Irineu BATISTA
1. INTRODUCTION
Silage is the product of the process of preserving and storing wet fodder and its utilization in agriculture has been known for a long time. Analogous products prepared with whole fish or parts of fish have been called fish silage.
Fish silage production was initiated in the 1920s when A. I. VIRTANEN used sulfuric acid/hydrochloric acid to preserve green fodder1. This method was adopted by EDIN2 in the 1930s to preserve fish waste. Acid fish silage us commercially manufactured in DENMARK, NORWAY AND POLAND with lesser amounts produced in other European Countries. Its production is being introduced also in Southeast Asian Countries using waste, by-catch, and surplus fish.
2. PRODUCTION PRINCIPLES
Fish silage can be prepared by the following method:
Addition of acid, inorganic and/or organic acid which lowers the off sufficiently to prevent microbial spoilage. The liquefaction of fish tissue is made by enzymes naturally present in the raw material.
Bacterial fermentation with lactic acid bacteria which are naturally present in the fish but it may be advisable, however, to add a starter culture of proper lactic acid bacteria. To favour the growth of these bacteria, it is essential to add a fermentable sugar because fish contain little free sugar. The lactic acid bacteria and the reserve of fermentable sugar suppress amino-acid degradation.
3. ACID-PRESERVED FISH SILAGE
In the production of acid-preserved fish silage, inorganic or organic acids or mixtures of these can be used. If inorganic acids are used the pH of silage is lowered to 2, or below in order to obtain a fully preserved product2. The quantity of acid required to lower the pH to 2 depends on the content of protein and ash in the raw material and before this type of fish silage can be fed to animal it must be neutralized. However, the high ash level of the neutralized product is undesirable in nutrition and neutralization is an additional laborious operation. Handling of mineral acids is also very hazardous and corrosion problem are more severe at the low pH of these fish silages.
Formic, acetic, and propionic acids are the organic acids usually utilized in the preparation of fish silage. If formic acid is used, the silage is stable at pH 3.5–4.03–6 and 4.5 with propionic acid7. These organic acids are more expensive than mineral acids, but a previous neutralization is not necessary before feeding the animals.
The antibacterial effect of weak organic acids is associated with undissociated molecules. Cell membranes are in general impermeable to the anion of the weak acid and to hydrogen ions (H+), but non-charged and undissociated molecules can freely pass through cell membrane. At low pH, a certain percentage of molecule of organic acid is undissociated which pass through cell membranes and inside the cell, pH is neutral and the organic molecule will dissociate, giving a hydrogen ion and an anion which will be trapped there. pH in cytoplasm, therefore, gradually decreases and the anion of the organic acid accumulates which contributes to the antimicrobial activity of these acid. In some instances the anion may be metabolized by the cell counteracting in this way its toxicity. This probably happens with acetate whose antimicrobial activity is considerably lower than propionate and formate. According to this theory of the mode of action of weak organic acids, their antimicrobial activity should increase with falling pH and it should be very high when pH is lower than the pKa of the weak organic acid. More than 50% of the propionic acid (pKa = 4.86) will exist in its undissociated form form at pH values below 4.86. In the case if formic acid (pKa =3.75) the pH must be below 3.75 in order to obtain its maximum anti-microbial activity.
The percentage of formic acid required for adequate preservation depends on the ash content of fish and OLSSON4 derived the formula:
L = 0.25 + 0.3 × % Ash
Where L is the volume of the formic acid (90%) in liters required to treat 100 kg of fish. However,for reasons of safety, a reduction in the amount of acid appears inadvisable whilst for reasons of simplicity recommendation of different amounts of acid for various materials was undesirable, and although 3.5% of acid is known to be too much for some materials, it appears to be adequate for most fish or waste fish likely to be treated.
On account of the high price of organic acids it can be recommended to use a mixture or inorganic and organic acids. The inorganic acid lowers the pH sufficiently for the organic acid to become antimicrobial. The use of these mixtures has been referred to by many authors and such experiments have been carried out mainly with formic acid and sulfuric acid. Fig. 1 shows the results obtained with different mixtures of formic acid (85%) and sulfuric acid (50%) In this diagram the upper region corresponds to mixtures of acids which give silages of good conservation quality8.
4. PREPARATION OF ACID FISH SILAGE
The process steps in the production of acid fish silage are (1) mincing or chopping the raw material; (2) addition of acid homogenization (3) autolysis and lipid separation; and,(4) storage. De-oiling is not necessary if the fish silage has an oil content of less than about 2% of the wet weight9.
The production of fish silage starts with the mincing of fish and mixing the mince of this substance with the acid. However, when fresh fish is used, the muscle components become rubber-like after the addition of acid and the mince tends to form closed pockets in which the acid do not enter quickly enough to prevent spoilage. This problem can be overcome by cutting or chopping the fish into pieces before the addition of acid. If the raw material is not fresh, thawed fish for example, the mixture of minced fish with acid is more easily done and it is vital that the acid and fish be well mixed because pocket of untreated material will putrify10.
