


By
P. J. Wood
Senior Forestry Advisor
Overseas Development Administration
London, England
In order to assess the position of forestry in Africa today in relation to the threat from conifer aphids, it is useful to examine the history of forestry - that is, the management of forests and trees - in the continent.
With some few exceptions, the exploitation of forests during this long era was based on domestic and small industrial needs. Most of the forests under management were natural and there was only a little planting. Such planting as there was, was probably of fruit rather than timber trees (e.g. Mangifera indica), though there was considerable understanding of the benefits of agroforestry. For example, many stands of Faidherbia albida in West Africa were created through human endeavours. There would have been little large scale damage or clearing of forests and "slash and burn" agriculture was controlled and well within the capacity of the land to sustain.
The previous patterns of forest use continued, but "Forest Reserves" were now created, reducing popular access to the forests and increasing large scale exports of logs and lumber, as well as expanding local use. In West Africa, the large extent of the natural forests allowed this phase to continue until well after the second World War, but in east and central Africa it was clear early in this century that the natural forests were not able to supply national needs and experiments with "compensatory" plantations took place. The plantations "compensated" for the deficiencies in productivity of the natural forests and initially used indigenous species such as Podocarpus, Juniperus procera, Olea africana and Widdringtonia nodiflora. When the wood production yields of the indigenous species proved to be unacceptably low, exotic species were tried, especially tropical and subtropical conifers and eucalypts.
Since the majority of the countries achieved independence in the 1950's and 1960's, the emphasis on plantations for productive forestry has continued. Interest in natural forest management has intensified as areas of natural forest have dwindled. In West Africa, for instance, the last 20 years have witnessed Nigeria pass from being a major exporter of tropical wood to a large net importer. In the last 10 years, Rural Development Forestry has become more and more important throughout Africa. This is based on the identified objectives of industrial forestry which primarily aimed at supporting either national or overseas industries, and incidentally was often viewed by National Governments as a major source of revenue.
Most emphasis is laid both by national governments and by international donors on RDF, which, in simple terms, encompasses:
Community Forestry - in which natural forests or plantations are managed by and for communities.
Social Forestry - in which natural forests or plantations are managed (Usually by the Forest Department) for and on behalf of rural people.
Farm Forestry - which covers farm woodlots and trees on farms, usually in individual ownership.
The whole range of agroforestry practices can be applied in any of these situations, though they are most relevant in relation to farm forestry. The techniques used in managing trees and woodlots in RDF are not fundamentally different from those in industrial forestry, but there are many more isolated trees and much smaller areas are involved.
The change from mainly industrial objectives to mainly rural development objectives in the last few years, coupled with the rise in international concern over tropical deforestation has diverted attention away from industrial plantations and there is now a real risk that economically important forestry plantations are being neglected.
Recent initiatives for saving tropical forests include the Tropical Forestry Action Programme being coordinated by the FAO, and also National Forestry Sector Reviews from the World Bank. It is most important that such plans should incorporate planted forests for the benefits both in production and in incidental conservation that they can bring when wisely and strategically developed.
Without giving an exhaustive list of all species that have been used in tropical forestry plantations, it may be useful to give some of the major ones for the humid and drier parts of Africa (Table 1).
It should, however, be noted that the number of species is considerably greater than those of the major food crops in the same area.
PARTIAL LIST OF TREE SPECIES PLANTED IN EAST AND WEST AFRICA
|
Eastern and southern Africa |
Western (Humid) Africa |
|
Pinus caribea Cupressus "benthamii" Eucalyptus camaldulensis |
Azadirachta indica |
* Indicates an indigenous species.
This list is not exhaustive but indicates the greater number of indigenous species in use in west Africa, and also that more species tend to be in use in the drier and more seasonal climates and the more variable relief in the eastern and central parts of the continent.
The remainder of this workshop will take advantage of the expertise of many specialists to evaluate both the risks and the appropriate measures to be taken in the different countries represented here. It is worth noting, however, that forest plantations have been remarkably disease free and the exotic species have shown themselves to be remarkably resilient in their new environments. The value of the plantations in the national economies of different countries has been variable. International political difficulties have played their part, as, for instance, in the case of the Viphya Plateau plantations of Malawi. But overall the plantations occupy a tiny area in relation to countries as a whole. Yet they are a substantial productive asset deserving both good management and good science.
By
Clive Carter
Forestry Commission Research Station
Farnham, Surrey, England
and
Gillian Watson
International
Institute of Entomology
56 Queen's Gate
London, England
Species of the conifer aphids of the families Adelgidae and Aphididae are known to occur widely wherever conifers are used for forestry, and may give rise to severe growth problems. Examples are given where their life cycles may be adapted to suit favourable climatic conditions by omitting certain stages so they can take advantage of the whole period when the nutrition is favourable to aphid development. There are now indications that genetic differences between trees offer resistance to aphid attack by virtue of the plant's biochemistry, some of these differences have been shown to be inherited. The interactions between aphids and their host trees are briefly reviewed with the purpose of drawing attention to how such a long-lived plant can favour or discourage aphid feeding.
In North America, one of the most startling effects of aphid damage occurs on the stems of the true firs, Abies sp., and is brought about by Adelges piceae whose saliva influences cambial activity, altering the cell-size and wall thickness of the stem trachieds. As a result, the crown foliage of affected trees is unable to acquire sufficient sap flow, so the tree enters into a sometimes lethal physiological drought and decline spiral (Doerksen & Mitchell 1965). Other species may not kill the tree, but may cause severe defoliation that results in a significant loss in potential growth-increment and therefore lengthens the time between thinning operations or eventual harvesting (Carter 1977). Just a few conifer aphid species are welcomed since they are recognised for their honeydew production and the benefit this brings to beekeepers, for a short period each year, in providing a source of forest honey (Carter & Maslen 1982). The damage caused by aphids to conifers therefore ranges from a subtle unmeasurable amount to the possible death of the whole mature tree through a change in its anatomical and physiological condition.
Tree dwelling aphids in natural or semi-natural forest communities are seldom abundant. The cultivation trees in towns is often accompanied by acute infestations of aphids even when the host tree is growing within its natural geographical range. Such urban outbreaks frequently occur on both broadleaved and coniferous trees, examples being Eucallipterus tiliae on Tilia cordata and Cinara piceae on Picea abies. Between these two extreme environments are man made forests. Nowadays these are likely to consist of monocultures of silviculturally selected, non-native tree species where other vegetation is suppressed for the sake of the planted tree crop, a situation that applies to British forests as well as elsewhere in the tropics (Evans 1982). In these forest situations, an aphid species may be presented with a new host plant species that is removed from the assemblage of plants associated with its native habitat. This combination took place in Britain during the 1960's following the extensive afforestation programme of unplanted hill farms and heathen moors, when the green spruce aphid, Elatobium abietinum moved from the European Norway spruce, Picea abies, to the North American Sitka spruce, P. sitchensis. A reciprocal outbreak can result when the native host tree is confronted with an introduced aphid species without its attendant suite of predators and parasites. This appears to have taken place in North America when the European silver fir woolly aphid, Adelges piceae, spread across the continent on the native firs, Abies sp., causing considerable decline and mortality as it spread (Carter 1971).
Apart from the present problems of aphids on conifers in Africa, there are other examples of intercontinental translocations. For example, the green spruce aphid threatens the successful growing of spruce in New Zealand (Dumbleton 1932), Iceland (Austara pers. comm.), and the Falkland Islands (Carter unpublished); Cinara cronartii on pines in South Africa (Shaw 1984) and Adelges piceae on true firs in Chile (Cerda & Gara 1977). When both the host plant and the aphid are non-native, these intercontinental translocations may be even more traumatic which suggests, not only the aspects of the tree's ecology are overlooked, but also that driving variables are present that powerfully influence the population dynamics of the insect. It has been shown by Kidd (1988) that, in the case of Cinara pinea, aphid numbers from one year to the next can increase by as much as ten-fold if predators are absent; but the magnitude of increase depends upon tree quality as host to the aphid. (Fig 1).
