J. GABAUDAN
Let us consider the fish as a transformer of raw materials of various origins (often by-products of the food industry) into high quality proteins for human consumption. Firstly, the structure and function of the organs responsible for ingestion, digestion (hydrolysis) and absorbtion will be examined: A good understanding of the histology of the digestive system can also help to evaluate the nutritional status of the fish and detent possible nutritional deficiencies or imbalances:
Secondly digestibility of raw materials will be discussed as a quality criterion and quantitative data required for accurate fish formulation. This will include a description of the methods used for digestibility measurements. Techniques for the determination of gastro-intestinal evacuation time will be mentioned as well with nutritional and environmental factors affecting this parameter. Lastly some aspects of the nutritional bioenergetics of fish will be presented, including energy metabolism, energy requirements and sources of energy.
* Fish with a stomach
The digestive tract includes the following parts: oral cavity, pharynx, esophagus, stomach, pyloric ceca and intestine. Besides, there are two digestive glands: liver and exocrine pancreas, the latter beings often a disseminated organ. The shape of the stomach varies with the species and can be described as:
- straight
- Y shaped
- siphon shaped
Roughly, the lenght of the digestive tube depends on the feeding regime (Smith, 1980)
- 0. 2 to 2. 5 times the Length of the fish for the carnivores,
- 0. 6 to 8. 0 times for omnivores,
- 0. 8 to 15. 0 times for herbivores:
However, other factors affect the length of the digestive tube, such as the proportion of ingested sediments:
* Stomach-Less fish
The general organisation is the same except that the stomach is missing. It is sometimes replaced by an expansion of the intestine called the intestinal bulb but its histology does not differ from that of the anterior intestine. Therefore, stomach-less fish have no acid digestion of their food. The absence of a stomach cannot be related to the feeding regime (Cyprinidae, Scaridae, Sc-mberesox, some species of Syngnatidae).
Since histologic differences exist among species, only the most commonly encountered structures will be described here. Further details can be found in the reviews of Kapoor et al. (19750, Fange and Grave (1979) and Gas and Noaillac Depeyre (1981). The wall of the digestive tube is constituted by 3 layers: (1) the mucosa which includes the epithelium and the sub epithelial connective tissue, (2) the muscularis externa formed by a circular and a longitudinal muscular layer and (3) the serosa
a) Esophagus
* Structure
- mucosa : stratified squamous epithelium, (inthe eel, the epithelium becomes simple after adaptation to seawater); numerous goblet cells present in the epithelium; sub epithelial connective tissue: generally formed by an inner dense layer and an outer loose layer. Presence of numerous leucocytes.
- muscularis externa : composed by two layers of striated muscle, an inner longitudinal-one and an outer circular-one.
- serosa : mesothelium plus connectivetissue
* Function
The esophagus conveys the food to the stomach. This is made easier by its ability to distend and its strong layers of striated musce.
b) Stomach
* Structure
The esophagus to stomach transition is abrupt for the epithelium while it is progressive for the muscularis externa.
The stomach can be devided into 3 regions: cardiac and fundic which contain gastric glands and pyloric region which has no glands.
- mucosa : high columnar, simple epithelium. The luminal surface of the epitheliocytes has short and widely spaced microvilli and it is covered by a mucous coat. Usually there are no goblet cells but the apical cytoplasm contains mucous granules which are secreted into the gastric lumen by exocytosis. Exocrine glandular cells are located in the connective tissue. They are radially arranged around the gland lumen and of a single type.
- muscularis externa : it is composed of two layers of smooth muscle, an inner circular-one and an outer longitudinal-one.
- serosa : it is made of a relatevely thick connective tissue layer and a masothelium.
* Function
Gastric glands secrete hydrochloric acid pepsinogene which becomes pepsin in the presence of a low pH. Therefore the stomach is the site of acid digestion of proteins. The pH in its lumen is equal to 2. The presence of lipases and chitinase has also been reported in the stomach of some species.
c) Intestine
* Structure
On the basis of ultrastructural and functional criteria, the intestine can be divided in 3 regions: (1) anterior intestine, (2) posterior intestine and (3) rectum.
- mucosa : Anterior intestine: it forms numerous branched folds. The epithelium is composed of a simple layer of high columnar cells, the enterocytes, among which lay goblet cells. The apical plasma membrane forms microvilli which constitute the brush border thus increasing the exchange surface between the lumen and the enterocytes. The cytoplasm contains lipid vacuoles and lipid particles.
Posterior intestine: the folds of the mucosa are thicker and shorter. The cytoplasm of enterocytes has numerous supranuclear eosinophilic globules. Transmission electron microscopy reveals in this region of the cells numerous small channels and electron dense vacuoles. Histochemical methods demonstrated that these enterocytes are able to ingest macromolecules by endocytosis.
Rectum : the epithelial cells usually secrete mucopolysacharides in abundance. This region is very short and just precedes the anal opening.
- muscularis externa and serosa : these layers are similar to those found in the stomach. At the anal opening, the circular smooth muscle thickens to form a sphincter.
* Function
The intestine has two major functions: digestion and absorption.