4.1. Liquefaction of fish silage
During storage of silage there is a gradual liquefaction due to the degrading enzymes present in fish, mainly in guts. The increased liquefaction (antolysis) of silage can be measured by a fall in viscosity or by an increase in the volume of the aqueous phase after centrifugation. In Fig.2, we can observe the liquefaction of blue whiting silage prepared with formic acid (3%)11. Viscosity after two weeks of storage is relatively low, but in the case of snipe fish (a fatty species) the viscosity values are higher which means that the fat content is the main contribution to the final viscosity.
Protein breakdown can be measured by determining total nitrogen12 or trichloroacetic acid (TCA) soluble non protein nitrogen (NPN)13 present in the aqueous phase after centrifugation or filtration.
The rate of liquefaction depends on the type of raw materials, its freshness, the activity of digestive enzymes in the fish, the physiological condition of the fish at the time it was caught, the pH, the temperatures and the nature of the preservative acid10, 11. Changes in low molecular nitrogenous substances depending on catching season and storages temperature can be remarked in Fig.314. Prior freezing of the raw material did mot influence the rate of autolysis (Fig 4)15.
Proteases are the main enzymes responsible for autolysis which have an optimum in the pH range 2 to 4, when assayed with hemoglobin as substrate10. This activity decreases sharply above 4. The pH curve of autolysis of different tissue components does not coincide with that of a standard protein assay because the proteases have different pH optima according to the tissue components. The percentage of soluble material as a function of pH is plotted in fig.516 showing a maximum solubilization near pH 3.5.
4.2. Stability of amino-acids
It has been reported that amino-acids are very stable in an acid fish silage. The percentage of amino nitrogen released as ammonia are small but this may imply a significant reduction of the nutritional value of the silage if the ammonia is derived from essential amino-acids. In fact, there are a few reports referring to the decomposition of tryptophan, methionine cystine and histidine. Tryptophan which degrades at low pH when it is free17 and its rate of degradation increases marked with temperature12.
Low levels of methionine and cystine in liquid herring protein were detected, but this fact was not observed in fish silage prepared from white fish which had similar levels of isoleucine, threonine, cystine+methionine and lysine to the levels in white fish meal18.
Histidine is quickly destroyed by spoilage bacteria and may be thé limiting amino acid in fish silage prepared from spoiled fish19.
4.3 Changes in the oil
During the storage of fish silage, the fish oil present becomes rancid and oxidized. Glycerides in the oil are decomposed by lipases in free fatty acids which increase regularly during storage. Fig.6 shows the free fatty acid content of a snipe fish silage stored at room temperature15.
Unsaturated fatty acids in lipids react also with oxygen to form hydroperoxides as the initial products. These hydroperoxides in foods or feed decompose readily through a free radical chain giving stable secondary products. The rate of decomposition of hydroperoxides accelerates as its level increases whereas the rate of formation decreases as more Fatty acid become oxidized. This means that the level of hydroperoxides in foods and feed attains a maximum during storage, available oxygen, the presence of pro and anti-oxydants, reactivity of lipids, etc…
Fig.7 shows the evolution of the peroxide value during the storage of a fish silage prepared with snipe-fish20.
The presence of oxidized fat in animal rations causes loss of appetite, decreasing ability to gain weight, and death when the peroxide value exceeds 100 milli equivalents per kilogram of diet21–22.
The breakdown of hydroperoxide can be increased by heat, light, metal ions,and metalo-proteins and since oxidized lipids are responsible for the poor nutritional quality of fish quality, different ways has been tried so as to avoid its formation or to accelerate the decomposition23. Anti-oxidants like ethoxyquin or BHT has been used successfully to prevent the formation of hydroperoxides(see fig.7) and boiling of fish24 is also effective in the reduction of its formation in the oil.
The secondary products of lipid oxidation may react with proteins causing some reduction of the nutritional value23. These oxidized products may be also responsible for carcass taint.
4.4 Vitamin degradation
Only a few papers dealing with vitamin degradation in fish silage have been published. However special attention has been given to vitamin B1 (thiamine)
The tissue of clupied fish(herring, sprats, anchovy, etc…), carps, and fresh-water fish have frequently been shown to contain an enzyme which will destroy vitamin B1. This enzyme, thiaminase,can induce a deficiency of thiamine caracterized by damage to the central nervous system. These deficiency symptoms of thiamine were observed in salmon and rainbow trout fed on herring for extended periods.
According to the results obtained by ANGLESA and JACKSON25, the ensilation of fish containing thiamines does not inactivate the enzyme immediately, but after extended storage the thiaminase activity decreases below levels which can be reliably estimated. The presence of this enzyme in silage may not be a problem if the silage is then mixed with a dry meal to form moist pellets. If the dry meal contained a thiamine supplement, this would therefore remain intact until the feed was eaten by the fish.