The life cycles of true aphid (Aphididae, eg conifer lachnids) in their complete (holocyclic) sequence include males and egg laying females, which after pairing, give rise to overwintering eggs. This egg stage has two significant functions. Firstly it enables the species to survive adverse
climatic conditions or when the host plant is in an unsuitable condition for food supply, and secondly it enables some genetic variability of the aphid species to be manifested. Where favourable climatic and host plant conditions persist, the males and egg producing stages may be omitted altogether and anholocyclic colonies may persist for many generations. It is therefore possible that aphid clones of limited genetic variability will occur, provided conditions for their continued existence are met.
The life cycles of adelgids (Adelgidae, eg Pineus) in the complete sexual sequence comprise of seven forms and involve migration between two hosts, but in some species this may be reduced to two similar wingless asexual morphs. One, the progrediens, takes advantage of the plant in its active growing state; and the other, the sistens, has a first instar that can remain inactive during adverse conditions such as winter or plant dormancy. The first instar sistens can rest during the trees' dormant period with its stylet mouthparts implanted into the tissues, so that as soon as the tree resumes active metabolic growth that aphid can also develop and reproduce. Close parallels in the ability to respond to favourable conditions are to be found in the true aphids from the northern coniferous forests. This is especially so where their host plant has been introduced into regions with a mild maritime climate. In such places, parthenogenetic forms persist, or can even reproduce at a low ebb, throughout the winter months. Aphid growth and population increase can then take full advantage of irregular favourable host plant and climatic conditions free from the activity of predators. These circumstances are precisely those that precede green spruce aphid outbreaks in maritime regions of britain and Western Europe (Bejer-Petersen 1962, Carter 1972). Yet another strategy for an aphid is to invest in some storage material (body fat) in order to survive periods of inadequate food supply. This occurs with subterranean aphids that feed on the roots of herbaceous food plants. Their storage reserves enable them to survive several months of cold adverse conditions when plants are not in active growth (Judge 1967).
For an aphid to exploit successfully a host plant, it must either respond to renewed plant activity in the way the sistens form of adelgid does, or it must be phenologically synchronised with the host plant. Several specialist conifer aphids that feed on the young tissues do this. The eggs of Cinara pilicornis on Picea shoots hatch before bud burst, and the next two generations arising from these females feed and reproduce on the tender new shoot growth. But after six weeks, when the shoots are mature, the egg laying females start to appear again and eggs are laid that then remain unhatched for eight months spanning the hottest and the coldest periods of the year (Carter and Maslen 1982). Similarly, the new shoots of Abies and Picea are attacked by Mindarus abietinus and M. obliquus for just a brief period after bud burst. In a study plot of seedling Abies firma that was infested with M. abietinus, it was noticeable that certain trees escaped attack (Carter and Nichols 1985). By monitoring marked trees during the bud burst period over successive years, it was noted that the unattacked trees either came into leaf too early or too late for the aphids to exploit the particular stage of shoot growth. This type of phenological escape, or 'pseudo-resistance', could be exploited especially when working in harmony with other factors, such as a univoltine late emerging predator or regular climatic events.
Aphid growth rate and fecundity can be significantly different between individual trees of the same conifer species. Certain clones of Douglas-fir, Pseudotsuga menziesi, are consistently poor hosts for Adelges cooleyi while other clones are heavily attacked (Carter unpublished). These same clonal differences have been noted at three forest sites regardless of their soil type. Further evidence that poor aphid performance can be genetically controlled by the host tree has been shown with Cinara cronartii by Shaw and Marchant (1980) when different clonal trees from tha same parental crossings were shown to inherit resistant properties from the resistant parents. Evidence of heritable resistance of Pinus ellioti to Pineus sp. has also been recorded by Barnes et. al. (1976) in southern Africa. Identification of the precise resistance mechanisms of conifer host plants to aphids have yet to be made, but Nichols (1984) has shown a negative relationship between aphid performance and the concentration of certain phenolic and terpene compounds in the foliage of spruce.
Not all aphids necessarily produce large colonies on exotic trees. Certain aphid species may occur on the same plant at a low level for many generations. The larger juniper aphid, Cinara fresai, was first recorded from Britain at Alice Holt on a large old prostrate form of Juniperus sabina in 1954. Small numbers of the aphid can still today be found on the same tree at any time of the year. If these individuals are transferred to young potted plants of the same or related species in the same area under protected cultivation conditions (eg. polyhouse structures), substantial colonies will develop, causing significant death of shoots, and eventually the whole plant is overwhelmed.
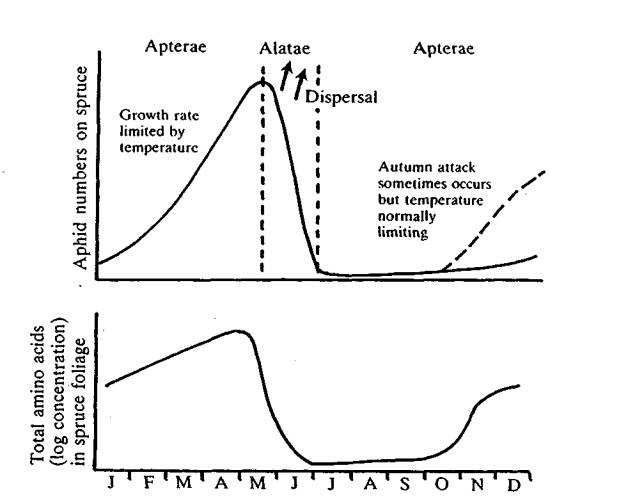
Injurious aphid colonies consist essentially of parthenogenetic females that have a short generation time and can respond quickly to certain nutrient conditions provided by the host plant. The growing and resting periods of the tree is often cyclic, whether brought about by the wet/dry season of the tropics or the cold/warm season of temperate zones. Fluid feeding insects, such as aphids depend entirely on soluble organic nutrients metabolized by the host plant. The inability of tree roots to take up nutrients, due to physical limiting factors, such as soil temperature and water availability influences host quality. Periods of plant stress in conifers, such as a sublethal drought, which is visibly difficult to recognize, has a profound effect on aphid fecundity and hence population increase (Major 1990). Soluble carbohydrates from photosynthesis are the aphids' energy source and are usually in plentiful supply; but it is the soluble amino-acids necessary for growth, which are at a relatively low concentration that can greatly influence aphid growth rate and fecundity. Seasonal changes in amino acid composition in conifer foliage significantly influences population increase and decline of the green spruce aphid (Carter and Nichols 1988) (Fig 2). An imbalance of major plant nutrients, especially nitrogen and potassium ratios, can significantly alter both the quality and quantity of available amino-acids and hence influence the growth rate of aphids (vanEmden and Bashford 1971).
Nutritionally modified conditions of the host plant can be brought about by an aphid of the same or even another species, a simple example being a delay in bud burst in trees following heavy aphid attacks the previous growing season (Carter 1988). Chlorosis of Picea caused by the green spruce aphid, Elatorium abietinum, improves the growth rate of the subsequent aphid generation by providing a better quality food source (Fisher 1987). Similarly in clustered aggregations of Schizolachnus pineti or Cinara pini, there is a temporary increase in amino acids concentration to 47% at their feeding sites, which then results in a 31% increase in nymphal growth rates (Kidd et al 1990). Eulachnus agilis favours senescing pine needles; its growth rate has been shown to increase when feeding on the same needles as Schizolachnus pineti (Kidd et al1985).
