R. METAILLER
It is group of elements linked together and organized in the following way:
- A question on nutrition is asked, objectives are defined
- An experimental protocol is elaborated
- An experiment is implemented, it begins proceeds and ends
- The results are analysed
- The conclusions obtained answer the question asked:
These different points will be the main objective of the present work. In order to avoid going into too much detail in this report, we shall only envisage the problem from a fish viewpoint leaving in the hands of those concerned by the rearing of other zoological groups, the task of carrying out the necessary transpositions and additions.
There exists a multitude of questions of variable complexity which a nutritionist or a group of scientists may have to answer on a given context. They result from diverse motivations of zoological, nutritional, economical, pathological type. We shall not try to list them here when faced with a question, two possible attitudes may be adopted:
- An attitude of research (explicative, analytical), which will lead us to study a small number of factors which will vary by placing “everything equal elsewhere”.
- A practical attitude (synthetic) which applies more to field work: here, we shall not study one or more factors, but compare the systems, the technical or factoral combinations without obligatory placing “everything equal elsewhere”
Indeed, the “border-line” between these two attitudes is not always evident, as they coexist within the same experiment. It is therefore of particular importance, from the beginning, depending on which attitude has been adopted, to formulate the question correctly, to situate the problem accordingly and to define properly, in distinct terms for everybody, the limits of the objectives which are to be set, the experimental protocol will depend on this along with the processing, the capacity and the generalization of the results.
The objectives being well established,the nutritionist, with the means available, will conceive a plan, an experimental protocol which will lead to the implementation of an experiment. Indeed, the elaboration of a protocol requires a team of workers of at least 3 people:
- The person in charge of the protocol
- The research worker who will be in charge of the adaptation of this protocol “in situ”
- A person who is well versed in statistics.
It is certaintly difficult to give a typical model of the experimental protocol as each problem will in itself be a particular case, it is however possible to acquire a certain number of recommendations:
- The protocol must situate the question asked in regards with what is known on the subject.
- It must be precise and written in clear terms
- It must be complete and take into account the question asked on the whole, and not only one particular point.
- It must be simple,it is advisable to avoid going into to much detail, concerning the different parameters
- It must enable an inventory being made and take into account all the real constraints linked with a given problem (the rearing enclosures, water quality, the animals, the food ingredients, raw material, the distribution of food, lighting…)
- It must enable the inventory being made of the measures to take and the means required to perform them.
- It must enable the distribution of different tasks
- It must enable the establishment of an agenda
- It must allow the scheduling of the way in which the results will be processed and interpreted
- It must eventually allow the estimation of the costs
- It must be logical and clearly comprehensive. Why should one try to compare uncomparable “things”?
When a protocol is formulated, the author must verify that the experiment scheduled has every chance of correctly answering the question asked. They must also ensure the no deviation could likely compromise the interpretation of the results or be misleading.
Tables 1a, 1b, 1c, and 2a, 2b, 2c, 2, d have but one objective, that is to visualize, as an example, the possible evolution of the experimental choices, in confrontation with a given problem: the testing of the different raw materials or the study of the protein requirements of a given species.
In all cases, it should be kept in mind that the formulation of an experimental protocol corresponds to a choice among the different possible options. The most important point is to define the consequences of this choice with regards to the question asked and the result expected.
Indeed, this concerns the practical application, “in situ”, of everything which has been thought and written in the experimental protocol.
It is very difficult to carry out experiments in production units. On one hand, they are too big and require a great number of animals and a large quantity of food; due to this fact, the experiment is naturally limited. On the the other hand it must be remembered that all experiments must receive a close follow up and more care must be taken than in ordinary routine rearing; the personal working in the production stations cannot always ensure this follow up correctly. Finally, the obligations demanded in production do not always comply with the experiment, (the animals must show good growth, bargain sales, etc…)
It is therefore advisable to have extra rearing structures installated for the experiment alone and specifically designated staff to operate them. The size of the“system” employed doesn't really matter; it can consist of set of 51 to 500 1 capacity tanks, a floating cage raft of 30 m3 or a series of ponds of 1 hectare. the essential point to remember is that there must be a clear distinction made between experiment and production.