Digestion : digestive enzymes active in the intestinal lumen at a pH close to neutrality are secreted either by exocrine pancreas or by the intestinal mucosa. The latter secretes:
+ proteases: aminopeptidase
di- and tripeptidase
+ alcaline and acid nucleosidases
+ polynucleotidases
+ lecithinase, lipase and esterase
+ amylase, maltase, laminarinase etc…
Absorption : nutriments are absorbed by the enterocytes and enter into the circulatory system through blood capillaries located in the subepithelial connective tissue. Then, they are carried to the liver by the portal vein.
Histochemical studies suggest that amino acids, fatty acids and glucose are mostly absorbed by the anterior intestine while macromolecules (including proteins), water and minerals would be absorbed by the posterior intestine.
Note concerning pyloric ceca :
Pyloric ceca are finger-like projections of the anterior intestine wall located just past the pyloric sphincter. Their histology and function is comparable to that of the anterior intestine. By retaining food, they increase the intestinal evacuation time.
d) Liver
* Structure
The hepatic parenchyma is composed of polygonal cells called hepatocytes. Hepatocytes are radially arranged around a central vein in two-cell thick laminae separated by sinusoids. The cytoplasm contains glycogen and lipids. The hepatic artery and the portal vein enter into the liver. The junction of several hepatocytes form the bile canaliculi which drain the bile to the gall bladder where it is stored. The bile is then delivered to the intestinal lumen by the common bile duct.
* Function
Bile secretion is the major digestive ole of the liver. The bile emulsifies the lipids in the intestinal lumen thus facilitating enzymatic activity. Th ebile also neutralizes the intestinal content which is strongly acidic whwn it enters into the intestine. The liver has numerous other functions which are not digestive:
+ storage of energy (in the form of lipids and glycogen)
+ protein synthesis (plasma proetins, for example)
+ site of gluconeogenesis and deamination of aminoacids
+ detoxification and inactivation of toxic substances.
e) Pancreas
* Structure
Pancreas is composed by endocrine glands which secrete hormones (glucagone and insuline) and exocrine glands which secrete digestive enzymes. Exocrine pancreas is usually diffuse and consists of scattered acini in the esemteries and within the liver surrounding branches of the hepatic portal vein. A pancreatic canal opens into the intestine next to the common bile duct. The pyramidal exocrine cells secrete their products into the lumen of the acini. Their nucleus is basal and spherical, the cytoplasm is basophilic except at the apex where numerous acidophilic granules can be found. These granules are zymogen granules containing the digestive proenzymes.
* Function
The exocrine pancreas secretes the following digestive enzymes:
+ proteases : trypsine, chymotrypsine, carboxypeptidase and elastase (all are stored and secreted as proenzymes and activated in the intestianl lumen)
+ amylase
+ lipases
+ chitinases (in some species)
The process of digestion is well described in the papers of Fange and Grove (1079). Table 1 shows the origin and the role of major digestive enzymes and thus indicates how proteins, carbohydrates and lipids of raw materials are hydrolized into aminoacids, glucose and fatty acids.
Absorption of digestion products takes place by diffusion, active transport and endocytosis.
* Proteins : free amino acids and peptides od low molecular weight are actively absorbed by enterocytes of the anterior intestine. In some species, proteins and polypeptodes are absorbed by endocitosis and digested within the cells. This process would allow, among other things, to partially recycle some digestive enzymes.
* Lipids : fatty acids would be absorbed by enterocytes of the anterior intestine and pyloric ceca.
* Carbohydrates : absorption of glucose occurs by active transport.
The nutritional value of a feed ingredient depends on its chemical composition and on the ability of the fish to digest and absorb its nutrients.
Digestibility represents all the processes leading to absorption by the intestinal mucosa of the ingested food. Therefore it can be evaluated indirectly as the difference between ingested nutriments and nutriments excreted in the faeces.
The apparent digestibility coefficient (DCA) is calculated with the following formula:
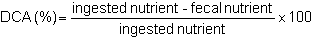
The true digestibility coefficient (DCT) takes into account excreted endogenous material such as spent enzymes, mucus, desquamated cells and bacteria. Its formula is:
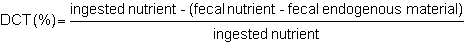
Since it is technically difficult to measure endogenous excretions, apparent digestibility is usually determined.
Measuring digestibility coefficient is simple in its principle: one must collect the faeces and titratethe substance of interest in the feed and in the faeces. Technically it is not simple since the faeces are released into the water and therefore leaching of soluble compounds must be controlled.
Two methods, direct and indirect, and several techniques for faeces collection allow the evaluation of apparent digestibility.
* Direct method
All of the ingested food and excreted faeces must be quantitatively determined. This is done in a metabolism chamber which allows the simultaneous and separate collection of faecal, gill and urinary excretions (Smith, 1971). The chamber (fig.1) consists of body of the fish. Gill excretions are collected in the anterior part, faeces (and water) in the posterior part and urine is collected through a catheter introduced in the ureter.
The collection of excretory products is therefore qualitative and quantitative. This makes possible the determination of apparent digestibility coefficients of any desired nutrient and the matabolisable energy of the tested diet.