Aphids are also capable of inducing chemical changes in the tissue of their conifer hosts which may be their detriment. After a while, highly infested sites on the tree become unfavourable and the colonies decamp to another site (Larsson 1985). With Cinara cupressi on the vigorous hybrid Leyland cypress, x Cupessocyparis leylandii, and Cinara fresai on Juniperus virginiana, this behaviour is quite conspicuous, as small branchlets of foliage discolour and are shut down in response to feeding (Carter unpublished). This phenomenon is less obvious with Cinara pini on Pinus sylvestris; but anatomical inspection of the plant tissue beneath the aphid colony has shown extensive lignification develops with the cortex parenchyma cells as a result of aphid feeding (Smith 1991).
The question foresters usually ask first is: "What is the aphid doing to the host plant?" Perhaps they should also remember to ask: "What is the host plant doing to the aphid?"
The first step in the study and control of a new pest is its accurate identification *. But what is the taxonomic status of anholocyclic aphid populations? In favourable conditions they may prevail for many years on long-lived, perhaps geographically isolated hosts. should they be regarded as the same species as that found elsewhere on similar hosts, or as a geographical race or sub species? In the absence of sexual reproduction, the traditional concept of a species being unable to breed with other species cannot be applied.
Where a pest population originated from a single introduction, all members of subsequent anholocyclic generations are normally genetically identical. The isolated population will possess only a portion of the total gene pool for the species and may differ in some small aspects from some specimens in the main population. The anholocyclic pest often behaves differently in response to new ecological conditions, a new suite of potential hosts and no natural regulating mechanisms. However, there is no good reason to treat the isolated population as a separate taxon unless mutation has caused significant changes in its characteristics. Such mutations are extremely rare in asexual populations, and can only be detected and given taxonomic status if they result in important changes in biology, host preferences, impact or insecticide resistance.
Some quite simple silvicultural proposals which can reduce problems caused by aphids involve basic ecological factors. For example, young silver fir, Abies alba, from natural regeneration appear to suffer far less from Adelges normannianae when growing as an understorey than in exposed situations. Picea abies suffers from the green spruce aphid in hot, dry parts of Britain. These examples are both likely to be due to water stress problems that bring about an amino- acid imbalance that favours aphid development but is then detrimental to the trees. Water availability is only one of many components of the ecological system and only some of these factors can be modified for a particular site. Careful site selection could avoid problems of this kind. Furthermore, as the aphids have been shown to respond directly or indirectly through the plant to climatic and microclimatic factors, so in turn will their natural enemies. Therefore and understanding of which regulatory components operate in the place of origin of these aphid pests is essential to introducing a system of pest management.
Barnes, R.D., R.F. Jarvis, M.A. Schweppenhauser and L.J. Mullin. 1976. Introduction, spread and control of the pine woolly aphid, Pineus pini (L.) in Rhodesia. South African Forestry Journal, 96:1-11.
Bejer-Petersen, B., 1962. Peak years and regulation of numbers in the aphid, Neoniyaphis abietina (Walker) Oikos 13:155-168.
Carter, C.I., 1971. Conifer woolly aphids (Adelgidae) in Britain. Forestry Commission Bulletin 42, 51 pp.
Carter, C.I., 1972. Winter temperatures and survival of the green spruce aphid. Forestry Commission, Forest Record 84, 10 pp.
Carter, C.I., 1977. Impact of green spruce aphid on growth. Forestry Commission Research and Development Paper 116, 8 pp.
Carter, C.L., 1988. Aphids and conifers:Summary of a workshop meeting. Forestry Commission Research Division, 26 pp.
Carter, C.I. and N.R. Maslen, 1982. Conifer lachnids. Forestry Commission Bulletin 58, 75 pp.
Carter, C.I. and J.F.A. Nichols, 1985. Some resistance features of trees that influence the establishment and development of aphid colonies. Z. Angew. Entomol. 99:64-67.
Carter, C.I. and J.F.A. Nichols, 1988. The green spruce aphid and Sitka spruce provenances in Britain. Forestry Commission Occasional Paper 19, 7 pp.
Cerda, M.L. and R.I. Gara, 1977. El afido Adelges: un problema serio para los Abies chilenos. Chile Forestal, 3:27.
Doerksen, A.H. and R.G. Mitchell, 1985. Effects of the balsam woolly aphid upon the wood anatomy of some western true firs. Forest Science 11:181-188.
Dumbleton, L.J., 1932. Report on spruce-aphis investigation. N.Z. Jour. Sci. Tech. 13:207-220.
van Emden, H.F. and M.A. Bashford, 1971. The performance of Brevicoryne brassicae and Myzus persicae in relation to plant age and leaf amino acids. Entomologia Experimentalis & Applicata 14:349-360.
Evans, J., 1982. Plantation forestry in the tropics. Oxford, 472 pp.
Fisher, M., 1987. The effect of previously infested spruce needles on the growth of the green spruce aphid Elatobium abietinum and the effect of the aphid on the amino acid balance of the host plant. Annals of Applied Biology 111:34-41.
Judge, F.D., 1967. Overwintering in Pemphigus bursarius (L.). Nature 216:1041-1042.
Kidd, N.A.C., 1988. The large pine aphid on Scots pine in Britain. In: Dynamics of Forest Insect Populations. A.A. Berryman ed., pp 111-128.A.
Kidd, N.A.C., G.B. Lewis and C. Howell, 1985. An association between two species of pine aphid, Schizolachnus pineti and Eulachnus agilis. Ecological Entomology 10: 427-432.
Kidd, N.A.C., S.D.J. Smith, G.B. Lewis and C.I. Carter, 1990. Interactions between host-plant chemistry and the population dynamics of conifer aphids. In: Population Dynamics of Forest Insects, A.D. Watt et al, eds, Andover, pp 189-193.
Larsson, S., 1985. Seasonal changes in the within-crown distribution of the aphid Cinara pini on Scots pine. Oikos 45:215-222.
Major, E.J., 1990. Water stress in Sitka spruce and its effect on the green spruce aphid, Elatobium abietinum. In: Population Dynamics of Forest Insects, A.D. Watt, et al, eds, Andover, pp 85-93.
Nichols, J.F.A., 1987. The performance of the green spruce aphid on various spruce species and the effect of foliar amino-acids and secondary compounds. M. Phil. Thesis, University of Reading.
Nichols, J.F.A., 1987. Damage and performance of the green spruce aphid, Elatobium abietinum on twenty spruce species. Entomologia Experimentalis & Applicata 45:211-217.
Shaw, M.J.P., 1984. Some effects of infestation by the black pine aphid, Cinara cronartii (Tissot and Pepper) Proc. 17th Int. Congress Entomol. Hamburg, pp 607.
Sidje, H.A. van der, M.J.P. Shaw and G. van Wyk, 1985. Reaction wood in Pinus taeda - a preliminary report. South African Forestry Journal, 133:27-32.
Smith, S.D.J., 1990. Variable host quality in Pinus sylvestris and its relevance to the population ecology of Cinara pini L., PhD Thesis, University of Wales, Cardiff.
By
William M. Ciesla
Forest Protection Officer
FAO, Rome, Italy
The cypress aphid, Cinara cupressi (Buckton) is presently causing extensive die back and mortality of Cupressus lusitanica, a tree which is native to Mexico and Guatemala and has been widely planted as an industrial, agroforestry and ornamental tree in portions of southern and eastern Africa. Other trees of the family Cupressaceae are also affected. Purpose of this paper is to provide an overview of the state of knowledge of this insect, its potential for damage and pest management opportunities.