The number of experimental enclosures is often a limiting factor when developing an experimental protocol, more so, as the results can vary greatly and it is not possible to test a given parameter in only one structure. It is absolutely necessary to carry out replications. The minimum number of replication necessary can be theoretically calculated in accordance with the variability of the parameters measured, by the difference that we wish to emphasize and by the threshold chosen. In practice, a minimum of three replication is generally necessary for each experimental group with all the consequence that this entails, in number of animals, quantity of food, upkeep time.
Only when the experimentation itself has this as objective, the environment for the animals must be as similar as possible for all the groups: flow, quality, temperature chemical composition of the water, dissolved oxygen, lighting, form, size, colour and position of the experimental enclosures, phonic environment and more so than ever this is necessary when the objectives is axed on research.
So as to eliminate a certain number of badly controlled factors, inevitably linked with a given rearing structure, it is necessary to divide up, at random, the different experimental groups (and even better still, the different replicas) in the rearing unit (Diag.1). This distribution can be done at random or by means of a table of numbers which are also picked at random. In this case, we resort to the most simple (from a statistic viewpoint) experimental system: this is a totally randomized system.
It is also highly advisable to regularly permute (when weighting especially) the experimental groups, but this implies having at disposal a certain number of free supplementary rearing enclosures (Diag.2).
It is also possible when the variation of a factor is inexorable but defined (ex. Natural light gradient in the rearing room or tidal current in the floating cage raft) to use more or less complex statistic models, permitting to take into account these factors at implementation level of the experimental plan. Some of these plans (block systems) are highly recommended even when the gradient of the secondary factor has not been clearly defined.
Another point, which is often forgotten, is that of the experimental unit. From a statistic viewpoint, the different animals in an enclosure are rarely correct uncertain variables. On the contrary, the weight, length averages are much more reliable experimental units; this remark reinforces the necessity of replicas.
They must be healthy and of the same origin; densities should be adapted to the rearing enclosures and defined in such a way so as to ensure good conditions for the animal throughout the experiment. It is necessary to schedule eventually the animals for sampling during the experiment. A histogramme of the population must be carried out; the distribution must be normal and as coordinate as possible so as to favour the apparition of significant differences between the groups and so as to minimize competition between the animals.
It must be remarked that the above recommendations are valid if research is the prime objective, (Chap.2) and requires a homogenous population responding to the problem studied. On the contrary, in practice, there will be a tendency to employ a representative group of the population studied which will then be well defined in the protocol.
Experimental food differs from the classical food normally employed, by a certain number of criteria. It must be simple, in other words, it must contain many raw materials. To establish standard diets or to study food requirements, the raw materials must be obligatory known, of very good quality, and have a good intrinsic value (from year to year and during one year). This is one of the reasons why casein and gelatine are employed in purified food.
However important or insignificant the experiment scheduled may be, the manufacture of the food (whether 1 kg or 1 ton) will always be difficult to perform by means of the general routine conveyor belt production system; therefore, it is necessary to have at disposal an apparatus adopted to the dimension of the experiment (mixers, meat mincers, press, dryer, crushing mill, sieve, …)
For a given experimentation, which lasts not more than 3 to 4 months, on general, it is advisable to schedule the manufacture of all the food at the same time and to ensure good processing and slocking throughout the whole period of experimentation When the food is prepared daily, the same unique stock of raw materials shall be employed throughout the whole period.
A precaution very often neglected while very useful consists in the biochemical analysis (very summarily: humidity, total proteins and lipids, ashes) of the food before starting the experiment so as to verify that it is conform to the standards demanded. In all cases, the humidity rates should be regularly determined for the calculation of the ration distributed.
When manufacturing the food, especially if there is a great difference between the different diet formulations, a homogeneity in the food size along with its consistancy and stability must be respected, so as not to induce any errors in the experimentation. The use of diverse binders permits avoiding this type of artefact.
