abundance of these elements in soils, and especially in soil solution (see Fig. 2).
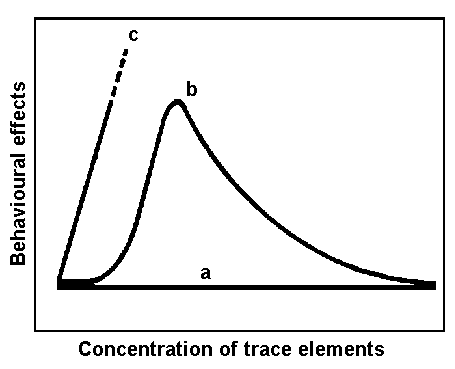
Although plants exert control over the uptake or rejection of some chemical elements by appropriate physiological reactions, they are known to be passive receptors of trace elements due to root absorption or fall-out interception. Thus, atmospheric deposition of trace elements directly on the plant surface contributes significantly to chemical stress on plants. Even though many trace elements are essential for growth, they can also have toxic effects on plant metabolism at higher concentrations. Hypothetical schemes of the reactions of plants to increasing concentrations of the essential and nonessential trace elements arc presented in Figure 3.
Metabolic disorders of plants are effected by both micro-nutrient deficiencies and by their excesses. An adequate balance among all chemical elements in plant tissues is also of great significance, because all elements are interlinked in a body either by synergism or by antagonism - or by both.
Basic reactions related to the toxic effects of trace-element excesses in both plant and animal organisms are the following:
· changes in permeability of the cell membranes;
· reactions with thiol groups resulting in denaturation of proteins:
· competition for sites with essential metabolites and reaction with essential chemical compounds:
· replacement of essential ions, and occupation of sites for essential chemical compounds.
Figure 2. Trace - element uptake by plants as a function of their concentrations in nutrient solutions.
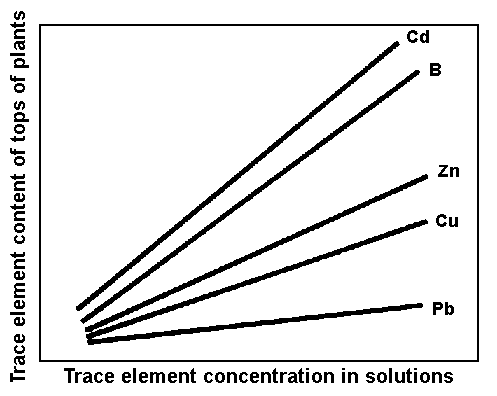
Figure 3. Schematic diagram of behavioural plasticity of plants under chemical stress: (a) completely chemical tolerant species exhibit no behavioural changes and hence suffer no damage; (b) initially chemical-intolerant species develop behavioural tolerance in time; (c) in species intolerant of chemicals behavioural changes cause damage and plants/ail to develop tolerance in time.
In spite of a great diversity in toxicity levels, it can be stated that the most toxic elements for both higher plants and certain micro-organisms are nickel, mercury, copper, lead, cobalt, columbium, silver, beryllium and tin. A great health risk to animal organisms, including man, is correlated primarily with increased environmental concentrations of mercury, cadmium, lead, beryllium, chromium, copper, nickel, silver, arsenic, antimony, thallium, zinc, fluorine, selenium, tellurium and radionuclides.
A common feature of plants is their ability to survive even under conditions of excess trace elements in their environments, mainly in soils. Lower plants - especially micro-organisms, mosses, liverworts and lichens - reveal an extremely high level of adaptation to chemical stresses. Although higher plants are believed to be less tolerant, they are also widely known to accumulate trace elements and to survive in soils contaminated by toxic quantities of various trace elements.
A constant increase of trace - metal concentrations has been reported in certain plants. For lead, the highest rate of increase was observed in moss sampled in Sweden between 1875 and 1965 (Rühling and Tyler, 1969). Selected groups of trees in both rural and urban localities in the United States accumulated several times more lead during the growth period from 1963 to 1973, as compared to 1910-20 (Boggess and Wixson, 1977).
The evolution of trace-element tolerance in plants is responsible for the presence of new species and genotypes in polluted sites. Mechanisms involved in trace-element tolerance are related to both external factors (e.g. a low solubility of ions surrounding plant roots), and to internal ones, namely several metabolic processes protecting organisms from the excess of an active micro-ion.
Plant resistance to trace-element stress and their ability to accumulate extremely high amounts of trace elements (see Table 3) may lead to a great health risk, because they can produce a contaminating]ink in the food-chain.