C. cupressi is one of a large number of species in the genus Cinara (Homoptera:Lachnidae). Commonly known as the giant conifer aphids, the adults range from 2 to 5 mm. in size and are dark coloured, long legged insects which are either naked or covered with a powdery wax. According to Eastop (1972), there are about 200 described species. Approximately 150 species are described from North America, 20 from Japan and the oriental region and 30 species are of European or Mediterranean origin.
Giant conifer aphids occur wherever conifers are found. They are widely distributed in Asia, Europe and North America. All species feed on twigs, branches and occasionally roots of conifers of the families Cupressaceae and Pinaceae. The following genera of conifers have been reported as hosts (Browne 1968, Eastop (1972) Furniss and Carolin 1977, Notario and Baragaņo 1990, Kabungo n.d.):
Family Cupressaceae:
Callitris
Chamaecyparis
Cupressus
Cupressocyparis
Juniperus
Thuja
WiddringtoniaFamily Pinaceae:
Abies
Cedrus
Larix
Picea
Pinus
Pseudolarix
Pseudotsuga
Tsuga
These insects apparently are easily transported, presumably on planting stock, and some species have been introduced into areas where conifer plantations have been established and have become pests. For example, C. cronartii, a rare species which infests stems of southern yellow pines infected with lesions or cankers of the rust fungus Cronartium fusiforme, is native to the southeastern United States. In 1974, this insect was discovered in South Africa, where it has become a pest of pine plantations (Kfir et al 1985). C. juniperi has been found in New Zealand (Browne 1968).
The giant conifer aphids usually have several generations a year. In temperate climates, winter is spent in the egg stage on the needles or bark. During the summer generations, females give birth to living young by parthenogenesis.
Most species of Cinara feed in colonies, usually on twigs and branches and also occasionally on the roots of their host plants. Many species confine their attacks to one genus of conifers and some will attack only one species of host plant. Feeding and oviposition site and species of host tree are helpful for species identification (Furniss and Carolin 1977).
In areas of alternating wet and dry periods, colonies of Cinara tend to be more abundant and damaging during the dry periods (Browne 1968, Kfir et al 1985).
Colonies of Cinara produce copious amounts of honeydew, a sweet, sticky material that covers the surrounding branches and foliage. This material provides a medium for the growth of dark coloured sooty molds which cover branches and foliage. Ants are also attracted to the honeydew which they use as food. Some species of Cinara are tended by ants which move adult aphids from branch to branch to start new colonies (Furniss and Carolin 1977).
Adults and nymphs suck plant juices from the phloem tissue of host plants. Feeding causes desiccation of plant tissue. Heavy populations of Cinara are capable of causing branch die back and tree killing.
Sooty molds associated with Cinara colonies cause foliage discoloration and interfere with photosynthesis and gas exchange.
In their native habitats, species of Cinara are usually kept in check by natural factors including weather, parasitoids and predators. However several species have been reported as pests. In North America, C. strobi, is capable of killing branches and young seedlings of Pinus strobus (Drooz 1985). C. pilicornis, which infests Picea in Europe, can be a pest in Christmas tree plantations because trees become sticky and covered with sooty mold (Speight and Wainhouse 1989).
When introduced into new locations, in the absence of natural enemies, several species of Cinara have become pests of economic importance. Two examples are the introduction of C. cronartii into South Africa (Kfir et al 1985) and C. cupressi into eastern and southern Africa.
C. cupressi is a large aphid. Two forms of female adults have been described; a brownish or yellowish wingless form and a brownish coloured winged female. Both forms give birth to living young (Browne 1968).
C. cupressi occurs in Africa, Asia, Europe, the Middle East and possibly North America (Fig 1). Places where it occurs naturally and where it may have been introduced difficult to determine. The insect feeds on a wide range of host plants in the family Cupressaceae (Tables 1-2).
ASIA - C. cupressi has been collected in the state of Sikkim, which is located between Bhutan and Nepal in northern India in 1975. The host plant is Juniperus recurva (Agarwala and Raychaudhuri 1982), a tree native to the eastern Himalayas.
EUROPE - In Europe, C. cupressi is reported from Belgium (Latteur 1980), Czechoslovakia (Eastop 1972), England (Browne 1968), France (Rabasse 1980), Germany (Eastop 1972), Italy (Binazzi 1978), the Netherlands (Eastop 1972), Poland (Eastop 1972) and Spain (Notario and Barango 1990). Most of the collections have been made on exotic host plants.
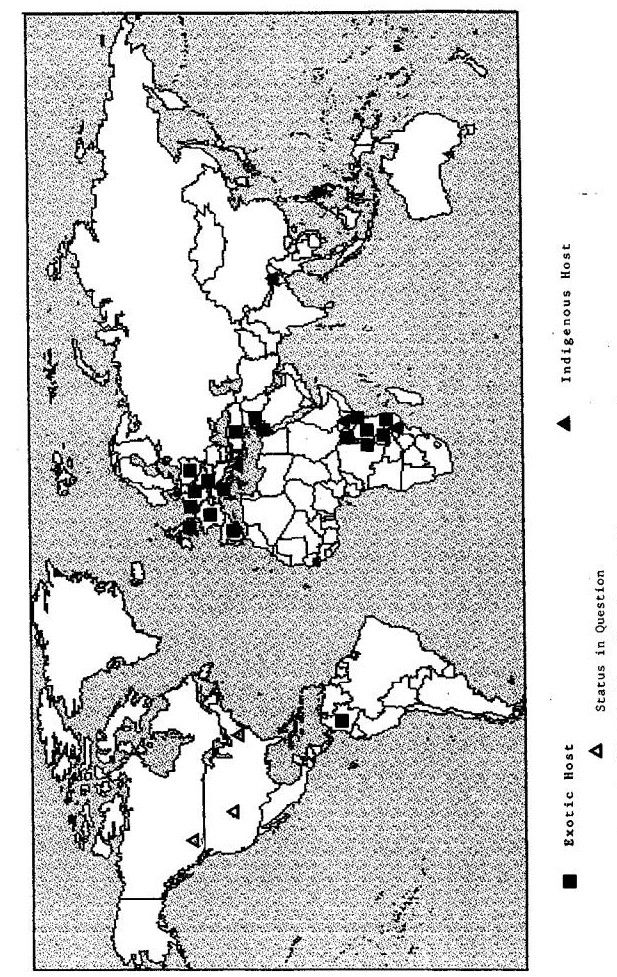
Fig. 1. Worldwide distribution of Cinara cupressi.
Browne (1968) reports that C. cupressi occurs in southern England where it occurs on the stems, branches and shoots of Cupressus macrocarpa, a tree native to California, USA, which has been widely planted in parts of England. In 1988, damage was reported on several exotic hosts; Cupressocyparis leylandii, Chamaecyparis lawsoniana and Thuja occidentalis.
Notario and Barango (1990) list C. cupressi as part of the aphid complex which infests conifers in Spain. Hosts are Cupressus arizonica and Thuja occidentalis. Neither of these hosts are native to Spain however and there is no indication of the degree of damage being caused to these hosts by this insect.
C. cupressi is also widely distributed in Italy where its hosts are Cupressus arizonica, Cupressus macrocarpa, Cupressus sempervirens, and Chamaecyparis lawsoniana and several species of Juniperus (Binazzi 1978). This insect has been reported as being the primary cause of dead twigs and branches on Cupressus in parts of Italy (Inserra et al 1979).
NEAR EAST - In 1980, C. cupressi was reported causing severe damage to C. sempervirens and C. arizonica in Israel (Mendel and Golan 1983). C. cupressi has also been reported from Jordan (Mustafa 1987) and Turkey (Eastop 1972).