The distribution of the food is an important point to determine as the choice made (fixed ration or ad libitum feeding) has consequential effects on the experiment itself and on the interpretation of the results.
The distribution of fixed rations (for example 1% of the biomass per day) is easy (food doses ready for manual or automatic distribution) but requires a regular follow up of the fish stock so as to adjust the quantity distributed according to the growth of the fish. This method of feeding will be employed especially when one wishes to distribute isoproteic or isoenergetic rations… It implies on the contrary the regulation of the food ration which will be less consumed.
This risk causing certain groups being “underfed” and thus limits the differences in growth at the end of the experiment. Finally, it must be remarked that lose of appetite will cause slight differences in the quantity of food really ingested by the animals.
Ad libitum distribution will be adopted when the quantity of food ingested is the objective of the experimental result. This can however cause difficulties in the interpretation. The distribution while employing automatic devices (self feeders) limits human intervention. Manual distribution is more time demanding while permitting at the same time to give a better appraisement of the behaviour of the population
This has been defined in the protocol, but different arrangements can be envisaged in accordance with the evolution of the population.
- For animals weighting less than one gram (wearing) 3 weeks to one month can be sufficient for the experiment: the stoppage in mortality is often chosen as a criterion to define the end of the trial
- For animals weighting a few grams, 2 or 3 months must be scheduled for the experiment and 3 to 4 months for animals weighting more than one hundred grams. In these cases, it is the doubling or tripling in weight which is scheduled. In fact, if we have opted for a regular follow up of the stock, it is possible to to stop the experiment as soon as significant differences of the factor in question have been established.
- In certain cases, longer duration periods are necessary, for example so as to observe the deficiency symptoms for which the multiplication by 10 of the initial weight is sometimes necessary.
It is absolutly necessary to keep daily notes on the rearing in a copybook (and not on loose pages) where all the data may be clearly and precisely found, concerning the experiment form the general outline principles to the smallest details.
They must be taken at random, from a graded stock of animals (§ 4.2), which are perfectly adapted to this environment. For salmonids for example, the acquirements of groups must be avoided when they are at sea. So as to obtain a good homogeneity in the different experimental groups, they must not be collected in one go, but little by little (Diag. 3). This operation, although it may seem simple, should be well prepared in advance and great care taken so as to avoid errors being made in the count.
The establishment of the initial weight of the animals (from day zero -beginning of the experiment) can be carried out as soon as the groups have been obtained while applying the method chosen, perhaps with the delay in time so as to avoid supplementary stress caused to the animals.
In all cases, the animals should be weighed while fasting (24 or 48 hours) so that the true weight of animals may be correctly estimated.
- If the distribution is perfectly homogenous and if there is a sufficient number of animals available, it will be possible to schedule a supplementary group, which shall receive the same treatment as the others and killed so as to define the initial average weight and the initial biochemical composition (for all the groups).
- It is also possible to weigh the different groups. This can be done by weighting individually the animals; in this case, this operation also serves to verify the number of fish. Each group alone can also be weighed while counting them or not. Finally a sample can be taken from each group as long as a sufficient number of animals have been scheduled at the beginning. The samples are killed, weighed and kept for analysis.
As in all rearings, solutions causing the less stress possible are researched.
So as to avoid all parasite parameters which have an influence on the experiment, the upkeep of the animals will be greatly taken into account (Cleanliness of the rearing tanks, food distribution, collection and deduction of dead animals).
Throughout the diverse manipulations, it should be advisable to schedule a light anesthetic for the animals so as to avoid causing repeated and great stress.
- 1 mg/1 of quinaldine (1 ppm)
- 0.2 ml/1 of phenoxyethanol (200 ppm) Ethyleneglycol monophenylic ether.
These doses are given only as indications and should be adapted according to the specimen, its age, and the anesthetic degree desired.
It is also advisable during each intervention, to carry out antiseptic treatments:
- Furazolidone: a 20 mm bath in a solution of 20 ppm.