Fluorine emitted from various industrial plants (e.g. aluminium smelters, brickworks, glassworks, and phosphate fertilizer factories) is considered to be the most common, hazardous and phytotoxic trace pollutant. The great chemical activity of hydrofluoric acid, which is formed by both solid and gaseous fluoride, is responsible for a high acidification of the soil and for the destruction of clay minerals and humic mineral complexes.
Figure 4. Generalized effects of trace-metal concentrations in nutrient solution upon yield and metal content of plants.
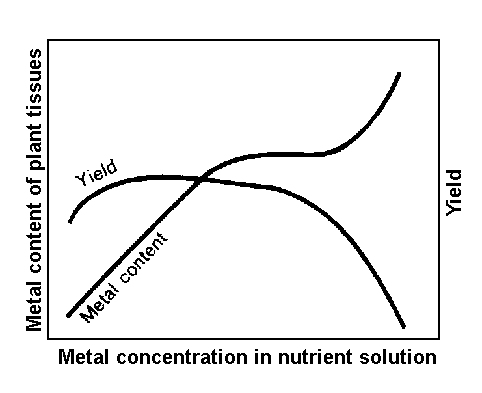
Figure 5. Response of young barley plants to concentrations of four different heavy metals in their tissues. The concentration of the metals is given in powers of 10. (Based on data from Beckett, Davis and Brindley, 1979)
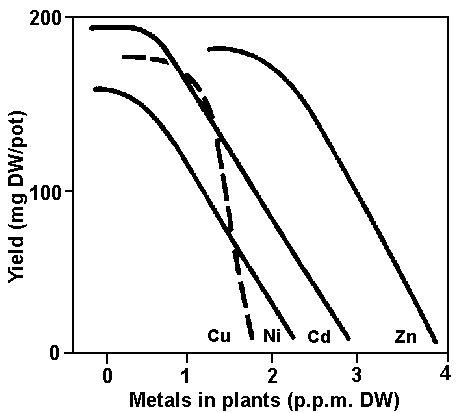
Table 3. Excessive levels of trace metals in food plants grown in contaminated sites (p.p.m. DW)
|
Element |
Concentration |
Plant and part |
Pollution source |
Country |
|
|
Excessive1 |
Background |
||||
|
Cadmium
|
45 |
0.3 |
Lettuce, leaves |
Lead smelter |
Australia |
|
4.2 |
0.08 |
Rice, grain |
Zinc smelter |
Japan |
|
|
6.4 |
0.4 |
Spinach, leaves |
Zinc/lead smelter |
Zambia |
|
|
Copper
|
64 |
8 |
Lettuce, leaves |
Copper smelter |
Australia |
|
14 |
5 |
Radish, roots |
Urban garden |
United Kingdom |
|
|
4 |
2.8 |
Rice, grain |
Irrigated farmland |
Japan |
|
|
Lead
|
1000 |
2 |
Lettuce, leaves |
Lead smelter |
United Kingdom |
|
57 |
1.8 |
Carrot, roots |
Lead smelter |
Poland |
|
|
66 |
3 |
Spinach, leaves |
Zinc/lead smelter |
Zambia |
|
|
Mercury
|
0.4 |
0.008 |
Lettuce, leaves |
Chlor-alkali work |
Switzerland |
|
0.8 |
0.05 |
Carrot, roots |
Mercury mine |
Yugoslavia |
|
|
0.6 |
0.01 |
Oats, grain |
Seed treatment |
Sweden |
|
|
Zinc
|
710 |
25 |
Onion, bulb |
Lead smelter |
United Kingdom |
|
316 |
40 |
Lettuce, leaves |
Zinc smelter |
Australia |
|
|
114 |
35 |
Soybean, grain |
Sludged farmland |
United States |
|
1Maximum found contents.
Source: Kabata-Pendias and Pendias (in press).
"The impact of trace-element stress on the biosphere in arid and semi-arid regions should especially be of great concern. Specific bio-geochemical processes occurring in these regions may lead to fast, and irreversible, alterations of the natural environment."
Although fluorine is known to be readily available to plants when occurring in soluble forms in soils, there is no evidence of fluorine toxicity when this element is absorbed by roots only. However, airborne fluorine is highly toxic to plants.
Fluorine pollution is known to inhibit the growth of various plants. The most significant damage is known to be caused by the combined stress of fluorine and sulphur dioxide. Pine trees seem to be especially sensitive to this combination.
Although plants can develop mechanisms of tolerance to trace element stress, their health and productivity may nevertheless be significantly reduced. The generalized effects of trace-metal concentrations in nutrient solution on yield and metal content of plant tissues are shown in Figures 4 and 5.
In fact, anthropogenic sources of trace elements entering the biosphere are one of the most serious aspects of environmental contamination or pollution. The impact of trace-element stress on the biosphere in arid and semiarid regions should especially be of great concern. Specific bio-geochemical processes occurring in these regions may lead to fast, and irreversible, alterations of the natural environment.
