H. I. Joly1
Laboratoire INRA-ENGREF de sciences forestières
Unité de génétique des population d'abres forestiers
associèe au CIRAD-Forêt, France
Present address: CIRAD-Forêt,
45 bis avenue de la Belle Gabrielle,
94 736 Nogent-sur-Marne, France
SUMMARY
In connection with an overall evaluation of the genetic resources of Faidherbia albida, its genetic variation and breeding system were studied using isozymes as genetic markers.2 The 19 populations studied showed a high level of variation in isozymes within and between populations. The main differences occurred between western and eastern populations. The outcrossing level of 4 populations from Niger was studied and found to be unexpectedly high for an insect pollinated species, especially considering the fact that the morphology of the inflorescence and the dynamics of the flowering would seem to favour a high degree of selfing. It is suggested that Faidherbia albida possesses a flexible breeding system involving some self-incompability.
INTRODUCTION
Faidherbia albida (syn. Acacia albida) is widely distributed in Africa (Wickens 1969) where it grows under varying conditions of rainfall, soils and altitude. Its importance as a multipurpose tree in agroforestry in Africa is well known. As the species is in leaf and flowers during the dry season, both leaves and pods are available as forage; this is especially important at the end of the dry season, when other sources of fodder are scarce. The tree also positively affects soil fertility and produces a wide range of other products for the use by rural communities.
Due to threats to parts of its genepool, its importance in rural development and scope for domestication and improvement, F. albida has been listed as a priority species by the FAO Panel of Experts on Forest Gene Resources since 1974 (FAO 1974) It is also one of the species covered in the ongoing FAO Project on Genetic Resources of Multipurpose Woody Species in the Sahelian and North Sudanian Zones of Africa (de Framond 1990). In spite of these activities, however, there is still a lack of basic information on this species, particular in information related to genetic variation and breeding systems.
Genetic variation can be assessed through provenance and progeny trials, established on a range of sites. After systematic collection work, complementing collections made within the framework of the FAO project mentioned above, carried out by national research institutes in West Africa and CTFT (now CIRAD-Forêt), such trials were established in Burkina Faso and in Zimbabwe in 1985. In Burkina Faso, trials were established at Gonsé (rainfall 800 mm) and at Dindéresso (rainfall 950 mm), using 19 populations from West Africa (Burkina Faso, Mali, Niger, Senegal), Central Africa (Cameroon) and East Africa (Burundi, Ethiopia, Zimbabwe). Some of the same populations were also tested in Zimbabwe. Additional collections are under way in eastern Africa under an Overseas Development Administration (U.K.) scheme (Fagg & Barnes 1990). Recently field trials have also been set up in other countries. Although results were not conclusive, partly due to the unsuitability of one of the sites in Burkina Faso where mortality was high, there seems to be a clear distinction in the behaviour of eastern and western populations of the species, evident both in the trials in Burkina Faso (Billand 1991) and in Zimbabwe (Sniezko & Stewart 1989).
Progeny trials have been established in Burkina Faso since 1987 to estimate the heritability of some important traits in a few provenances. Early results of these trials showed a heritability of 0.26 for height, at age 42 months (Billand, 1991).
The field trials described above will go some way towards estimating genetic variation and heritability in Faidherbia albida. However, field studies of genetic diversity can be complemented through studies on genetic markers, and this latter methodology also allows for estimation of the gene flow within and between populations. The present paper describes work carried out on the genetics of populations of F. albida at the INRA-ENGREF Laboratory in Nancy by analyzing seeds using isozymes as genetic markers.
MATERIAL AND METHODS
Seeds from 19 populations have so far been analyzed. The seeds were supplied by CIRAD/CTFT. One seed per tree and about 20 trees per population were used in the study (see Table 1).