- Quaternary ammonium: a 20 mm bath in Ca solution of 1 ppm of the active principle (which is 5 mg/1 of CETAVLON at 20 %, for example). The doses indicated must be followed for these products.
This is of assistance so as to follow the evolution of the different groups and necessary if the food distribution method employed is fixed rations. It must also be remarked that the attainment of a growth curve permits a better estimation of the initial and final weight.
Live animals can be weighed dry (or by means of a damp cloth or in a container containing water. As for all manipulation, the animals should receive a light anesthetic. Taking into account the local conditions, the solution causing the less stress will be chosen.
As has been stated in § 5.2, the animals may be weighed individually, the whole group together or a sample taken from each group and weighed, associated or not with the deduction of the animal. It must be remarked that the replacement of the samples back into the container must be avoided as this not comply with the conditions imposed by the statistic calculations.
The weighing of a doomed sample presents many advantage, on the contrary
- Authentification of the statistic treatment
- Study of the homogeneity of the population
- Limited stress for the animals kept in the rearing
- Possibility of biochemical analysis.
It is difficult to define outside a given context, the dimension of the sample. This can only be defined through experience and by means of an appropriate statistical investigation; Let us however remark that samples, counting around 30 animals at a time, is a good basis to begin with. Evidently, a sample must be taken correctly, which is not always an easy task and may call for a test being performed beforehand. It is necessary to them in the population into one corner of the tank, pond or cage before taking the sample.
The precision of weighing itself must neither be taken too seriously nor too carelessly but a just medium must be found. Even with the use of a mg electronic scales, it is not reasonable to employ such precision for an aquatic animal (even dry) which weight no more than a fews tens of gm. Even a 50 g precision balance must not be employed to weigh one kilo only.
When the weighing is carried out on land, the research scientist can choose his own method of precision. When the weighing is carried out at sea (boat, raft, floating cage) a problem will arise whatever the precision method chosen may be, due to the movement caused by the water. If the doomed sample solution (weighed on land) can not be adopted, a method employing several distinct samples must be utilized.
“Weight” alone can not allow the judgement of the real effect of the treatments. A ponderal increase has indeed a completely different signification depending on whether it corresponds to an increase in tissue proteins or in fat deposit. Thus, in every experiment, it is necessary to take an initial and final sample so as to carry out the biochemical and eventually the clinical and histological analysises which will allow a better comparison of the different groups. The intermediary samples (weighing of doomed samples 5.4), can be used so as to follow up the state of health of the animals during the period of the experiment.
As for the samples taken for the biochemical analysis, the best compromise to adopt between:
- the necessity of having to carry out a number of individual analysises so as to compensate for the heterogeneity of the composition of the animals.
- and the difficulty in carrying out numerous analysises (time, price, personnel), seems to initiate the division, into experimental groups of four pools of around 10 animals with an average group weight. The animals in each pool will be minced and homogenized. Only one aliquot pert will be conserved per analysis (deep freezing, lyophilization).
Before the sample is taken, the animals are not fed for 24 hours, or even 48 hours beforehand, so that the digestive content will have no effect on the analysis scheduled. However long the animals are kept fasting, it is essential that this period be the same for all the samples taken in the same experiment.
Let us point out that depending on the analysis method chosen a few tens of g (live weight) will suffice out the obligatory elementary analysis: rates of humidity, proteins, lipids and ashes.
They will be treated in accordance with the demand:
- mean weight gain
- Final average weight (FAW) - initial average weight (IAW).
The fact of using the average weight instead of the total weight allows the use of the notion “gain” even when there exists a certain mortality, provided however that there is a feeble rate.
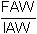
Not employed much by scientists, the multiplier coefficient is sometimes employed by aquaculturists:

This parameter is hardly ever employed
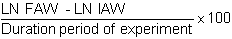
or 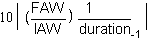
SGR is greatly employed. It permits the comparison between the results of the different experiments not having the same time lengths as one another. This in fact is slope B of the exponential aquation which characterizes the growth of the animals
Y = aeBx
where Y is the weight of the animals at time (X)
and a the initial weight.