Photochemical oxidants
Photochemical oxidants occurring in the atmosphere as pollutants include: ozone (O3), nitrogen dioxide (NO2), and peroxyacetyl nitrates (PAN). Several other materials may also be involved in photochemical reactions, but they are of much less importance.
These compounds, which are being released mainly by industrial plants and motorized vehicles, have recently been recognized as the most harmful substances to the plant and animal kingdoms, particularly when they occur as photochemical smog. Photochemical smog consists of a mixture of gaseous, aqueous and solid-particle pollutants reacting with ultraviolet light and producing compounds that are both irritating and toxic to living tissues.
Meteorological factors control the concentrations, transport and toxicity of photochemical oxidants in air.
Usually, their formation and volatility are increased in hot weather, However, other factors, such as light and water, may also have an effect.
The environmental effects of oxidants, especially their absorption by plants, their phytotoxicity, and the mechanism of plant resistance to other oxidants, have been the subject of broad studies (Mudd and Kozlowski, 1975). In general, it can be said that photochemical oxidants decrease photosynthesizing tissues, such as through leaf tissue injuries, and also interfere with processes of gas exchange at the leaf surface. It has also been shown that amino-acids, proteins, and unsaturated fatty acids are susceptible to ozone oxidation, resulting in various changes on the plasma membrane and affecting metabolic processes.
Because pollutants rarely exist in isolation, several phytotoxic gases and substances may have diverse effects, depending on their proportions and interactions and on the resistance of a given ecosystem, Nevertheless, it should be emphasized that all oxidants, especially ozone, are responsible for extensive vegetation damage as well as for decomposition of manufactured materials.
Natural photochemical compounds such as ozone are known to volatilize from forests as a result of the action of sunlight on terpenes and other hydrocarbons produced by large masses of certain forest vegetation.
|
Glossary adsorption: the adhesion, in an extremely thin layer of molecules (as of gases, solutes or liquids), to surfaces of solid bodies or liquids with which they are in contact; differs from absorption", in which there is penetration beneath the surface of the contact solid bodies or liquids. anion: specifically, the ion in an electrolysed solution that migrates to the "anode" or positively charged pole; broadly, a negatively charged ion. antagonism: when the combined effect of two or more things, i.e. pollutants, chemicals, nutrients, etc., is less than the sum of their effects when acting independently; the opposite of 'synergism" (see below). anthropogenic: of, relating to, or involving the impact of man upon nature. calcareous: consisting of or containing calcium carbonate. cation: specifically, the ion in an electrolysed solution that migrates to the "cathode" or negatively charged pole, i.e. the opposite of "anion" (see above); broadly, a positively charged ion. colloid: a substance which is in a state of division and which prevents the passage through a semi-permeable membrane; it consists of particles too small for resolution with an ordinary light microscope; in suspension or solution it diffracts a beam of light and fails to settle out. DW: the air-dried weight basis of samples. humic: of, relating to, or derived at least in part from humus. ferralitic soils: well-drained soils formed originally from basic igneous rocks, consisting mainly of hydrated oxides of iron and aluminium with low silicon dioxide content. metabolite, metabolic: a metabolite is a product of metabolism, or the sum of the processes in the building up and/or breaking down of protoplasm incidental to life; something that is metabolic is undergoing a metabolism related change. necrotic: causing localized death of living tissue. PAN: peroxyacetyl nitrate. PCH: polycyclic hydrocarbons. phytotoxic: poisonous to plants, p.p.m.: parts per million. radionuclides: a radioactive type of atom characterized by the particular constitution of its nucleus, i.e. by its energy content and by the number of protons and neutrons it contains. sesquioxides: hydrous oxides of iron, aluminium and titanium. synergism: when the combined effect of two or more things, i.e. pollutants, chemicals, nutrients, etc. is more than the sum of their effects when acting independently; the opposite of antagonism" (see above). thiol groups: also called mercapto groups"; any of a class of compounds analogous to the alcohols and phenols but which contain sulphur in the place of oxygen; they may have very disagreeable odours. volatile, volatilize: something is volatile when it is easily converted to vapour or gas at relatively low temperatures; to volatilize something is to make it volatile, or capable of easy transformation into gas or vapour. |
Particulates
Many pollutants occur in the atmosphere as aerosol suspensions consisting of solid particulates or droplets of liquid in the air. Suspended particles of dust, chemicals, liquids, sea-salt, pollens and bacteria are abundant in the atmosphere, and their behaviour and impact on living organisms are governed by physical properties. The environmental effects of suspended particulates are the least understood aspects of air pollution.