Thiaminase becomes compltely inactivated after 5 min. at 82°C 26.
Thiamine is also decomposed by physical/chemical conditions such as intensive light, heavy metals, sulphite and some carbohydrates. It is heat stable under acid conditions but labile under neutral and alkaline27–28.
The deficiency of vitamine E observed in chicken fed on a silage/cassava meal diet was attributed to the decomposition of this vitamin by oxidized lipids10, but the same results were not obtained in another case11.
Riboflavine, pyridoxine and niacin seem to be stable in the acid silage29 and also vitamin B12 which has a half life of 2 months at 10°C 30.
5. FEEDING TRIALS WITH FISH
Fish silage has been used in fish feeds with varying success depending upon the species of fish, the type of acid used in the ensiling process, and the method of processing.
Silage based moist pellets have been proven excellent. diet for salmonid fish in Norway and a few papers have been published on the utilization of fish silage in feeds for these species.
RUNGRUANGSAK and UTM31 used acified feeds treated with hydrochloric, formic or sulfuric acid(2.5%w/w) to feed rainbow trout to test the effects on protease activities, growth and feed utilization. Hydrochloric acid had no both growth and proteolytic activities. Sulfuric acid showed similar effects, with the exception that the protease activity in the stomach was not depressed.
The results obtained by HARDY et al., 32 indicate that the lenght of storage of fish silage affects its nutritional value, suggesting that if silage is allowed to liquefy and is stored for a long period before dried and used in trout diets, total replacement of fish meal by silage should not be attempted. Stopping the liquefaction process before completion by heat treatment may allow higher levels of dried fish silage to be used in the diet without affecting fish growth. This study showed also that fish silage made with sulphuric acid can be neutralized with Ca(OH)2 without reducing the whole body levels of zinc in the fish.
According to AUSTRENG and ASGARD29, silages containing propionic acid do not seem to be accepted by salmon, while silages preserved by formic acid alone or in combination with sulphuric acid have given good results. Rainbow trout seems to be more tolerant to different acids than salmon. For both species, silage from trash fish, fish offal, blood and casein have been used with success.
TORRISSEN et al.33 demonstrated that astaxanthin is stable in an acid silage of shrimp processing waste. The digestion by rainbow trout of the astaxanthin present in this waste material was improved by ensiling to about 71% as compared to 45% in the corresponding fresh or dried material. Also the rate of accumulation of the pigment in the fish muscle was marked higher in fish fed the silage diet than those given fresh or dried shrimp waste.
Identical results ware obtained by CHEM and MEYERS34 in the acid ensilage of crawfish waste in which the astaxanthin pigment present was stabilized.
In diets for carps, fish silage has been shown to be equally good as a source of protein35.
6. FERMENTED FISH SILAGE
The utilization of lactic bacteria to preserve fish was invented by CARL36, who obtained a fermented product with good keeping quality and no unpleasant fish odour. On media containing sugars, lactic acid bacteria produce large amount of lactic acid which decreases the pH(fig.8)37 and thus renders the medium unsuitable for the growth of most microorganisms. These bacteria preserve food or feeds, preventing its microbial spoilage, add flavour to the products and may also prevent rancidification and other undesirable chemical reactions38–40.
Fish has a very low level of free sugars available for fermentation by bacteria. While spoilage bacteria utilize amino-acids as a source of energy, lactic acid bacteria have limited ability to decompose them, but the presence of fermentable sugars like glucose, fructose, and ribose, enhance its growth even when the environmental is rich in amino-acids. If the environment is anaerobic, spoilage bacteria ferment also glucose producing acids and lactic acid bacteria become predominant.
At the early stages of fermentation the presence of hetero fermentative bacteria can occur, producing CO2, acetic acid and ethanol in addition to lactic acid. These kind of bacteria must be avoided and may be suppressed by adding 5% sodium chloride. This can be also accomplished added small quantities of organic acids and boiling the fish prior to inoculating with a suitable starter culture.
Fermented fish silage have an acceptable hygienic quality and it appears that coliforms, typhoid bacteria and coagulase positive staphylococci are destroyed along with spores of Clostridium botulinum. If the silage is exposed to air, the growth of yeast and fungi41 may occur.
As referred to before, a relatively high percentage of carbohydrates must be added to the fish in order to ensure a successfull preservation. A few sources of fermentable sugar have been used including mixtures of malt meal and oats meal, mollases, cassava meal, and whey powder. The quantities of carbohydrates used are quite variable but it seems that at least a 10% addition of mollases is required to produce a stable silage9.
As with acid fish silage there is also in fermented fish silage, a protein digestion to soluble compounds but it seems that is significantly lower than for acid silage.Fig.942 shows the results obtained in a silage prepared with snipe fish and cassava meal as the carbohydrate source.