- Weighing and measuring of the animals.
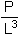
where P is expressed in g and L in cm.
It will be necessary to define if L corresponds to the total fork or standard lenght. This parameter permits to somehow visualize the state of “good health” of the animals. It is often linked with the fattening state of the animals.
* Conversion rate CT (or sometimes known as feed conversion)
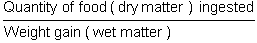
The conversion rate is largely employed; it permits having a global idea of how the food is used. one mustn't be alarmed to obtain for certain species values of less than 1, this can appear aberrant at first glance for heterotrophic species. it is due to the fact that the numerator is counted in dry matter weight, while the denominator is counted in wet or raw material weight.

This parameter is less employed than the preceding one.
Hepatosomatic index RHS
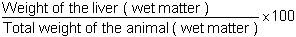
This index is often employed (easily worked out) to estimate the good state of the liver which is one of the keys organs for the metabolism of food.

This will enable to define the state of the animals from a fattening viewpoint but it is often difficult to establish, as depending on the species, pervisceral fat is often more or less attached to the digestive tract:

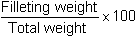
The two latter practical notions permit the economic estimates depending on how the product is commercialized.
Protein efficiency ration (PER)
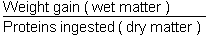
This permits a global estimation of the transformation of food proteins.
This concerns pools of animals (§ 5.5)
* Protein utilization coefficient (PUC)
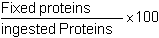
| with Fixed proteins | % animal nitrogen whole at the end of the experiment | 1 × 6.25 × FAW | % animal nitrogen - whole at the beginning of the experiment | × 6.25 × IAW |
This allows the precise appreciation of the transformation efficiency of food proteins.
The use of total lipids allows an objective global appreciation of the fattening state of the animals and its evolution throughout the experiment.
- Hepatic lipids
- Carcass lipids
- Muscular lipids
- Visceral lipids
- Hepatic glycogen
All this data will permit a better comprehension of the phenomena remarked.
- Hematocrit: gives a global idea of the eventual state of anemia of the animals.
- Circulating blood sugar
- Circulating free amino-acids
This permits the appreciation of the impact at cell level of the different treatments.
6.10 Digestibility measures by incorporating a trace element in the food and collecting the faeces
* Apparent digestibility coefficient
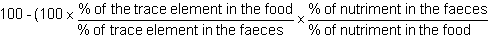
This enables to define the digestibility of different nutriments.
When the data has be obtained in the appropriate way (correct sampling: § 5.4) and sufficiently repeated), it is necessary to do an adequate statistic processing of this. By means of a varience analysis, it will be possible to verify if the results obtained differ in a significant way on the whole. If this is so, a test of multiple comparisons must be carried out and this will permit the comparison of the different results with one another. The Newman Keuls test is particularly suited to problems which are frequently encountered in nutrition. A example of calculation is given in the chapter on “Statistic exploitation from experimental plan to a factor studied with repetitions.