NORTH AMERICA - Eastop (1972) considered two widely distributed North American species, C. canadensis Hottes and Bradley and C. sabinae (Gillette and Palmer) to be synonymous with C. cupressi. However in a later publication Eastop and Hille Ris Lambers (1976) once again consider C. sabinae to be a separate species and list the status of C. canadensis as questionable. C. canadensis has been reported on Juniperus virginiana in the eastern portion of the continent and C. sabinae has been reported on Juniperus scopulorum in the western regions (Voegtlin and Bridges 1988). Gillette and Palmer (1924) report a number of collections of Lachnus sabinae (= C. sabinae) from in Colorado including Denver, Estes Park, Ft. Collins and Longmont. Collections were made at elevations of up to 2590 m. There are no reports of damage by either of these at least closely related species in North America.
SOUTH AMERICA - Damaging populations of a species of Cinara have been detected in plantations of Cupressus lusitanica in Colombia. First collections were made from 1973 to 1975. These were initially identified as C. fresai (Bustillo 1975) and later re-identified as C. cupressi (Bustillo, PC 1990). Species has been confirmed as C. cupressi by taxonomists with the International Institute of Entomology in the UK (Watson, PC 1991)
|
COUNTRY |
HOSTS |
REFERENCES |
|
-ASIA- |
||
|
India |
Juniperus recurvara |
Agarwala and |
|
-EUROPE- |
||
|
Belgium |
Thuia occidentalis* |
Latteur 1980 |
|
Czechoslovakia |
None listed |
Eastop 1972 |
|
England |
Chamaecyparis lawsoniana* |
Browne 1968 |
|
France |
Cupressus arizonica* |
Rabasse 1980 |
|
Germany |
Thuja occidentalis* |
Eastop 1972 |
|
Italy |
Cupressus arizonica* |
Inserra |
|
Netherlands |
Thuja occidentalis* |
Eastop 1972 |
|
Poland |
Thuja occidentalis* |
Eastop 1972 |
|
Spain |
Cupressus arizonica* |
Notario and |
|
-MIDDLE EAST- |
||
|
Israel |
Cupressus arizonica* |
Mendel and |
|
Jordan |
Cupressus sempervirens* |
Mustafa 1987 |
|
Turkey |
Cuoressus goveniana* |
Eastop 1972 |
|
-NORTH AMERICA- |
||
|
(Designated as C. canadensis and C. sabinae) |
||
|
Canada |
Juniperus scopulorum |
Eastop 1972 |
|
United States |
Juniperus virgriniana |
Eastop 1972 |
* Indicates a host tree exotic to that country.
Observations on the life stages of Lachnus sabinae (= C. cupressi), a possible synonym of C. cupressi, in Colorado, USA, indicates that both winged and wingless females (apterous and alate virgogenia) are present during the growing season (April - September). Live nymphs are produced by parthenogenesis. Both sexes (alate males and apterous females) are present during October and November and females produce eggs (Gillette and Palmer 1924).
Studies of the reproductive biology of C. cupressi in Jordan indicates that in warm climates, parthenogenic reproduction continues throughout the year. Females have a mean fecundity of 23.5 and individuals have an average life span of 21.9 days at 20° Cand 12 hours of alternating daylight and darkness. From 8 to 9 generations are believed to occur in Jordan (Mustafa 1987).
COUNTRIES AFFECTED - In Africa, C. cupressi has been confirmed from the following countries in the following years:
|
Burundi1 |
1988 |
|
Rwanda |
1989 |
|
Kenya1 |
1990 |
|
Malawi1 |
1986 |
|
Tanzania1 |
1987 |
|
Uganda |
1989 |
|
Zaire |
1990 |
|
Zimbabwe1 |
1990 |
1 Identified or confirmed by the International Institute of Entomology (HE) London, UK
The insect was first discovered in Malawi in 1986 (Odera (1991). In 1987, infestations were detected in Tanzania (Kabungo nd). C. cupressi was discovered in Kenya in 1990. The potential for spread to other countries with suitable host plants through transport of infested Materialor of insects by air currents is high.
HOSTS - A large number of exotic and indigenous trees of the family Cupressaceae are attacked by C. cupressi are attacked in Africa. These include species in the genera Callitris, Cupressus, Cupressocyparis, Juniperus, Thuja, and Widdringtonia. There is considerable variation between susceptibility of the various hosts (Table 2). Cupressus lusitanica, which has been widely planted in eastern and southern Africa is highly susceptible to feeding by C. cupressi. Indigenous trees which are attacked include Widdringtonia nodiflora, Malawi's national tree and Juniperus procera, which occurs in Kenya and, to a lesser degree, Tanzania and Malawi.
DAMAGE - Colonies of aphids feed on the smaller twigs and branches in the main part of the crown. This results in branch die back followed by tree mortality. C. cupressi seems to be a shade loving insect. Aphid colonies tend to be more abundant in the inner and lower crowns. Therefore, dead branches first appear in these portions of the crown. The terminal branch tips are usually the last portion of the tree to become discoloured. The presence of sooty mold has also been reported.
In Tanzania, populations of up to 80 aphids/10 cm branch occur. Damage appears to be more severe during the June to September dry season (Kabungo n.d., Kessy 1990).
IMPACTS - Feeding causes desiccation of the stems and a progressive dieback of heavily infested trees. Dieback usually occurs from the inner crown outward and from the lower crown upward. Unless infestations are treated promptly, death of trees which are sensitive to the feeding of the cypress aphid is imminent.
C. lusitanica is extremely sensitive to feeding by cypress aphid. This tree is a highly favoured plantation species because of its rapid growth rate and excellent form. Trees mature in about 25 years and plantations on good sites are capable of producing an average of 300 m per ha at the time of final harvest. In Kenya, which has the largest area of industrial forest plantations of the countries presently affected by this insect, C. lusitanica is planted on about 86,000 ha. This comprises approximately 45 % of total area of this country's industrial forest plantations. Loss of these
(FROM DATA BY ODERA 1991)
|
Genus |
Species |
Susceptibility* |
|
|
Muguga, Kenya |
Malawi |
||
|
Callitris |
glauca |
- |
M |
|
pressi (robusta) |
H |
- |
|
|
rhomboidea |
H |
- |
|
|
Cupressus |
airizonica |
M |
- |
|
benthamii |
H |
- |
|
|
columellaris |
M |
- |
|
|
endlicheri |
M |
- |
|
|
forbesii |
M |
- |
|
|
funebris |
M |
T |
|
|
intratropica |
H |
- |
|
|
cashmeriana |
- |
T |
|
|
lindlyei |
M |
- |
|
|
lusitanica |
H |
H |
|
|
macrocarpa |
H |
- |
|
|
nevadensis |
M |
- |
|
|
sempervirens |
|||
|
sempervirens |
- |
S-M |
|
|
sempervirens |
|||
|
horizontalis |
H |
- |
|
|
sempervirens |
|||
|
pyramidalis |
- |
M |
|
|
torulusa |
T |
M |
|
|
Cupressocyparis |
leylandii |
T |
- |
|
Juniperus |
procera |
M |
T |
|
Widdringrtonia |
cupressoides |
H |
- |
|
juniperoides |
H |
- |
|
|
nodiflora |
H |
M |
|
* H - Highly susceptible
M - Moderately susceptible
S - Slightly susceptible
T - Tolerant
plantations could have serious effects on the region's domestic wood supply.
In addition, C. lusitanica is a key agro-forestry species in eastern and southern Africa and is widely planted for windbreaks, as a source of fuel wood and as living fences and hedges.