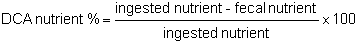
ME = IE - ( FE + ZE + UE )
where : IE gross intake energy
FE fecal energy
ZE energy in gill excretions
UE urinary energy
* Indirect method
The addition in the diet of an inert, undigestible and not absorbable marker eliminates the need for quantitative faeces collection. Indeed the variation of the ratio nutrient maker in the diet and in the faeces measures the digestibility as follows:
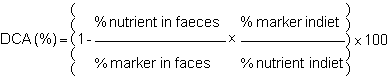
The most frequently used marker in digestibility studies is chromic oxyde (Cr2o3). It is usually incompored into the diet at a rate of 1‰ It can be titrated rapidly and easily by the method of Bolin et al. (1952) and it is believed not to interfere with the digestive process and gastro-intestinal transit.
Faeces collection in fish, even when not quantitative, is more problematic than for land animals. Indeed, leaching of soluble compounds occurs just after release and can lead to significant overestimations of CDA. Therefore, particular attention must be paid to the choice of a collection technique and to its possible adaptation to a given situation.
In order to eliminate leaching losses, the fish can be taken out of the water and the faeces removed by:
- stripping : the fish being under light sedation, pressure is applied with the fingers on the abdomen in the antero posterior direction
- analsuction : a glass tube is introduced into the anus and the rectum content is removed by suction
- dissection : the fish is sacrified, the rectum is dissected out and its content sampled.
Faeces collection can also be done continously by one of the following methods:
- netting: the faeces are picked up with a net as soon as they are released
- settling : figure 2 shows the experimental setup used at IFREMER which is derived from the disign proposed by Cho et al. (1982). A settling column is connected to the base of a cylindro-conical tank. The water flows through the column in which faecal material settles. Faeces remain in stagnant water until they are collected with a little water as possible through an opening located at the bottom of the column. The sampling, therefore does not cause any disturbance to the fish.
- continous filtration : the aparatus, shown in fig.3, was designed by Choubert et al.(1982). The water flows through screens moving linearly, thus separating the faeces and faeces and dropping them into a collecting pan.
* Validity of the methods
The methods described above were compared using the same raw materials and sea bass coming from the same origin at IFREMER, Brest. The results are summarized in table II. This work and data available in the literature (Windell et al. 1978, Smith et al. 1980, Vens Capell, 1985) lead to table III which lists the advantage and incovenientsof the different collection methods.
There is probably no perfect method. It can be seen in table II that stripping, suction or dissection tend to underestimate protein CDA since they may be further absorbed in the posterior intestine. On the other hand, it is very difficult to avoid completely leaching from faeces naturally released into the water. Continous filtration seems to be quite efficient but the faeces must be cohesive for them not to stick on the screens. The incorporation of an undigestible binder into the diet contributes to the good physical properties of the faces but can affect digestibility. In sea bass, 4% sodium alginate improve the cohesion of the fecal material without altering protein digestibility, while 6% result in a decrease of protein DC.
To conclude, a method for faeces collection should be selected keeping the following ideas in mind:
- handling of the fish must be kept to a minimum in order to reduce stress,
- the faeces collected must be representative of physiological process,
- leaching of soluble substance must be avoided as much as possible
Feed formulation by linear programming takes into accounts the fish nutritional requirements, the chemical composition of feedstuffs, their digestibility and price.
The DCA values given for protein (table IV), lipids (table V) and carbohydrates (table VI) in various sources illustrate the effects of a few factors on nutrient digestibility and the relevance of these data in fish feed formulation.
Gastric evacuation time is the time taken by a meal to evacuate completely the stomach. Gastric evacuation rate represents the evacuation kinetics of the food, it is expressed in units of weight per unit of time. These two parameters can also be determined for the intestine or for the whole digestive tube. In the intestine, the evacuation rate is difficult to measure since absorption must be accounted for. Therefore gastric evacuation is the usually preferred parameter. Also it is useful to quantity gastric evacuation is studies concerning feed intake, return of appetite and digestion.
The objective is to examine the gastric content at given time intervals. Several methods have been reviewed in detail by Talbot (1980).
Serial slaughter
A group of fish is fed ad libitum and sub-samples (8 to 10 specimens minimum) are killed immediately after in order to measure consumption and at intervals thereafter. The digestive tube is dissected out and gastric and intestinal contents are removed and weighed. The fish are usually starved before and after the tested meal so that the results are not confused by additional food intake.
This method has several incovenients:
- it requires the slaughteringof a large number of animals,
- the data are collected on different animals at each sampling time,
- food deprivation before and after the tested meal may introduce an error since normal feeding frequency is altered.
Gastric flushing
In order to avoid killing the fish, the stomach content can be pumped out from live animals. Giles (1980) described a device made of two syringes strapped together. Water is alternately forced into the stomach with one syringe and remove with food with the other syringe. According to Giles (1980), 95% of the gastric content can thus be recovered.
X-Ray radiography
A radio-opaque compound must be incorporated to the feed. Barium sulphate is often used at a level of 20% minimum which can require forcefeeding. Such a high incorporation rate has two incovenients: (1) it modifies substantially the composition of the diet and (2) it has been shown that forcefeeding can decrease up to 50% the gastric evacuation time. Talbot and Higgins (1983) used metallic iron powder (particle size 100 to 200 μm) at a low incorporation level (5–8%) and established the relationship between iron particle number and food weight. Since iron particle are comratevely heavy, they may separate from the food and be retained in the stomach longer than the food. Grove et al.(1985) solved this possible problem by using barium suphate coated polystyrene spheroids. The position of the food in the digestive tube is followed by X-raying the live fish at successive time.