The physical composition of air pollution is highly variable, and particles can comprise from 10 to 50 percent of total pollutants, depending on pollution sources and controls.
Apart from the chemical stress exerted by suspended particles, their deposition on plant surfaces results in a reduction of sunlight entering plant tissues. Health effects of aerial dusts are also of great concern.
Among dozens of substances that have been identified as minor particle components of air, the most harmful pollutants are soot, polycyclic hydrocarbons (PCH) and asbestos. Asbestos like fibres, consisting of silicate minerals in fibrous form, have been known to cause severe sickness when inhaled by animals and man (Maxwell, 1976).
Trace organic particulates, and especially those formed during coal conversion processes, even when their concentration is low, may adversely affect the environment. Of this group of particles, hydrocarbons and PCH are of special health concern. Fly ash from coal combustion or other coal conversion processes contains variable, usually small, proportions of such PCH compounds as pyrene, perylene, fluoranthene and coronene. Some of these are known to be carcinogenic. All interact with trace inorganic pollutants, forming organo-metallic compounds. All PCH compounds exhibit high absorption and adhesive properties and are therefore increasingly being recognized as harmful.
Particulates emitted from cement kilns have a very specific impact on the environment. Cement dust is composed of relatively coarse particles and contains a great proportion of calcium and magnesium oxides, increases soil pH and reduces vegetation growth.
SANITATION RESEARCH IN BRAZIL with support from UNEP, UNDP and WHO
At no time in history has there been a greater need for simultaneously increasing food and fuel production while preserving the biosphere. Developments during the past decades have demonstrated clearly the rapid destruction of man's natural resources. It is now well understood that chemical degradation of the biosphere may become the most important factor limiting food production on a worldwide basis. Soil contamination with chemicals, and particularly with trace elements, should be of great environmental concern. All methods of soil reclamation following chemical degradation tried up to now have failed to achieve success.
A statement by Y. Laulan (1982) best describes the present situation: "If current rates of land degradation continue, nearly one-third of the world's arable land will be destroyed in the next 20 years."
Until now, technology has led to wasteful use of resources, to soil exhaustion, spoilage, or contamination; to air and water pollution. Now these effects, formerly isolated, may rapidly extend to a large region, to a continent, or even to the whole Earth -as in the case of radioactive fall-out.
The nature of chemical stresses on the biosphere is such that no local controls and measures can now be wholly effective unless they are considered within the entire global system. Therefore, man urgently needs to integrate the planning of industrial and socioeconomic activities.
One final hope is that by learning from the industrialized countries, the developing countries can avoid, as far as possible, the negative consequences of large-scale industrialization and urbanization.
BECKETT, P.H.T., DAVIS, R.D. & BRINDLEY, P. 1979. The disposal of sewage sludge onto farmland: the scope of the problem of toxic elements. Wat. Pollution Control, 78: 419425.
BENGLEY, S. & LA BREOQUE, R.1982. Watch out for acid fog. Newsweek, 13 December, p. 57.
BJOR, K., HORNTVEDT, R. & JORANGER, E. 1974. Distribution and chemical enrichment of precipitation in a southern Norway forest stand. SNSF Project, Res. Rep. 1, Ås, Norway. 28 p. (In Norwegian, English summary)
BOGGESS, W.R. & WIXSON, R.G. (eds). 1977. Lead in the environment. Rep. NSF/RA-770214, Washington, 264 p.
GRODZINSKA, K.1979. Tree bark-sensitive biotest for environment acidification. Environment Int., 2: 171-176.
HUTCHINSON, T.C. & COLLINS, F.W. 1978. Effect of H+ ion activity and Ca2+ on the toxicity of metals in the environment. Environ. Health Perspect., 25: 47-52.
KABATA-PENDIAS, A. & PENDIAS, H. Trace elements in soils and plants. Boca Raton, FL, CRC Press. (In press)
KITAGISHI, K. & YAMANE, I. 1981. Heavy metal pollution in soils of Japan. Tokyo, Japan Scient. Soc. Press. 302 p.
LAULAN, Y.1982. Economic consequences. Back to the dark ages. Ambio, 11: 149-151.
MAXWELL, K.E. 1976. Environment of life. Encino, CA, Dickenson Publ. 489 P.
MUDD, J.B. & KOZLOWSKI, T.T. 1975 Response of plants to air pollution. New York, Academic Press. 383 p.
RÜHLING, A. & TYLER, G. 1969. Ecology of heavy metals: a regional and historical study. Botaniska Notiser, 122: 218-269.
TAYLOR, O.C. 1980. Phytotoxic air pollutants and their source. UNECE, Symp. on Effects of Air-Borne Pollution on Vegetation, Warsaw, p. 51-58.