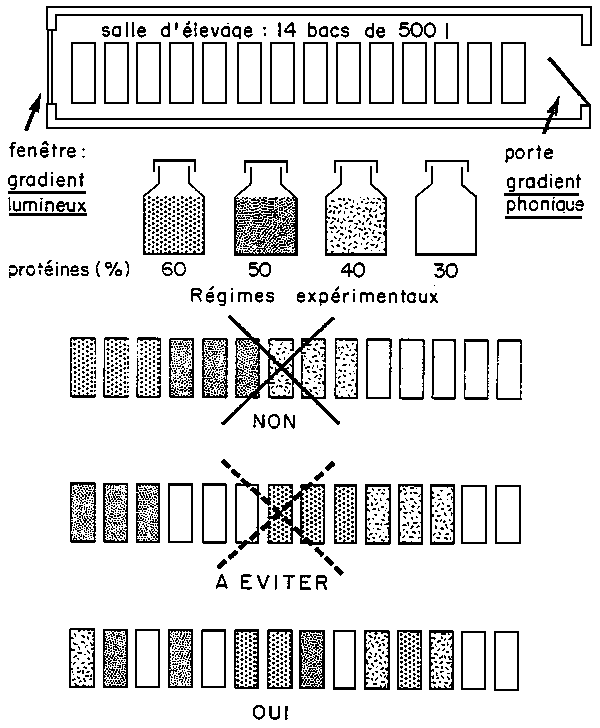
Figure 1 - Répartition des lots expérimentaux
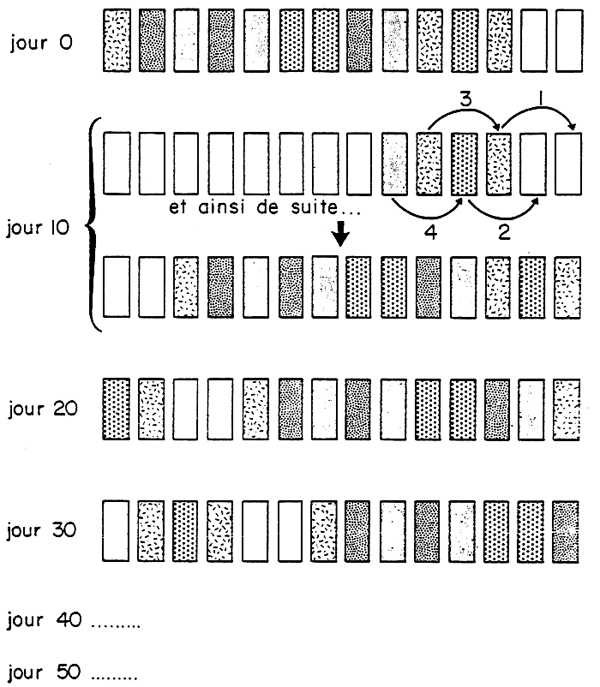
Figure 2 - Permutation régulière des lots expérimentaux
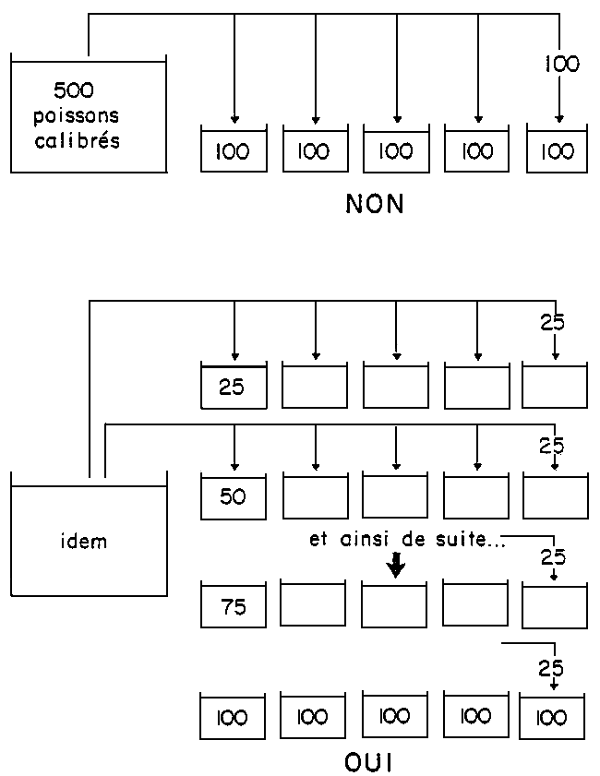
Figure 3 - Constitution des lots expérimentaux
| REGIMES | A | B | C | D | E |
| INGREDIENTS | |||||
| Caséine | 38 | 18 | 18 | 18 | 18 |
| Dextrine | 28 | 28 | 28 | 28 | 28 |
| Gélatine | 12 | 12 | 12 | 12 | 12 |
| Huile de foie de morue | 9 | 9 | 9 | 9 | 9 |
| Cellulose | 8 | 8 | 8 | 8 | 8 |
| Prémélange minéral | 4 | 4 | 4 | 4 | 4 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 | 1 |
| Farine de poisson | - | 20 | - | - | - |
| Levure de bière | - | - | 20 | - | - |
| Tourteau de soja | - | - | - | 20 | - |
| Blé | - | - | - | - | 20 |
| Protéines | 43 | 41 | 36 | 36 | 29 |
| Lipides | 10 | 11 | 9 | 10 | 10 |
Tableau 1a : Testage de différentes matiéres premiéres. Composition (%) deg régimes expérimentaux.