This method is elegant and simple. It is quantitative and therefore allows the measurements of gastric evacuation rates on small as well as large animals.
Grove et al.(1978) compared return of appetite and gastric evacuation rate in rainbow trout (fig.4). The graph describing the return of appetite is the mirror image of that describing the gastric evacuation. This demonstrates that the strong correlation existing between gastric content and appetite. Although gastric evacuation, return of appetite and feed consumption are correlated in time, the control mechanisms of appetite are most likely of metabolic or nervous origin (Flether, 1984). Knowledge of gastric evacuation can thus allow a rational approach to determining feeding frequency and ration size. This illustrated by the diagram in fig.5 which gives gastric evacuation time for rainbow trout in ralation to body weight and temperature.
The following factors modify gastric evacution:
Temperature: gastric evacuation time and temperature are inversely related (table VII)
Body weight: gastric evacuation time increases with increasing body weight for a meal size expressed as a percentage of body weight (Flowerdew3 and Grove/1979)
Ration: GET is longer for when the ration is increased, but the relationship is nonlinear (Jobling et al.,1977)
Energy concentration of the diet: diet with low energy content move more rapidely through the stomach than highly energetic diets (jobling, 1981)
The energy metabolism of fish differ from that of higher vertebrates in three major aspects:
- fish do not maintain a constant body temperature,
- it spends little energy to maintain its position in water,
- the major product of nitrogenous excretion is ammonia rather than urea or uric acid.
These three features al low the fish to “save energy” which results in more protein deposited per unit of energy intake than for other farm animals (Lovell, 1979).
The energy flow in fish is schematically represented in fig.6 (from Cho and Kauslik, 1985).
Digestible energy is the difference between gross energy intake (IE) and energy excreted in the faeces (FE):
DE = IE - FE
Hence DE depends on protein, Lipid and carbohydrate digestibility.
Metabolizable energy (ME) is a more defined evaluation of available energy since it takes into account nitrogenous losses due to amino acid oxidation and which are exerted through gills (ZE) and in the urine (UE):
ME = DE - (ZE + UE)
ME is therefore related to the proportion of dietary proteins used as source and can be modified by changing, for instance, the protein to Lipid ratio.
The heat increment of feeding (HiE) represents the energy spent by digestive and absorption processes, metabolic transformations and interconversions of compound and formation of metabolic excretory products: The determination of HiE can be done either by measuring directly the heat released by the fish. Net energy is then calculated as follows:
EN = EM - HiE
Net energy is the most accurate estimate of the dietary energy available for maintenance (basal metabolism and resting activity) and production (growth and reproduction). Unfortunately it is technically very difficult to measure and its use is still controversial.
Cho and Kaushik (1985) suggest a method to evaluate ME, NE and maintenance energy which does not require the collect of the gill and urinary excretions and the measurement of HiE. Their procedure is as follow:
- measure digestibility coefficient of the diet
- analyse diet and whole body composition at the beginning and end of the feeding trial
- calculate digestible N (DN) and digestible energy (DE) consumed the fish
- calculate retained N (RN) and energy (RE)
- non-fecal nitrogen Loss = DN - RN
non-fecal energy loss (ZE + UE) = (DN - RN) × 25 KJ/gN
ME = DE - (ZE + UE)
- HiE = DN × 28 KJ/gN (salmonids at 15c)
NE = ME - HiE
- maintenance energy =NE-ER
It is important, when formulating feeds, to be able to determine dietary energy losses since they depends to a large extend on the balance between digestible protein and energy (HiE resulting from dietary protein is much greater,than that from dietary Lipids or carbohydrates). Generally speaking, energy Losses for ZE+UE represents 4 to 8% of DE and HiE represents 9 to 14% of DE.
A fish will first use available dietary energy to meet the maintenance requirements. An energy deficient diet will therefore penalize growth. On the other hand, an excess in dietary energy will reduce food consumption since fish, as other animals, tend to adjust their feed intake on their energy requirements. If the feed is highly concentrated in energy,feed intake may decrease and intake of essential nutrients may become insufficient.
The determination of energy requirement is not simple since they depends on species, temperature, ration, rearing conditions, etc…Few data are available in the Literature and they must be applied with caution. For a warmwater fish such as catfish the DE/Protein ratio should be 8 to 9 Kcal/g protein for diets containing 32 to 35% proteins(NCR, 1981). For seabass juveniles the ratio is 7 to 8 Kcal Me per g protein.
Energy is “stored” in the chemical structure of feed ingredient molecules which are digested, absorbed and oxidized. Oxidation release energy. Lipids, proteins and carbohydrates are all providing energy.
Lipids
Lipids are the most energy concentrated compounds. Their gross energy value is 9.1 Kcal/g. A good quality oil will usually provide 8 to 8.5 Kcal DE/g.
Lipids are therefore an appropriate source of energy to spare proteins. Dietary levels are usually 9 to 10; for sea-bream, 12% for sea -bass and 15 to 18% for rainbow trout.