Table 1. Origins of the population studied.
| Country | Population | Latitude | Longitude | Altitude | Rainfall |
| Burkina Faso | Diou | 11°45'N | 02°56'W | 275 m | 800 mm |
| Dori | 14°02'N | 00°02'W | 275 m | 800 mm | |
| Safané | 12°08'N | 03°13'W | 293 m | 800 mm | |
| Cameroon | Bibémi | 09°16'N | 13°50'E | 220 m | 875 mm |
| Guétalé | 10°57'N | 13°55'E | 450 m | 850 mm | |
| Makary | 12°34'N | 14°28'E | 286 m | 500 mm | |
| Mora | 11°00'N | 14°13'E | 400 m | 680 mm | |
| Moulvouday | 10°23'N | 14°50'E | 330 m | ||
| Zamay | |||||
| Mali | Barouéli | 13°05'N | 06°51'W | 250 m | 800 mm |
| Kémény | 12°58'N | 05°40'W | 270 m | 760 mm | |
| Kolongotomo | 13°49'N | 05°48'W | 280 m | 650 mm | |
| Samaye | 13°50'N | 04°45'W | 260 m | 570 mm | |
| Sarro | 13°42'N | 05°15'W | 250 m | 585 mm | |
| Niger | Bouza | 14°25'N | 06°07'E | 300 m | 690 mm |
| Kollo | 13°18'N | 02°21'E | 210 m | 593 mm | |
| Matameye | 13°25'N | 08°28'E | 450 m | 560 mm | |
| Tera | 14°00'N | 00°45'E | 240 m | 458 mm | |
| Zimbabwe | Mana pools | 15°45'S | 29°20'E | 360 m | 780 mm |
For the sub-study concerning gene flow within populations, the 4 available populations from Niger were chosen. Six seeds per tree were analysed using electrophoresis on emerging seedlings 5 days of age. Eight enzymatic systems, representing 10 loci, out of 15 for which staining procedures have been described by Joly et al (1992), were investigated in the present study.
For each population the following genetic parameters were calculated: number of polymorfic loci, mean number of alleles per locus, observed heterozygosity, expected heterozygosity, and fixation indices (see Table 2). Distribution of variation between and within populations was estimated according to Wright (1965). A principal component analysis was also carried out on the observed allele frequencies of the populations.
The gene flow within a population was analysed following the multilocus outcrossing rate estimation model of Ritland & Jain (1981), using a programme provided by Ritland (1990).
Table 2. Genetic characteristics of population studied
| Mean heterozygosity | ||||||
| Population | Mean no. of alleles per locus | Percentage of loci polymorphic* | Direct-count | Expected** | Fixation index | |
| 1. | DIOU (BF) | 2.8 | 80.0 | 0.298 | 0.371 | 0.357 |
| 2. | DORI (BF) | 3.3 | 90.0 | 0.330 | 0.444 | 0.227 |
| 3. | SAFANE (BF) | 2.5 | 80.0 | 0.228 | 0.293 | 0.392 |
| 4. | BIBEMI (C) | 2.9 | 80.0 | 0.365 | 0.437 | 0.175 |
| 5. | GUETALE (C) | 3.0 | 90.0 | 0.419 | 0.461 | 0.195 |
| 6. | MAKARY (C) | 2.6 | 90.0 | 0.200 | 0.343 | 0.417 |
| 7. | MORA (C) | 2.6 | 90.0 | 0.197 | 0.343 | 0.426 |
| 8. | MOUVOULDAY (C) | 3.3 | 90.0 | 0.360 | 0.446 | 0.193 |
| 9. | ZAMAY (C) | 3.3 | 90.0 | 0.274 | 0.377 | 0.165 |
| 10. | BAROUELI (M) | 2.9 | 90.0 | 0.267 | 0.415 | 0.091 |
| 11. | KEMENY (M) | 3.4 | 90.0 | 0.359 | 0.446 | 0.273 |
| 12. | KOLONGOTOMO (M) | 3.4 | 90.0 | 0.360 | 0.466 | 0.146 |
| 13. | SAMAYE (M) | 2.9 | 90.0 | 0.250 | 0.411 | 0.191 |
| 14. | SARRO (M) | 3.6 | 90.0 | 0.395 | 0.479 | 0.213 |
| 15. | BOUZA (N) | 3.0 | 90.0 | 0.340 | 0.398 | 0.345 |
| 16. | KOLLO (N) | 3.3 | 90.0 | 0.368 | 0.452 | 0.136 |
| 17. | MATAMEYE (N) | 3.3 | 90.0 | 0.325 | 0.405 | 0.257 |
| 18. | TERA (N) | 2.8 | 90.0 | 0.269 | 0.414 | 0.197 |
| 19. | MANA-POOLS (Z) | 2.4 | 50.0 | 0.229 | 0.265 | 0.222 |
* A locus is considered polymorphic if the frequency of the most common allele does not exceed 0.95
** Unbiased estimate (see Nei, 1978)
(From Joly, 1991)
RESULTS
Genetic variation within populations
The results showed that the populations were highly variable for the loci examined. In most cases, the percentage of polymorphic loci was 90%; only the population of Mana Pools, Zimbabwe, exhibited a low level of diversity (see Table 2); this was also the population with the lowest number of alleles per locus. The level of heterozygosity, which can be considered a measure of allelic diversity (Nei 1977), was very high for the west African populations (0.400), as compared to the east African one (0.265).