Substitution de 20% de diverses matiéres premiéres à une quantité équivalente, …sont différentes pour chaque aliment. Lea différences éventuelles que l'on peut mettre en évidence ne sont interprétables au plan nutritionnel.
| REGIMES | A | B | C | D | E |
| INGREDIENTS | |||||
| Caséine | 38 | 21 | 27 | 26, 6 | 35 |
| Dextrine | 28 | 28 | 24 | 24,4 | 19,4 |
| Gélatine | 12 | 12 | 12 | 12 | 12 |
| Huile de foie de morue | 9 | 7, 3 | 9 | 9 | 8, 6 |
| Cellulose | 8 | 6, 7 | 3 | 3 | - |
| Prémélange minéral | 4 | 4 | 4 | 4 | 4 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 | 1 |
| Farine de poisson | - | 20 | - | - | - |
| Levure de biére | - | - | 20 | - | - |
| Tourteau de soja | - | - | - | 20 | - |
| Blé | - | - | - | - | 20 |
| Protéines | 43 | 43 | 43 | 43 | 43 |
| Lipides | 10 | 10 | 10 | 10 | 10 |
Tableau 1b : Testage de différentes matiéres premiéres. Composition (%) des régimes expérimentaux (isoprotéiques et isolipidques).
Dans ce cas, il eat tenu compte de la composition en protéines, lipides, … des diverses matiéres premiéres lors la composition des aliments. La substitution se fait au niveau protéique, lipidique, glucidique, …; elle fait done intervenir la caséine, l'huile de foie de morue, la dextrine et la cellulose (qui sert de ballast).
Ainsi, c'est bien l'effet “matiéres premiéres” qui est étudié; dependant chaque matiére premiére n'étant testée qu'à un seul taux d'incorporation, il, est nécessaire d'être prudent dans l'interprétation et la généralisation des résultats.
| REGIMES | C0 | C5 | C10 | C15 | C20 |
| INGREDIENTS | |||||
| Levure de biére | - | 5 | 10 | 15 | 20 |
| Caséine | 38 | 38, 25 | 32, 5 | 29, 75 | 27 |
| Dextrine | 28 | 27 | 26 | 25 | 24 |
| Gélatine | 12 | 12 | 12 | 12 | 12 |
| Huile de foie de morue | 9 | 9 | 9 | 9 | 9 |
| Cellulose | 8 | 6, 75 | 5, 5 | 4, 25 | 3 |
| Prémélange minéral | 4 | 4 | 4 | 4 | 4 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 | 1 |
| Protéines | 43 | 43 | 43 | 43 | 43 |
| Lipides | 10 | 10 | 10 | 10 | 10 |
Tableau 1c : Testage d'une matiére (levure de biére: gradient d'incorporation). Composition (%) des régimes expérimentaux.
Chaque matiére premiére testée. Il est possible ainsi d'étudier son efficacité réelle et son taux optimal d'incorporation pour la formule de base considérée
| REGIMES | A | B | C | D |
| INGREDIENTS | ||||
| Farine de poisson | 50 | 61, 1 | 72, 2 | 83, 3 |
| Amidon cuit | 2 | 2 | 2 | 2 |
| Cellulose | 36 | 26 | 16 | 6 |
| Huile de poisson | 7 | 5, 9 | 4, 8 | 3, 7 |
| Prémélange minéra | 1 | 1 | 1 | 1 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 |
| Liant | 3 | 3 | 3 | 3 |
| Proteins | 36 | 44 | 52 | 60 |
| Lipides | 12 | 12 | 12 | 12 |
Tableau 2a : Besoin protéique du bar. Composition (%) des régimes expérimentaux: gradient de farine de poisson centre gradient inverse de cellulose.