Proteins
Fish utilize proteins very efficiently as energy source. Gross energy value is5.65 Kcal/g and DE commonly varies from 4 to 4.5 Kcal/G
Carbohydrates
Carbohydrates are an inexpensive source of energy. Unfortunately they are poorly utilized by fish. It has been found that fish have Limited glucidase activity and do not control well glicaemia. Groos energy of carbohydrates is 4.5 Kcal/g but DE values varies from 1.2 to 3.6 Kcal/g.
Table I Summary of digestive processes
| Organ | Enzyme | Activation | Substrate | Product |
| Stomach | Pepsine (HCL) | Acidity pH=2 | Protein:peptide bond between TYR, TRP, PHE and between ASP and GLU | polypeptides |
| Liver | (secreates bile which emulsifies lipids and neutralizes intestinal content) | |||
| Pancreas | Trypsine | enterokinase from int.mucosa | Proteins:peptide bond when carbonyl group belongs to ARG or LYS. | polypeptides |
| Chymotrypsine | by trypsine | proteins:peptide bond when carbonyl group belongs to TYR, TRP, PHE | ||
| Carboxypeptidae | " " | polypeptides:from the end with a free carboxyl group | amino acids | |
| Elastase | " " | elastine | ||
| Amylase | " " | starch and glycogen | glucose maltose dextrines | |
| Chitinase | " " | chitine | di-and trimere of N-acetyl-D glucosamine | |
| Lipases and Esterases | triglycerides | fatty acids monoglycerides glycerol | ||
| Intestine | Aminopeptidase | polypeptides:from the end with a free amino group | amino acid | |
| Dipeptidase | dipeptides | amino acids | ||
| Tripeptidase | tripeptides | amino acids | ||
| Maltase | maltose | glucose | ||
| Nucleotidase | nucleic acids | nucleotides | ||
| Nucleosidase | nucleosides | purine pyrimidine pentose | ||
Table II comparison af different faeces collection methods
| Stripping | Anal suction | Dissection | Netting | Setting | Continous filtration | |
| DCA Proteins % | 82 | 87 | 84 | 91 | 94 | 92 |
| DCA lipids % | 94 | 96 | 95 | 97 | 96 |
Table III Advantages and Incovenients of seven methods for faeces collection
| Method | Inconvenients | Advantages |
| Stripping | • Contamination of sample with urine, blood, sperm: underestimation of DCA • Stress • Little sample collected • is the sample representative of naturally released faeces? | • simple to do |
| Anal suction | • stress • very little sample collected • is the sample representative ? | • simple |
| Dissection | • requires large number of fish • little sample collected • is the sample representative ? | • simple |
| Netting | • Labor intensive • tedious • stress | • simple • faeces are released naturally |
| Setting | • Some Leaching may occur | • faeces released naturally • no stress • continous collect |
| Continous filtration | • cost of apparatus • Leaching ? | • same as sattling |
| Matabolism chamber | • stress • fish may not adapt to confining • requires excellent water quality | • quantitative collect • ME can be determined |
Table IV Digestibility of proteins
| Source | Crude protein (%) | DCA (%) | ||
| Alfaalfa meal | 17.0 | 61.0 | effect of the source (1) (rainbow trout) | |
| Cotton seed meal | 50.5 | 77.7 | ||
| Anchovy meal | 70.1 | 83.5 | ||
| Meat and bone Meal | 52.9 | 70.3 | ||
| Whole soybean | 127c,10mn | 42.9 | 45.4 | effect of processing (1) (destruction of anti-trypsic factor(ranibow trout) |
| 175 c,5mn | 42.5 | 56.6 | ||
| 232 c,8mn | 41.1 | 75.1 | ||
| Commercial feed | 50.0 | |||
| ration = | 3.3k/kg | 71.20 | effect of ration (2) (catfish) | |
| 10.0g/kg | 67.26 | |||
| 16.7g/kg | 60.03 | |||
(1) Smith, 1976.