Juniperus procera, is a major indigenous species of high elevation forests in portions of eastern Africa. In Kenya alone, there are 280,000 ha of plantations and natural forests. This species is damaged by cypress aphid, but to date damage has not been as severe as that which is occurring on C. lusitanica. Loss of this tree in water catchment areas could result in soil erosion and loss of water quality.
In addition to direct losses to forest resources, the presence of a large number of dead trees in rural and urban areas provides a large volume of fuel which can increase the destructive potential of wildfire. Many homes in rural areas are surrounded by cypress hedges which are dying. The presence of dry hedges poses an imminent fire hazard and could result in loss of life and property.
Several approaches; biological, genetic, silvicultural and chemical offer promise for managing the cypress aphid in Africa. Since the insect is a recent introduction, the development of pest management programs will require research support. An integrated approach; one which will afford protection to existing plantations and ensures the viability of Cupressus lusitanica, Juniperus procera, and other hosts as viable forest, agroforestry and ornamental trees, should be considered.
This approach involves the identification and evaluation of natural enemies already effecting C. cupressi in Africa and the introduction of parasites and predators which might supplement the existing natural control complex. Kessy (1990) reports that six families of insects, representing four orders (Diptera, Coleoptera, Hymenoptera and Neuroptera) have been observed as natural enemies of C. cupressi in Tanzania. Larvae of the family Sirphidae (Diptera) are commonly seen feeding on colonies of C. cupressi in Kenya.
Parasitoids are an important part of the natural control complex of aphids of the family Lachnidae. According to Mills (1990), these offer the greatest potential for biological control.
Braconid wasps of the genus Pauesia attack all lachnid aphids (Mills 1990). During the early 1970's, the black pine aphid, C. cronartii, was discovered damaging plantations of Pinus taeda, P. patula, and P. elliotii plantations in South Africa. This aphid is native to the southeastern United States where it is found in or near bark lesions or cankers caused by fusiform rust, Cronartium fusiforme. An unidentified species of Pauesia was discovered to be a parasitoid of C. crontartii and was introduced and established in South Africa. This was followed by a collapse of the aphid population (Kfir et al 1985). The parasitoid has seen been identified as P. bicolor (Mills 1990).
Two species of Pauesia. P. cupressobii, and P. juniperorum have been recorded from Cinara juniperi, an aphid closely related to C. cupressi which infests Juniperus communis. These two parasitoids are considered to be specific to Cinara sp. which feed on plants of the family Cupressaceae. Another parasitoid, Aphidus sp. has been recovered from C. cupressi in Germany. This parasitoid is apparently heavily attacked by hyperparasitoids.
Identification and propagation of strains of cypress resistant to attack by C. cupressi is a long range measure which, if successful, would ensure the future of the Mexican cypress as a viable alternative for afforestation and reforestation programs in Africa. This line of investigation should explore the seed sources which have been used to establish cypress plantations, opportunities for evaluation of alternative provenances, mechanisms of resistance and ultimately, the establishment of seed orchards for breeding resistant strains.
Kessy (1990) reports that in some infested areas in Tanzania, individual trees were not infested or damaged. Consequently there may already be resistant individuals present within the infested area.
Since cypress aphid appears to be a shade loving insect and colonies are found in the inner crown amid dense foliage, thinning of plantations could possibly could create conditions less favourable for population buildup. Establishment of plantations on sites where soils are shallow or there is insufficient moisture may place host trees under stress and make them more sensitive to attack. Site factors which make certain trees more sensitive attack should be identified. Sites having these characteristics should not be planted to species sensitive to aphid attack in the future. Use of species which are less sensitive to cypress aphid attack is another also possibility. For example, Cupressus torulusa Don., a species native to the Himalayas, and planted in Africa, appears to be more tolerant to attack (Table 2). These approaches also require testing and evaluation before they can be recommended however.
Some chemical control has already been undertaken in Tanzania (Kabungo n.d., Kessy 1990). In Kenya, the following insecticides have been found to be effective against C. cupressi (Anon 1990):
Fenitrothion
Fenvarerlate
Malathion
Cypermethrin
Lamdacyhalothrin
Bifenthrin
Carbosulfan
Timing of insecticide application apparently is critical to prevent branch dieback. Studies in Italy indicate that chemical treatments with aphicides applies when aphids were beginning to colonize the plants prevented tree damage. On the contrary, chemical applications 20 - 30 days after aphid colonization gave excellent control but did not prevent death of twigs that had been inhabited by this insect (Inserra et al 1979).
While chemical control may be effective in the short term, it is not considered to be a viable long term control tactic. This is because of the problems associated with environmental contamination, applicator safety and the potential for the target insect to develop resistance to chemical pesticides. In addition, chemical control is counter productive to the development of long term biological control tactics.
Effective management of cypress aphid, like any other destructive pest, is best conducted within the context of integrated pest management (IPM). IPM consists of a number of ecologically sound biological, cultural, genetic and mechanical tactics used alone or in combination to reduce pest populations to tolerable levels. Use of chemicals can be part of an IPM programme but they are generally used sparingly and as a last resort.
Long term management of this insect will undoubtedly rely on a combination of silvicultural, genetic and biological tactics. These must be developed and tested through a comprehensive programme of research and development. In the interim, several short term tactics can be applied which will afford protection to the forest resource. These include:
* Accelerated harvesting of cypress plantations which are mature or nearly mature and are in imminent danger of dying to recover values and reduce fire hazard.
* Removal of dead and dying hedges from around home sites to reduce fire hazard.
* Judicious use of chemical insecticides to protect high value stands such as seed orchards and hedges around rural home sites.
In response to a request from the government of Malawi, FAO has fielded a consultant to evaluate the status of aphids, including cypress aphid, in that country's conifer plantations and indigenous forests. A special project involving both research and operational aspects has been recommended (Odera 1991). In addition, the International Institute of Biological Control (IIBC), an organization based in the United Kingdom, has begun a search for natural enemies of cypress aphid and two species of pine infesting aphids for possible introduction in Malawi.
The Government of Kenya has requested an emergency Technical Cooperation Project (TCP) from FAO to help initiate emergency control measures and serve as a bridge to a longer term project which has been developed. This project contains components which address operational control, research and development and technology transfer with an ultimate goal of having a working IPM system for cypress aphid within five years. FAO specialists have provided technical assistance to the Kenya Forest Research Institute (KEFRI), the Department of Forestry and the International Centre for Insect Physiology and Ecology (ICIPE) to develop a master plan for this five year project which will be funded by several donors.
FAO was a technical collaborator in conjunction with IIBC and USDA Forest Service in a special workshop on conifer aphids in Africa which was organized by KEFRI. One of the primary objectives of this workshop was to strengthen networks between scientists and practitioners of the countries affected by these insects.
Once long range pest management options are developed and are ready for operational use, provisions must be made for their application. Transfer of research information to operational use is best accomplished through the development of a strong sub-regional network of research scientists and pest management specialists working toward a common goal; the effective management of this new threat to the forest resources of eastern and southern Africa.
Agarwala, B.K. and D. Raychaudhuri, 1982. Two species of Cinara, Curtis, from India with a description of a new species (Homoptera:Aphididae, Lachnidae). Akitu Journal:46 2.
Anonymous, 1990. Chemical control of cypress aphid in Kenya. Kenya Forest Research Institute. 7 pp.
Binazzi, A. 1978. Contibuti alla conoscenza degli afidi delle conifere. 1. Le specie dei Genn. Cinara Curt., Schizolachnus Mordr., Cedrobium Remaud ed Eulachnus D. Gu. presenti in Italia (Homoptera:Aphidoidea Lachnidae). Redia LXI 291-400.