The production of volatile bases and ammonia in a fermented fish silage is considerably higher than in an acid fish silage. The evolution of total volatile bases in both types of silage from capelin is represented in fig.1037. Nevertheless, the ammonia or volatile bases are not derived necessarly from essential amino acids, since the nutritional value of fermented silage is not affected43.
Lactic acid bacteria are naturally present in the fish in very low numbers when compared to potential spoilage bacteria44. To enhance the preservation process, it is advisable to add suitable bacteria unless the carbohydrate additives carry such bacteria as natural microflora. Starter culture must, of course, be used when the fish is boiled prior to preserving41. The inoculation of fish/carbohydrate can be done by the addition of pure starter cultures produced commercially, or by reinoculation from a previous silage of acceptable quality. Another method is to mix it with a “sauerkraut”45–46 which can be produced by mixing sliced cabbage with vinegar, sodium chloride, cassava meal and some sugar.
7. FISH SILAGE VERSUS FISH MEAL
According to RAA and GILDBERG9, fish silage is a means of utilizing waste fish in situations where conventional fish meal production is inappropriate or unavailable. Such situations are characterized by scattered and irregular landing of fish meal plant. Fish silage has some advantage on fish meal:
Acid preserved fish silage does not putrefy, retaining a fresh acidic smell even after storage for weeks at tropical temperatures. As well, there are not the same environmental problems with silage as with fish meal manufactures.
A fish silage is almost sterile and pathogens like Salmonella are efficiently killed in it.
The scale of production of fish silage can be varied at will without the economy of the process being greatly affected. The capital investment in equipment may be anything from a homemade drum with a chopper to a sophiscated plant designed for the de-oiling of large quantities of fish silage.
The energy requirements of silage production are very low compared with fish meal.