(2) Henken et al. 1985
Table V digestibility of lipids (for rainbow trout. from Austreng et al., 1979)
| Source | DCA Lipid (%) | ||
| Soybean oil | 87.9 | effect of source | |
| Cod liver oil | 90.6 | ||
| Hydrogenated capelin oil | |||
| fusion point 21 c | 74.6 | effect of fusion point | |
| " " 41 c | 46.4 | ||
Table VI Digestibility of starch (for trout, Jolivet, 1986)
| Source | DCA Starch (%) | |
| Cooked corn starch | 85.5 | |
| Raw corn starch | 47.6 |
Table VII Gastric evacuation time for 30 g. Tilapias fed 3% of body weigh (from Ross and Jauncey, 1981)
| Temperature (C) | Gastric evacuation time (h) | |
| 20 | 16.4 | |
| 25 | 10.8 | |
| 30 | 8.5 |
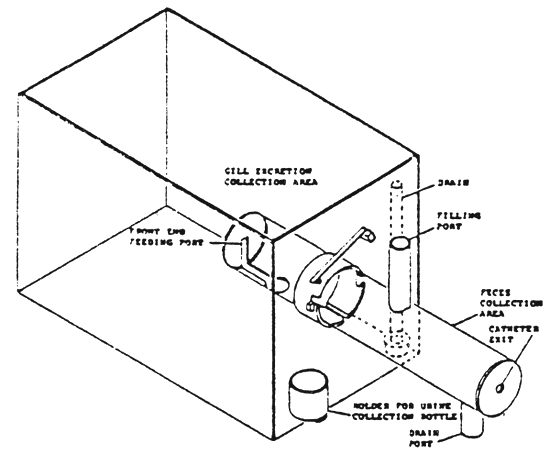 | Figure 1 - Metabolism chamber (in Gabaudan, 1979) |
Figure 2 - Experimental set-up for collection of faeces by setting (from Jolivet, 1986) | 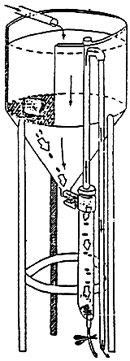 |
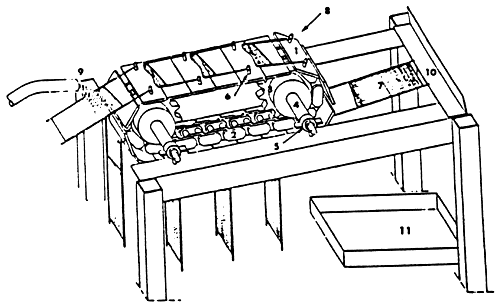 | Figure 3 - Apparatus for continous faeces collection by filtration (in: Choubert et al., 1982) |
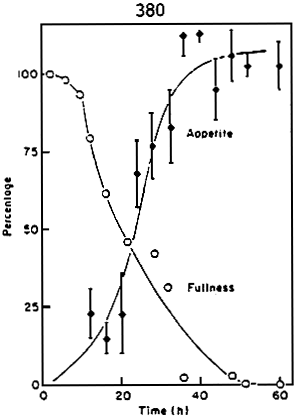
Figure 4 - Comparison between return of appetite and gastric evacuation rate for rainbow trout at 11 – 12 C . (in: Grove et al., 1978)
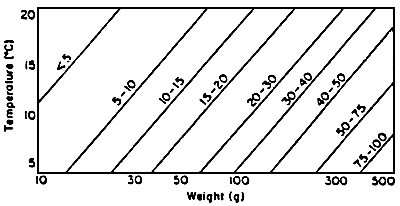
Figure 5 - Gastric evacuation time in hours at different temperatures and for different body weights. (in: Grove et al., 1978)
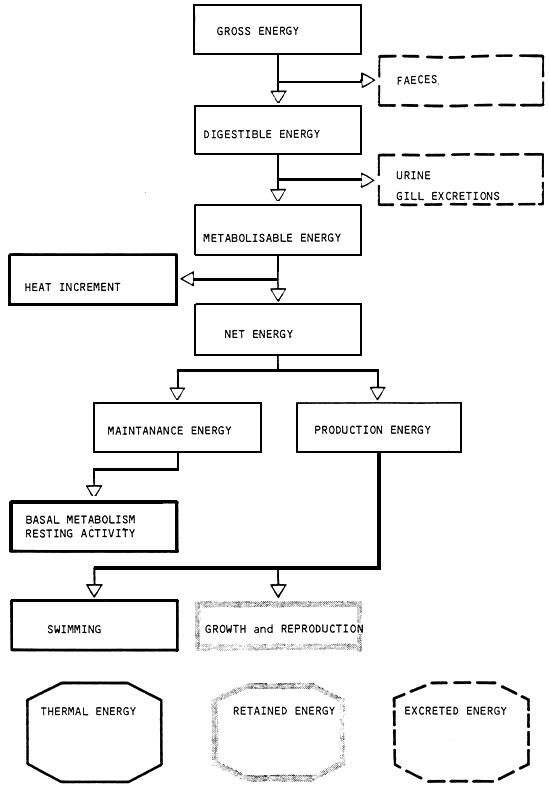
Figure 6 - Energy flow in fish
BIBLIOGRAPHY
AUSTRENG E., A. SKREDE and A. ELDEGARD, 1979. Effect of dietary fat source on the digestibility of fat and fatty acids in rainbow trout and mink. Acta Agriculturoe Scandinavica, 29: 119–126.
BOLIN D.W., R.P. KING and E.W.KLOSTERMAN, 1952. A simplified method for the determination of chromic oxide (Cr2O3)when used as an index substance. Science, 116: 634–635.
CHO C.Y., S.J. SLINGER and H.S. BAILEY, 1982. Bioenergetics of salmonid fishes energy intake, expenditure and productivity. Comp. Biochem. Physiol. 73B: 25–41.
CHO C.Y. and S.J. KAUSHIK, 1985. Effects of protein intake on metabolizable and net energy values of fish diets. In: Nutrition and Feeding in fish. Ed. by C.B. Cowey, A.M. Mackie and J.C. Bell. Academic press, London, U.K.
CHOUBERT G., J. De la NOÜE and P. LUQUET, 1982. Digestibility in fish: improved device foe the automatic collection of feces. Aquaculture, 29: 185–189.
FANGE R. and D. GROVE, 1979. Digestion. In Fish Physiology, ed. W.S. Hoar, D.J. Randall and J.R. Brett, vol. VIII, Academic Press, London, 162–241.
FLETCHER D.J., 1984. The physiological control of appetite in fish. Comp. Biochem. Physiol. 78A: 617–628.