Browne, F.G. 1968. Pests and diseases of forest plantation trees. Clarendon Press, Oxford. 1330 pp.
Bustillo, A.E., 1975. El pulgon gris del cipres, Cinara fresai Blanchard (Homoptera: Aphididae). Revista Colombiana de Entomologia 1:33-34.
Drooz, A.T. (ed), 1985. Insects of eastern forests. USDA Forest Service, Misc. Pub. 1426, 407 pp.
Eastop, V.F. 1972. A taxonomic review of the species of Cinara Curtis occurring in Britain (Hemiptera:Aphididae) Bull. British Museum (Natural History) Entomology 27:101-186.
Eastop, V.F. and D. Hille Ris Lambers 1976. Survey of the world's aphids. Dr. W. Junk b.v. the Hague 573 pp.
Furniss, R.L. and V.M. Carolin, 1977. Western forest insects. USDA Forest Service, Misc. Pub. 1339, 654 pp.
Gillette, C.P. and M.A. Palmer 1924. New Colorado Lachnini. Annals Entomol. Society America, 17:1-44.
Inserra, R.N., N. Luisi and N. Vovlas, 1979. Il roulo delle infestazioni dell afide Cinara cupressi (Buckton) nei deperimenti de cipresso. Informatore Fitipatologico 29: 7-11.
Kabungo, D.A. n.d. A preliminary report on cypress aphid in the southern highlands of Tanzania; 1987-88. The Uyole Agricultural Centre, Mbeya, Tanzania, SKU/1/9/18 Vol II, 5pp.
Kessy, B.S., 1990. Major pest problems: Tanzania. Proceedings, IUFRO Montreal, Canada, V2:259-269.
Kfir, R., F. Kirsten and N.J. Van Rensburg, 1985. Pauesia sp. (Hymenoptera:Aphidiidae), a parasite introduced into South Africa for biological control of the black pine aphid, Cinara cronartii (Homoptera:Aphididae). Environmental Entomology 14:597-601.
Latteur, G. 1980. About the recent presence of Cinara cupressi (Buckton) in Belgium. in Grasso, V. and P. Raddi. II Cipresso: malattie e defensa. Florence, Italy, EEC Agrimed pp 223-224.
Mendel, Z. and Y. Golan, 1983. The cypress aphid (Cinara cupressi), a new pest of cypress in Israel. Hassadeh Journal:63 (12).
Mills, N.J. 1990. Biological control of forest aphid pests in Africa. Bull. Entomolo. Research. 80: 31-36.
Mustafa, T.M. 1987. Reproductive biology and population studies of cypress aphid, Cinara cupressi (Buckton), and pine aphid, C. maritime (Dafour). Dirasat (Jordan) 14:99-105.
Notario, A. and J. Baragaņo, 1990. Los pulgones de las coniferas de Espana. Proceedings IUFRO Montreal, Canada V.2: 226-231.
Odera, J.A. 1991. Some opportunities for managing aphids of softwood plantations in Malawi. Assistance to Forestry Sector Malawi - MLW/86/020. FAO, Rome, 135 pp.
Rabasse, J.M. 1980. Les insectes ravaguers des cypress en France. in Grasso, V. and P. Raddi (ed). II Cipresso: malattie e defensa. Florence, Italy, EEC, Agrimed, pp 217-222.
Speight, M.R. and D. Wainhouse, 1989. Ecology and management of forest insects. Oxford: Clarendon Press, 374 pp.
Voegtlin, D.A. and C.A. Bridges, 1988. Catalog of the Cinara species of North America (Homoptera:Aphididae). Illinois Natural History Survey. Special Publication 8, 55 pp.
Winter, T.G. 1989. Cypress and juniper aphids. Arborculture Research Note, Dept. Environment UK #80, 3pp.
2 Reference not seen, quoted by Kabungo n.d.
By
S.T. Murphy, Y.J. Abraham
and A.E. Cross
International Institute of Biological Control
Silwood Park, Buckhurst Road
Ascot, Berks, SL5 7TA
United Kingdom
Until the recent expansion of forestry in eastern and southern Africa (Evans 1982, 1986), exotic pine plantations were relatively free of insect problems (Mills 1990). However this situation has changed markedly in the last few years with the dramatic spread within the region of two aphid pests which originate from the northern temperate zone: The pine woolly aphid, Pineus sp. (Homoptera:Adelgidae) and the pine needle aphid, Eulachnus rileyi Williams (Homoptera:Lachnidae) (Mills 1990). These aphids are the first specific conifer pests to have invaded Africa and are the most damaging of the insects currently feeding on the pines which are widely grown both in plantations and by small-scale farmers. As with all invading pests, the absence of their specific natural enemies in Africa permits them to become unusually abundant and damaging in this new environment, and as such, they pose a serious threat to the future of the various forest systems.
The purpose of this paper is to summarize the known ecology of the two pine aphids and to provide an economic assessment of the damage caused by these species.
The pine
woolly aphid was first reported simultaneously from Kenya and Zimbabwe in 1968 and was probably introduced
into Zimbabwe with loblolly pine, Pinus taeda, scions from Australia in 1962 (Barnes et al 1976). Since 1968, the aphid
has spread throughout African pine
plantations and the other countries now affected include Ethiopia, Tanzania, Malawi and
South Africa. This species was
originally identified as Pineus pini (Macquart) which is indigenous to
Europe and
possibly parts of Pakistan and India (R. Blackman, pers. comm.).
A number of studies have been completed on the adelgid in Africa (Odera 1974, Barnes et al 1976, Mailu et al 1980) and these have suggested that the life history of this species is similar to that of the European P. pini (Mills 1990). The African Pineus sp. is anholocyclic and autoecious; that is the species occurs on one host (pines) and reproduction occurs entirely parthenogenetically. Both apterous and alate forms of the aphid occur although the former is commoner than the latter. The aphid feeds at the base of the needles and on young bark and the apterous form, which is about 1 mm in length in the adult stage, produces a while "woolly" was which gives this species its common name. At high densities, this feeding behaviour causes a reduction in needle growth, needle drop, shoot death, dieback of branches and, sometimes, death of trees.
A wide range of pine species are attacked by the pine woolly aphid (Zwolinski 1989) but there seems to be a great variation in the susceptibility to attack between species (Odera 1974, Barnes et al 1976, Zwolinski 1989). Of the main industrial species, P. kesiya, P. elliottii, and P. radiata seem to be highly to moderately susceptible, while P. patula and P. taeda are only slightly susceptible to attack than mature plantation trees. Barnes et al (1976) note, however that the susceptibility of trees of the same species also differs between individuals planted on the same site.
In Zimbabwe and Kenya, population studies have indicated that the pine woolly aphid is most abundant during the dry season and tends to be reduced by rainfall during the wet season (Barnes et al 1976, Mailu et al 1980). Mailu et al (1980) also reported that nine species of indigenous predatory insects were found attacking the aphid and that together these predators were removing approximately 12 % of the aphid population. The most common predators were Exochomus sp. (Coleoptera:Coccinellidae).
First records of the pine needle aphid come from Zambia, Zimbabwe and South Africa in the late 1970's (Odendaal 1980, Loyttyniemi 1979, Marchant 1981) but the species has also subsequently spread to Malawi and Tanzania. This aphid originates from Europe and North America (Carter and Maslen 1982).