Mixture of acid-preserved fish silage and carbohydrate filters can be dried in open trays tropical conditions without fly infestation because flies are repelled by the evaporating acids.
On the other hand, fish meal is less bulky and thus cheaper to transport.
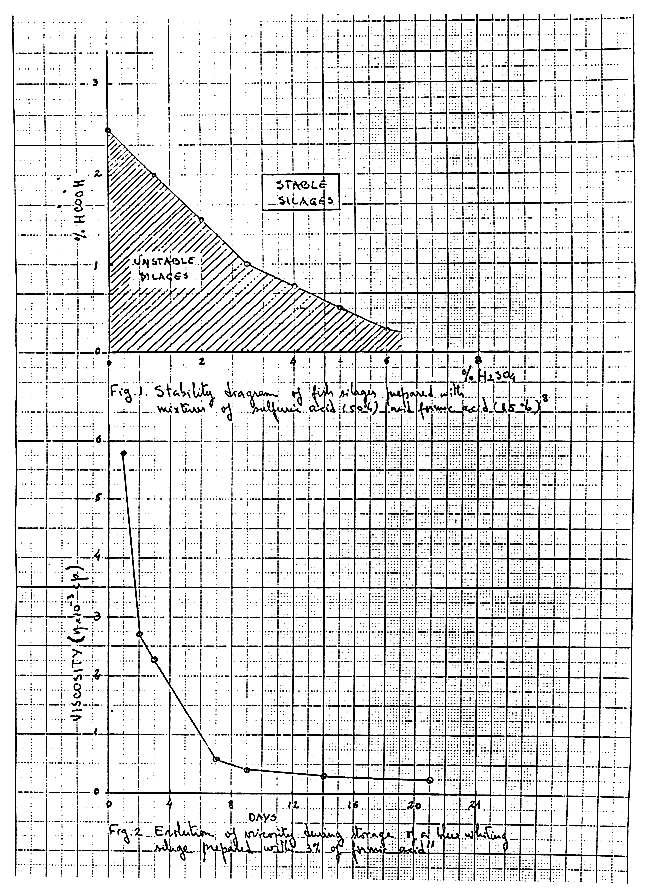
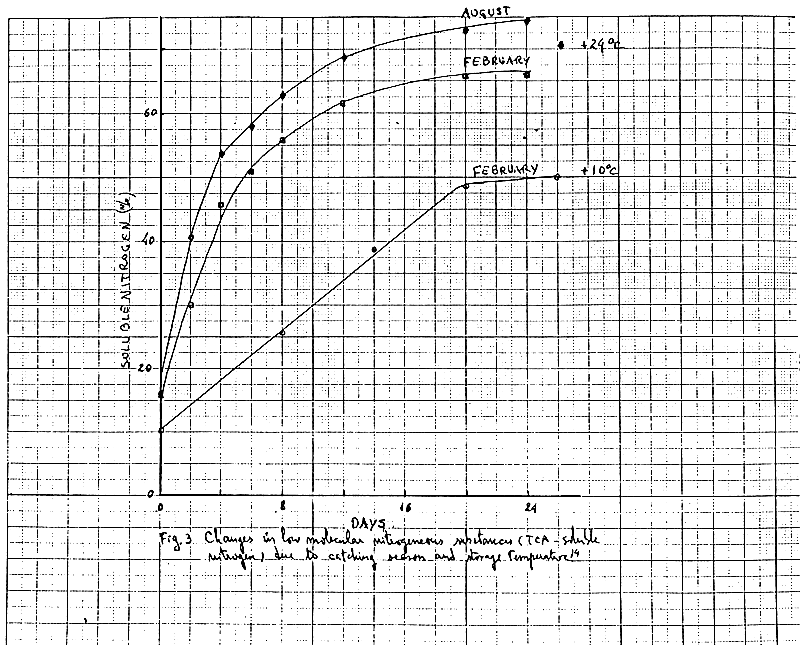
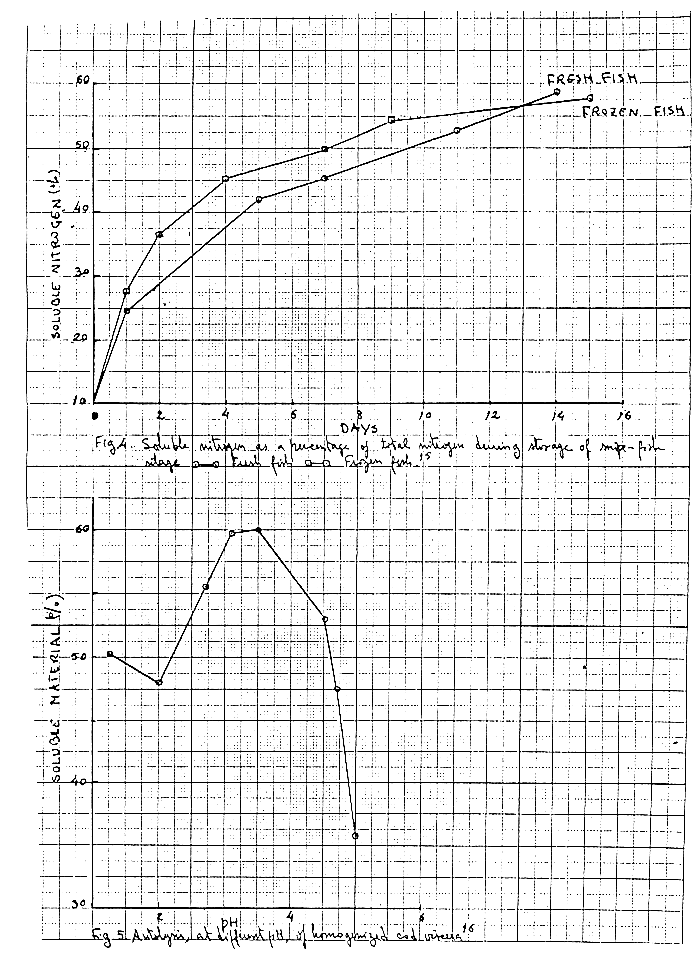
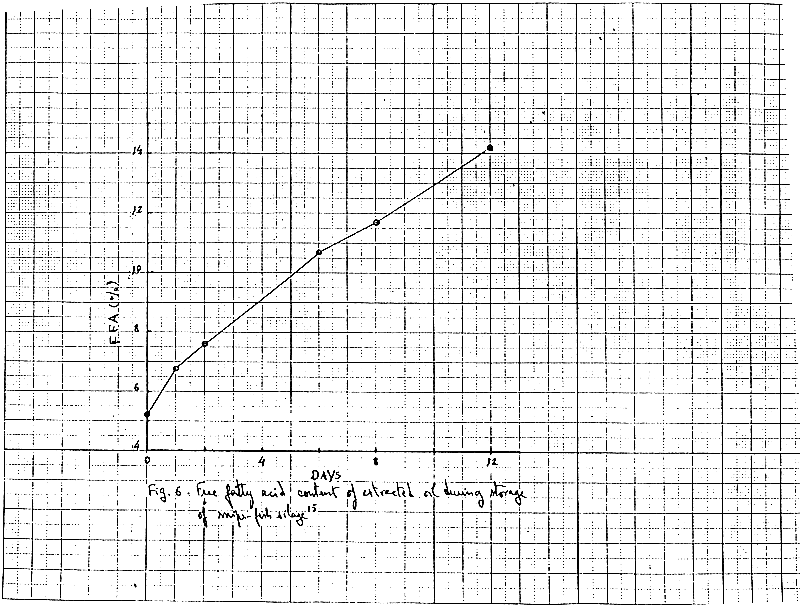
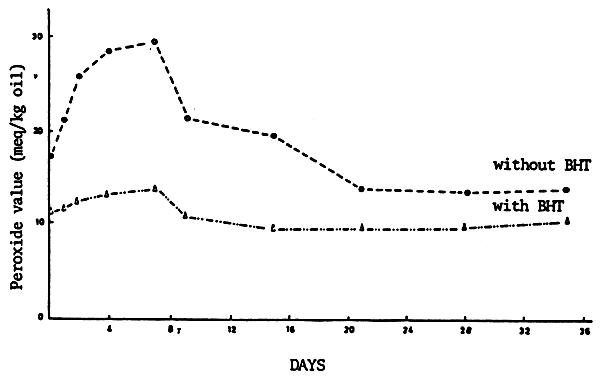
FIG. 7 - Peroxide value of extracted, oil during storage of snipe-fish silage. 20
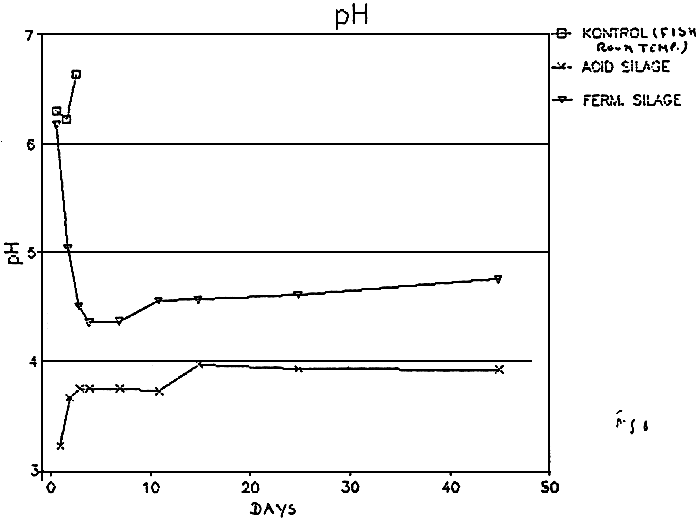
FIG. 8 - Evolution of pH in fermented silage in an acid silage. 37
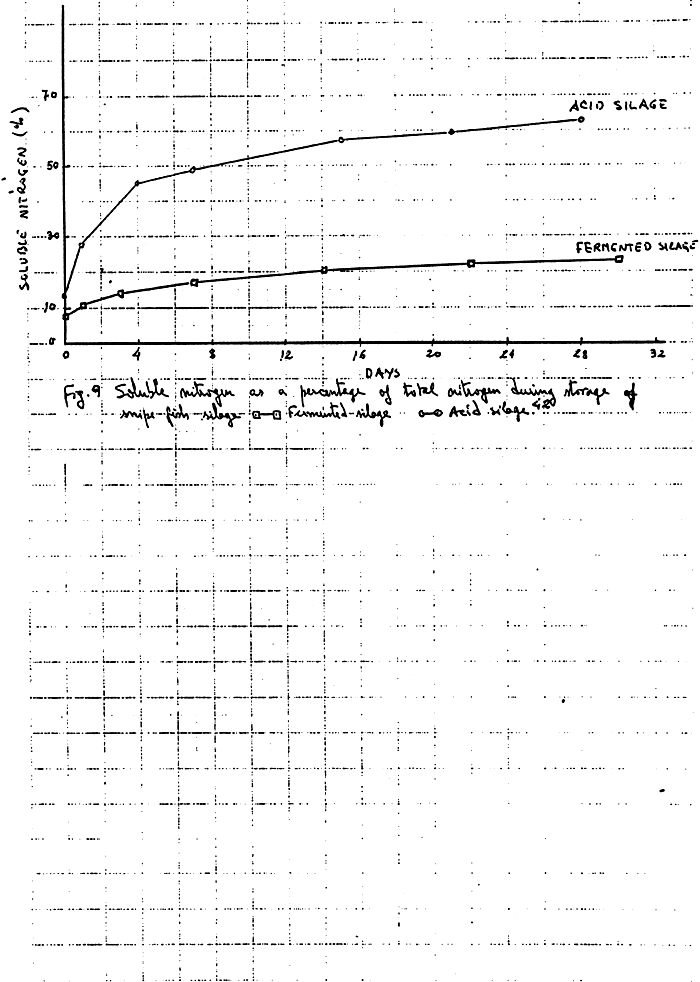
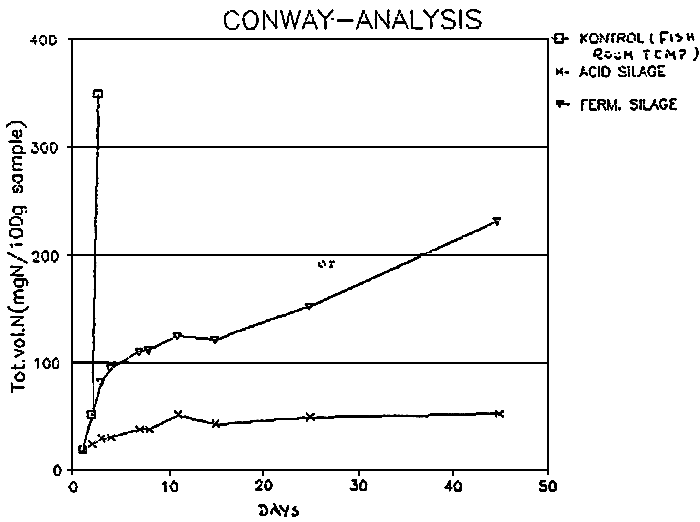
FIG. 10 - Evolution of TVN in a fermented silage and in an acid silage. 37
8. REFERENCES
1. PETERSEN H., 1953. Acid preservation of fish and fish offal. FAO Fish. Bull., 6 (1), 18
2. EDIN H., 1940. Unders â kingar angåande importa vsteningers ä ggviteproblem. Nord. Jordbr. Forsk,. 22/42
3. TATTERSON I.N., 1976. The preparation and storage of fish silage, in Proc. Torry Res. Stn. Symp. Fish silage, Torry Research station, ABERDEEN, I.