FLOWERDEW M.W. and D.J. GROVE, 1979. Some observations of the effects of body weight, temperature, meal size and quality on gastric emptying time in the turbot, Scophthalmus maximus (l. ) using radiography, J. Fish. Biology 14: 229–238.
GAS N. and j. NOAILLAC-DEPEYRE, 1981. Organisation, ultrastructure et fonction du tube digestif des téléostéens d'eau douce. In Nutrition des Poissons, Actes du Colloque CNERNA, Paris, Edition du CNRS: 19–43.
GILES N., 1980. A stomach sampler for use on live fish. J. Fish Boil., 16: 441–444.
GROVE D.J., L.G. LOIZIDES and J.NOTT, 1978. Satiation amount, frequency of feeding and gastric emptying rate in Salmo gairdneri. J. Fish Boil. 12: 507–516.
GROVE D.J., M.A. MOCTEZUMA, H.R.J. FLETT, J.S. FOOTT, T. WATSON and M.W.FLOWERDEW, 1985. Gastric emptying and the return of appetite in juvenile turbot, Scophthalmus maximus L., fed on artificial diets. J. Fish Boil. 26:339–354.
HARPER H.A., V.W. RODWELL and P.A.MAYES, 1979. Review of physiological chemistry. 17th Edition. Lange Medical Publications, Los Altos, U.S.A.
HENKEN A.M., D.W. KLEINGELD and P.A.T. TIJSSEN, 1985. The effect of feeding level on apparent digestibility of dietary dry matter, crude protein and gross energy in the african catfish Clarias gariepinus (Burchell,1822). Aquaculture, 51: 1–11.
JOBLING M., 1981. Dietary digestibility and the influence of food components on gastric evacuation in plaice, Pleuronectes platessa L.J.Fish Boil. 19: 29–36.
JOBLING M., D. GWYTHER and D.J. GROVE, 1977. Some effects of temperature, meal size and body weight on gastric evacuation time in the dab Limanda limanda (L. ). J. Fish Boil. 10: 291–298.
JOLLIVET D., 1986. Etude de la digestibilité de l'amidon chez le turbot (Scophthalmus maximus L. ). Mémoire de DEA, Université de Bretagne Occidentale. Brest, France.
KAPOOR B.G., H. SMIT and I.A. VERIGHINA, 1975. The alimentary canal and digestion in Teleosts. Adv. Mar. Boil., 13, 109–239.
LOVELL R.T., 1979. Fish culture in the United States. Science, 206: 1368–1372.
ROSS B. and K.JAUNCEY, 1981. A radiographic estimation of the effect of temperature on gastric emptying time in Sarotherodon niloticus (L) x S. aureus (Steindachner) hybrids. J. Fish Boil. 19: 333–344.
SMITH L.S., 1980. Digestion in Teleost fishes. In Fish Feed Technology, ADCP/ REP/80/11, FAO Rome: 4–18.
SMITH L.S., 1982. Introduction to fish physiology. T.F.H. Publications, Inc. Neptune, U.S.A.
SMITH R.R., 1971. A method for measuring digestibility and metabolisable energy of fish feeds. Prog. Fish Culturist, 33: 132–134.
SMITH R.R., 1976. Metabolizable energy of feedstuffs for trout. Feedstuffs, 48: 16–21.
SMITH R.R., C.PETERSON and A.ALLRED, 1980. Effect of leaching on apparent digestion coefficients of feedstuffs for salmonids. Prog. Fish Cult. 42: 195–199.
TALBOT C., 1985. Laboratory methods in fish feeding and nutritional studies. In: Fish Energetics New Perspectives, ed. by P.Tytler et P.Calow, Croom Helm, London, 125–154.
TALBOT C. and P.J. HIGGINS, 1983. A radiographic method for feeding studies on fish using metallic iron powder as a marker. J. Fish Boil. 23: 211–220.
VENS-CAPELL B., 1985. Methodical studies on digestion in trout. I–Reliability of digestion coefficients in relation to methods for faeces collection. Aquaculture Eng. 4: 33–49.
WINDELL J.T, J.W FOLTZ and J.A.SAROKON, 1978. Methods of fecal collection and nutrient leaching in digestibility studies. Prog. Fish Cult. 40: 51–55.