L'expérience envisagée est tout à fait valable; cependant les régimes ainsi composés sont peu rélistes au plan pratique et même théorique (taux de cellulose excessif).
| REGIMES | A | B | C | D |
| INGREDIENTS | ||||
| Farine de poisson | 50 | 61, 1 | 72, 2 | 83, 3 |
| Amidon cuit | 26 | 18 | 10 | 2 |
| Cellulose | 12 | 10 | 8 | 6 |
| Huile de poisson | 7 | 5, 9 | 4, 8 | 3, 7 |
| Prémélange minéral | 1 | 1 | 1 | 1 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 |
| Liant | 3 | 3 | 3 | 3 |
| Protéines | 36 | 44 | 52 | 60 |
| Lipides | 12 | 12 | 12 | 12 |
Tableau 2b : Besoin protéique du bar. Composition (%) des régimes expérimentaux: gradient de protéins centre gradient d'amidon.
Les régimes envisagés ici plus proches des régimes pratiques mais il n'est pas tenu compte de leur énergie, ce qui entranîne un bicis dans L'expérimentation.
| REGIMES | A | B | C | D |
| INGREDIENTS | ||||
| Farine de poisson | 50 | 61, 1 | 72, 2 | 83, 3 |
| Amidon cuit | 37, 7 | 25, 8 | 13, 9 | 2, 0 |
| Cellulose | 0, 3 | 2, 2 | 4, 1 | 6, 0 |
| Huile de poisson | 7 | 5, 9 | 4, 8 | 3, 7 |
| Prémélange minéral | 1 | 1 | 1 | 1 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 |
| Liant | 3 | 3 | 3 | 3 |
| Protéines | 36 | 44 | 52 | 60 |
| Lipides | 12 | 12 | 12 | 12 |
| Energie digestible (Kcal/100g régime) | 388 | 388 | 388 | 388 |
Tableau 2c : Besoin protéique du bar. Composition (%) des régimes expérimentaux
: protéines contre amidon, régimes isoénergétiques
(énergie digestible des protéines : 4, 76 Kcal/g;
de I 'amidon : 3, 2 Kcal/g;
des Iipides : 8 Kcal/g).
L 'objection précédente (cf tableau 2b) est supprimée; cependant à chacue taux de protéines ne correspond qu 'un taux de glucides: it faut done étre trés prudent lors de l 'interpretation des résultats.
| REGIMES | B1 | B2 | B3 | C2 | C3 | D3 |
| INGREDIENTS | ||||||
| Farine de poisson | 61, 1 | 61, 1 | 61, 1 | 72, 2 | 72, 2 | 83, 3 |
| Amidon cuit | 2 | 13, 9 | 25, 8 | 2 | 13, 9 | 2 |
| Cellulose | 26 | 14, 1 | 2, 2 | 16 | 4, 1 | 6 |
| Huile de poisson | 5, 9 | 5, 9 | 5, 9 | 4, 8 | 4, 8 | 3, 7 |
| Prémélange minéral | 1 | 1 | 1 | 1 | 1 | 1 |
| Prémélange vitaminique | 1 | 1 | 1 | 1 | 1 | 1 |
| Liant | 3 | 3 | 3 | 3 | 3 | 3 |
| Protéines | 44 | 44 | 44 | 52 | 52 | 60 |
| Lipides | 12 | 12 | 12 | 12 | 12 | 12 |
| Energie digestible (Kcal/100 g régime) | 312 | 350 | 388 | 350 | 388 | 388 |
Tableau 2d: Besoin protéique et glucidique du bar. Composition (%) des régimes expérimentaux: gradient de protéines et d' amidon.
Pour chaque taux de protéines, plusieurs taux de glucides sont testés. Les régimes indexés des mêmes chiffres sont isoénergétiques. Par souci de clarté, seuls les régimes B, C et D du tableau précédent sont envisagés.