4. OLSSON N., 1942. Försök rörande fiskeriprodukternas till raratagande och användbarhet som fodr för höns och kycklinger. Cantbrukshögskolan Husdjurförsöksanstalten (Sweden), Report № 7, 55.
5. ATKINSON A., LAMPRECHT E., MISPLON., 1974.Acid and alkali preservation of fish meal, in 28th Annua. Rep. of the Dir., Fishing Industry Res. Inst., CAPE TOWN. 44.
6. JENSEN J., SCHMIDTSDORF W., 1977. Fish silage, low fat and solube fish protein products, in Proc. I.A.F.M.M. Symp. Prod. Use Fish Meal, SZCZECIN, Poland.
7. GILDBERG A., RAA J., 1977. Properties of a propionic acid formic acid preserved silage of cod viscera. J. Sci. Food Agric. 28, 647.
8. BATISTA I., 1986. Actividades desemolvidases in ano de 1984 in Servico de Tecnologia das Productos Aquäticos. Relatoria interno. № 131, LISBOA.
9. RAA J., GILDBERG A., 1982. Fish silage: A review. CRC Critical Reviews in Food Science and Nutrition, 383.
10. WINDSOR M., BARLOW S., 1981. Fish silage, Introduction to Fishery By-products, Fishing News Books Ltd. 84.
11. BATISTA I., 1984. Ensilados de Badejo (Micromesestius poutassou, Risso) relat. INIP. LISBOA (32) Agosto, 14 P. il.
12. BACKHOFF M.P., 1976. Some chemical changes in Fish silage. J. Fd. Technol.,11. 353
13. TATTERSON I.N., WINDSOR M.L., 1974. Fish silage J. Sci. Fd. Agric. 25, 369.
14. LINDGER S., PLEJE M., 1983. Silage fermentation of fish or fish waste Products with Lactic Acid Bacteria. J. Sci. Food Agric. 34, 1057.
15. BATISTA I., MENDES R., unpublished data.
16. RAA J., GILDBERG A, 2976. Antolysis and proteolytic activity of cod viscera. J. Fd. Technology. 11, 619.
17. KOMPIANG I.P., ARIFUDIN R., RAA J., 1980. Nutritional value of unsilaged by-catch fish from Indonesian shrimp trawlers, in Advances in Fish Science and Technology. Connell J.J. Ed. Fishing News Books, FARNHAM - Surrey, England, 349.
18. SMITH P., ADAMSON A.H.,1976. Pig feeding trails with white fish and herring liquid protein (fish silage), in Proc. Torry Res. Stn. Symp. Fish silage, Torry Research Station, ABERDEED, III.
19. DISNEY J.G., HOFFMAN A., OLLEY J., CLUCAS E.J., BARRANCO A., FRANCIS B.J.,1978. Development of a fish silage/carbohydrate animal feed for use tropics. Trop. Sci. 20 (2), 129.
20. BATISTA I., MENDES R.,1986. Actividades desemovidas em 1985 in Servico de Tecnologia dos Produtos Aquäticos. Relator ïo interno № 132, LISBOA
21. DUGAN L., 1975. Lipids VI. Chemical properties and reactions, in Principles of Food Science. Vol. 1, Fennema O.R. Ed. Marcel Dekkir, NEW YORK, 166.
22. BARLOW S.M, PIKE I.H., 1977. The role of fat in fish meal in pig and popultry nutrition. Tech. Bull. Int. Assoc. Fish Meal Manuf. U.K.,4, 38.
23. GARDNER H.W., 1978. Lipid hydroperoxides and their degradation, in Encyclopedia of Food Science. Peterson M.S. and Johnson A.H., Eds. AVI publishing, WESTPORT. Conn. 467.