ANNEXES
ANNEX I
PROGRAMME
| 19/10/86 | - Welcome of participants | |
| 20/12/86 | - Opening of the session | |
| - Digestion and energetic metabolism | J. GABAUDAN | |
| 21/10/86 | - Proteins and amino-acids | C.B. COWEY |
| - Carbohydrates | C.B. COWEY | |
| - Lipids and fatty acids | C. LEGER | |
| 22/10/86 | - Vitamins and mineral salts | J.G. KOENIG |
| 23/10/86 | - Nutritional requirements of crustacea | M.J. CECCALDI |
| - Nutrition of molluscs | J.M. DESLOUS PAOLI | |
| 24/10/86 | - Raw materials | J. GUILLAUME |
| - Silage - Theory and practical work | I. BATISTA | |
| 25/26/10/86 | - Study tour | |
| - VIVAL - VIVEIROS de RIA S.A.R.L | ||
| Salgados - Boine, 8500 PORTIMAO (Mr. TEUNISSEN) | ||
| - SINEXPLAL Ltd | ||
| OLHAO (Mr. DELFIN AGOSTINHO) | ||
| - I.N.I.P - Centro de Investigacao Pesqueira de FAHO | ||
| FARO (Mr. POUSAO FERREIRA) | ||
| 27/10/86 | - Formulation - CRESPO FOLGADO | |
| - Fish feed processing and technology | J.P. MELCION | |
| 28/10/86 | - Feeding stimulants | A.M. MACKIE |
| - Microparticles | J.P. MELCION | |
| - Experimentation in nutrition | R. METAILLER | |
| 29/10/86 | - Presentation of commercial feed | |
| - AQUALIM (J. SABAUT) | ||
| - EWOS BIOTER S.A. (A. TIANA MARISCAL) | ||
| - TROUW International (H. HOGENDOORN) | ||
| - Visit | ||
| - QUIMICAL (CRESPO FOLGADO) | ||
| 30/10/86 | - Feeding in marine aquaculture | - C. de la POMELIE |
| 31/10/86 | - Departure of participants | |
ANNEX 2
PARTICIPANTS
| ABOUHALA ABDERRAHMANE | - S.té MAROST ATALAYOUN -B.P. n.4 NADOR (MAROC) |
| ABDELKADER ROUABAH | - C.E.R.P. DE BOUISMAIL WILAYA DE TIPAZA (ALGERIE) |
| ATTIA EL HILI HEDIA | - I.N.S.T.O.P. 2025 SALAMBO (TUNISIE) |
| MEDHJOUB AMEL | - C.N.A. de MONASTIR MONASTIR (TUNISIE) |
| WAGDI AHMED HAFEZ | - GENERAL AUTHORITY FOR AQUATIC RESOURCES 4, Tyran street - NASR CITY (Cairo) EGYPT |
| GAMAL AHMED TALAAT EL SAWAF | |
| GEORGE GEORGIOU | - FISHERIES DEPARTMENT NICOSIA (CYPRUS) |
| GEORGIOS ANASTASSIADES | |
| MURAT ERSOY | - AQUACULTURE RESEARCH INSTITUTE ANTALAYA (TURKEY) |
| CARMELO GALEA | - THE BIOLOGY DEPARTMENT, THE UNIVERSITY OF MALTA TAL AXOQQ - MSIDAQ (MALTA) |
| NICHOLAS BONANNO | - CARTER MDINA ROAD ZEBBUG (MALTA) |
| SKARAMUCA BOSKO | - BIOLOSKI ZAVOD DUBROVNIK (YUGOSLAVIA) |
| IVOS IGOR | - CENMAR ZADAR (YUGOSLAVIA) |
| DAMIR MUSIN | - BIOLOGICAL INSTITUTE DUBROVNIK (YUGOSLAVIA) |
| IVOS STERBIC | - MARIKULTURA MIRNA ROVINJ (YUGOSLAVIA) |
| MAFALDA FONSECA | - I.N.I.P. Avenida de BRASILIA - 1400 LISBOA (PORTUGAL) |
| TERESA GAMA PEREIRA |
ANNEX III
LIST OF LECTURERS
H.J. CECCADLI
Ecole Pratique des Hautes Etudes
Station Marine d'endoume
13 007 - Marseille (FRANCE)
Tél. (91) 521 294
G.B.COWEY
Institute of Marine Biochemistry
St. FITTICK ROAD
Aberdeen AB 1 3 RA
Tél. 0224/875 695
CRESPO-FOLGADO
Quimigal - Av. Infante Santo, 2
1300 - LISBOA (Portugal)
Tél. (1) 604 040
Tlx - 12 301
C.DE LA POMELIE
IFREMER
34 250 - Palavas Les Flots (France)
Tél. (67) 680 833
Tlx - 490 419
J.M. DESLOUS-PAOLI
IFREMER - station de la Tremblade
Mus du Loup - B.P. 133
17 390 - LA TREMBLADE (FRANCE)
Tél. (46) 361 841
J. GABAUDAN
IFREMER - Center de Brest
29 273 - Brest Cedex (France)
Tlx - IFREMER 940 627F
Tél. (98) 224 040
F.J. GATESOUPE
I.N.R.A. C/O IFREMER - Center de Brest
BP 357
29 273 - Brest Cedex (France)
Tél. (98) 224 040
Tlx - IFREMER 940 627 F
J. GUILLAUME
IFREMER - Centre de Brest
29 273 - Brest Cedex (France)
Tél. (98) 224 040
Tlx IFREMER 940 627 F
J. KOENIG
1, Cours de la République
69 100 - Villeurbanne (France)
Tél. (78) 933 754
B.IRINEU
INIP. Avenida de Brasilia
1400 - Lisboa (Portugal)
Tél. (1) 610 841
C.LEGER
I.N.R.A.
78 350 - Jouy en Josas
Tél. (16–1) 39 568 080
Tlx INRACRZ 695 431 F
A.M.MACKIE
Institute of Marine Biochemistry
St. Fittick's Road
ABERDEEN AB 1 3 RA
Tél. 0224/875 695
J.P.MELCION
I.N.R.A.
Chemin de la Gérodière
Nantes - (FRANCE)
R. METAILLER
IFREMER - Center de BREST
29 273 - BREST CEDEX (FRANCE)
Tél. (98) 224 040
Tlx - IFREMER 940 627 F