Studies on the pine needle aphid in Africa indicate that the species has a reduced life cycle reproducing continuously throughout the year without the sexual forms (Katerere 1984). The adult of the aphid is about 2.5 mm in length, grey and elongate. All stages feed on the underside of pine needles of all age classes. The aphid normally occurs as apterous adults but alates are sometimes produced. Infested needles turn yellow and the aphids produce copious quantities of honeydew, which induce a cover of sooty moulds on heavily infested trees. In Zambia, Zimbabwe and Malawi, the aphid has been found on P. caribea, P. chiapensis, P. elliottii, P. kesiya, P. merkusii, P. michoacana, P. oocarpa, P. patula, P. roxburghii and P. taeda (Loyttyniemi 1979, Katerere 1984, Mills 1989).
The population study by Katerere (1984) in Zimbabwe suggests that, in that country, there are two peaks of aphids each year; in July and November. In July the greatest densities are found on P. taeda and in November on P. patula. In 1978 and 1979 in Zambia, the build up of populations was rapid in May-June after the rains caused a dramatic decrease in population density in November and December (Lottyniemi 1979).
A number of studies have been conducted to determine the impact of the pine woolly aphid on wood production in Africa. In Kenya, Mailu et al (1978) showed that 6 % of the plantations of medium-aged P. patula, a major species grown for industrial purposes, were infested with the aphid and that those trees were losing approximately 5 % of their volume over a six year period. Again in Kenya, and more dramatically, Odera (1974) reported that 20% mortality occurred in some study plots in the centre of the aphid outbreak in 1968. More recently, Madoffe and Austara (1990) showed from studies in Tanzania, that the shoots and stems of seedlings of P. patula lost 20.9 % of their dry weight after 24 weeks.
Studies on other pines in the southern Cape region of South Africa in the early eighties (Zwolinski 1990) showed that 89.2 % of P. pinaster, 54.2 % of P. elliottii, and 27.2 % of P. radiata were infested with the aphid. P. pinaster was the most susceptible of the three pines and infested trees lost 19.5 % of the annual increment in volume. Zwolinski et al (1989) also reported that 31.8 % of P. pinaster cones were heavily infested and showed deformation, cracks and resinous outflow; the total number of seeds obtained from affected cones was reduced by 71.7%.
There is currently no information available about the quantitative affect of the pine needle aphid on its pine hosts. Studies on other tree-feeding lachnids do, however, give some insight on this subject. The most damaging attacks of aphid are generally on young trees and seedlings. For example, heavy infestations of the spruce aphid, Cinara piceae, (Panzer), reduces tree growth by up to 60 - 70 % in young spruce plantations in Japan (Furuta and Takai 1983) and the grey pine aphid, Schizolachnus pineti (F.) reduces growth of Scots pine to a similar extent (Thompson 1977). While no studies have been conducted on lachnid impact on older softwood trees, the sycamore aphid, Drepanosiphum platanoides (Schr.), reduces annual growth of mature sycamore by as much as 77 % (Dixon 1971) It is probable, therefore, that loss of growth is broadly equal in both young and older trees while the younger and seedling trees may be less able to survive this growth stress. It is likely that, at high densities, the pine needle aphid reduces growth in pines to the same extent as observed in these studies.
The presence of the two pine aphids at their current levels of abundance in the pine plantations of southern and eastern Africa is having a continuing effect on the economics of the countries concerned. Besides causing direct annual losses of valuable timber in established plantations, the aphids are also having several important indirect effects on forestry such as a set back in the planting programmes of some high yielding pine species (for example, P. radiata) that are susceptible to attack. Clearly problems such as these will become more acute with the inevitable expansion in the distribution of aphids.
Taking into account current timber losses due to aphid attack together with the many expanding forestry programmes involving exotic conifers in the region, sustainable, environmentally sound methods of control need to be introduced on a regional basis as a matter of urgency. In this connection, the International Institute of Biological Control (IIBC) is currently developing a regional biological control programme for southern and eastern Africa aimed at controlling and containing the two pine aphids together with the cypress aphid, Cinara cupressi (Buckton)
Barnes, R.D., R.F. Jarvis and M.A. Schweppenhauser, 1976. Introduction, spread and control of the pine woolly aphid, Pineus pini (L.) in Rhodesia. Journal of the South African Forestry Association 96:1-11.
Carter, C.I. and N.R. Maslen, 1982. Conifer lachnids. Forestry Commission Bulletin 42, 51 pp.
Dixon, A.F.G., 1971. The role of aphids in wood formation. 1. The effect of the sycamore aphid, Drepanosiphum platanoides (Schr.) (Aphididae), on the growth of Sycamore, Acer pseudoplatanus (L.). Journal of Applied Ecology 8:165-179.
Evans, J., 1982. Plantation ecology in the tropics. Oxford; Clarendon Press, 432 pp.
Furuta, K., and M. Takai, 1983. Population dynamics of Cinara bogdanowiezoana Inouye (Homoptera:Lachnidae) in plantations of Picea glehnii Masters and P. jezoensis Carriere. Zeitschrift fur Angewahdte Entomologie 95:238-249.
Katerere, Y., 1984. Biology and population dynamics of the pine needle aphid, Eulachnus rileyi (Williams) in Zimbabwe. South African Forestry Journal 129:40-49.
Loyttyniemi, K., 1979. Eulachnus rileyi (Williams) (Homoptera:Lachnidae) infesting pine trees in Zambia. Annales Entomologici Fernici 45:116.
Madoffe, S.S. and O. Austara, 1990. Impact of the pine woolly aphid, Pineus pini (Macquart) (Hom. Adelgidae) on growth of Pinus patula seedlings in Tanzania. Journal of Applied Entomology, 110:421-424.
Mailu, A.M., C.P.M. Khamala, and D.J.W. Rose, 1978. Evaluation of pine woolly aphid damage to Pinus patula and its effects on yield in Kenya. East African Agricultural and Forestry Journal, 43:259-265.
Mailu, A.M., C.P.M. Khamala and D.J.W. Rose, 1980. Population dynamics of the pine woolly aphid, Pineus pini (Gmelin) (Hemiptera:Adelgidae), in Kenya. Bulletin of Entomological Research 70:483-490.
Marchant, L., 1981. The pine needle aphid, Eulachnus rileyi Williams (Homoptera:Aphididae). Pests and Diseases of South African Forests and Timber. Directorate of Forestry and Environmental Conservation, Pretoria, Pamphlet 273, 4pp.
Mills, N.J., 1989. Report on a consultancy visit to Malawi, July 23 - August 6 1989, to assess the aphid problems in conifer plantations and the feasibility of biological control, on behalf of the British Development Division, Southern Africa. CAB International Institute of Biological Control, Unpublished Report, 22 pp.
Mills, N.J., 1990. Biological control of forest aphid pests in Africa. Bulletin of Entomological Research, 80:31-36.
Odendaal, M., 1980. Eulachnus rileyi: a new pest on pines in Zimbabwe. South African Forestry Journal, 115:69-71.
Odera, J.A., 1974. The incidence and host trees of the pine woolly aphid, Pineus pini (L.) in East Africa. Commonwealth Forestry Review 53, 128-136.
Thompson, S., 1977. The effect of an attack by the aphid Schizolachnus pineti Fabricius on the growth on young Scots pine trees. Scottish Forest 31:161-164.
Zwolinski, J.B., 1990. The pine woolly aphid, Pineus pini (L.), a pest of pines in South Africa. South African Forestry Journal, 151:52-57.
Zwolinski, J.B., 1990. Preliminary evaluation of the impact of pine woolly aphid on condition and growth of pines in the southern Cape. South African Forestry Journal, 153:22-26.
Zwolinski, J.B., D.C. Grey and J.A. Mather, 1989. The impact of the pine woolly aphid, Pineus pini (L.) (Adelgidae:Homoptera) on cone development and seed production of Pinus pinaster in the southern Cape. South African Forestry Journal 148:1-6.