24. SEN D.P., BHANDARY C.S., 1980. Lipid oxidation in raw and cooked oil sardine (Sardinella longiceps) fish during refrigerated storage, in Fats and oils in relation to Food Products and their Preparations. Central Food techn. Res. Inst. MYSONE, India, 132.
25. ANGLESA J.A., JACKSON A.J., 1985. Thiaminase activity in Fish silage and moist Fish Feed. Animal Feed Science and Technology. 13, 39.
26. GNAEDINGER R.H., KRZECZKOWSKI R.A., 1966. Heat inactivation of thiaminase in whole fish. Comm. Fish Rev. 28 (8), 11.
27. EVANS W.C., 1975. Thiaminase and their effects on animals, in Vitamins and Hormones. Vol. 33. Evans W.C. ed., 467.
28. ARNOLD R.G., 1978. Thiamine degradation chemistry, in Encyclopedia of Food Science. Peterson M.S. and Johnson A.H., eds. AVI Publishig. WESTPORT, Conn., 749.
29. AUSTRENG E., ASGARD T., 1984. Fish silage and its use. International Conference, October 12–13 VERONA.
30. HANSEN P., 1959. Ensilering of fisk og Fiskeaffald. Meddr. Fisk. Minist. Fors. Lab., Denmark, May,26.
31. RUNGRUANGSAK K., UTNE F. 1981. Effect of different acidified wet feeds on protease activities in the digestive tract and on growth rate of rainbow trout (Salmo gairdneri Richardson), Aquaculture 22, 67.
32. HARDY R.W., SHEARER K.D., STONE F.E.,WIEG D.H., 1983. Fish silage in aquaculture diets. J. World Maricult. Soc. 14, 695.
33. TORRISSEN O., TIDEMANN E., HANSEN F., RAA J., 1981/1982. Ensiling in acid - A method to stabilize astaxanthin in shrimp processing by-products and improve uptake of this by rainbow trout (Salmo gairdneri). Aquaculture 26, 77.
34. CHEN H.M., MEYERS S.P., 1983. Ensilage Treatment of Crawfish waste for imporvement of Astaxanthin Pigment Extraction. J. Food Sci.48, 1516.
35. DJAJASEWAKA H., DJAJADIREDJA R., 1980. Fish silage as a feed for fresh water' fish, in Proc. I.P.F.C. Workshop Fish Silage, FAD Fish Rep, № 230, 74.
36. CARL L.K.,1952. Fremgangs måde till knoservering of et foderstof. Dansk patentausøging № 34215 50. (Method for Preservation of a Feeding stuff. Application Danish Patent № 3415/50). Unpublished, can be seen at Direktoratet for Patent og Varemaerkeraesent, Nyropsgade 45, COPENHAGEN Y, Denmark.
37. MENDES R. unpublished data.
38. ROA P.D., 1965, Ensilage of fish by microbial fermentation. Fish News Int., 4 (3), 283.
39. JAMES M.A., IEYR K.M., NAIR M.R., 1977. Comparative study of fish ensilage prepared by microbial fermentation and formic acid ensilage, in Proc. Conf. Handling Process. Mark. Trop. Fish. Tropical Products Institute, LONDON, 273.
40. WIRAHADIKUSUMAH S., 1968. Preventing Clostriduim botulianum type E poisoning and fat rancidity by silage fermentation. Lantbi. Hö. Annls., 34, 55
41 DURAIRAJ S., SANTHANARAJ T., SULTAN K.M., DORAI RAJAH K.A.P.A.,. 1976. Utilization of trash fish. Fish ensilage - Some aspects of processing and storage. I. in Proc. Symp. Fish Process. Ind., Central Food Techn.
42. BATISTA I., MENDES R., 1986. Progress Report 1986. NATO PO FISHES. SUB-PROJECT NUTRITION, Action 4, Technology of fishfeed Production, INIP. LISBON.
43. KOMPIANG I.P., DARWANTO A., ARIFUDIN R., 1980. Nutritional value of fish silage in Proc. I.P.F.C. Workshop Fish Silage, FAD Fish. Rep. № 230, 44.
44. SCNRØDER K., CLAUSEN E., SANDBERG A.M., RAA J., 1980. Psychotrophic Lactobacillus plantarum from fish and its ability to produce antibiotics, in Advances in Fish Science and Tehcnology. Connel J.J. Ed. Fishing News Books; FARNHAM, Surrey, England. 480.
45. STANTON W.R., YEOH Q.L., 1977. Low salt fermentation method for conserving trash fish waste under SE Asian condition, in Proc. Conf, Handling Process, Mark, Trop. fish. Tropical Products Institute. LONDON, 277.
46. YEOH Q.L., 1980. The status of research on fish silage in Malaysia, in Proc. I.P.F.C. Workshop Fish Silage, FAO Fish, Rep, № 230, 19.