For all populations studied, the mean fixation indices found indicated a deficit in heterozygotes as compared to a panmictic population. Some populations, such as Makary and Mora from Cameroon, exhibited a very large deficit (F.0.400), while others followed a pattern expected after panmixis.
Genetic variation between populations
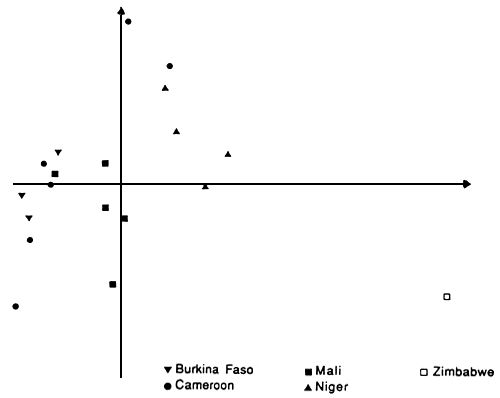
The principal component analysis (PCA) indicated that there were large differences between the populations from western Africa and eastern Africa. The first two axis of the PCA represented 47% of the total variation. The first axis opposed the western populations to the eastern one. The second axis included differences between the western populations; two populations from Cameroon (Bibémi and Guétalé) along with populations from Niger seemed to be different from the rest (Figure 1). The dendrogramme obtained with the absolute genetic distance between populations supported these findings (Joly et al 1992).
Figure 1. Projection of the population studied on the plane (1,2) of the principal component analysis
Breeding system
The estimated outcrossing rate (tm) for the four populations studied ranged from 0.893 to 0.996 (Joly and Aygalent 1992); this is higher than the expected value for an insect pollinated species (tm<0.90) (Surles et al 1990). The duration of flowering in F.albida is from 10–14 weeks (Zeh-Nlo and Joly, in press.); the number of hermaphroditic flowers in an inflorescence is large (around 100) and these flowers open progressively (Tybirk and Jorgensen, in press., Zeh-Nlo, unpublished data). In view of this, one would expect a large amount of self-pollination in F.albida. The results presented in this paper suggest that F.albida possesses a self-incompatibility system, which seems to operate at varying intensity for individual trees, which consequently vary greatly in their degree of outcrossing (Joly and Aygalant 1992).
CONCLUSION
This study indicates that F. albida is a genetically variable species with a high degree of genetic variation as compared to Australian acacias which have been subject to similar studies. While more results on eastern populations are still underway, these first results are comparable to earlier findings related to F. albida, pin-pointing large differences between western and eastern populations of the species in respect to growth traits. The results also indicate that F. albida is self-incompatible to varying degrees.
Further and much expanded studies will have to be carried out, using genetic markers as well as field trials, to gain a general picture of the patterns of genetic variation in F.albida. Controlled crosses should be carried out in support of such studies, including self and foreign pollen to clarify aspects related to the breeding systems (Joly 1991).
The present results can be considered a first step towards a better understanding of the species, and should be duly considered in the development of improvement and conservation programmes.
ACKNOWLEDGEMENT
This study has been funded under the EEC research scheme STD2 no. TS2*0207 -F(EDB).
REFERENCES
BILLAND, A. 1991 Variabilité génétique de Faidherbia albida en essais comparatifs de descendances au Burkina Faso. In the proceedings of the international workshop on Faidherbia albida in West Africa held in Niamey, 22–26 April, 1991 (in press).
BILLAND, A. & DE FRAMOND, H. 1990 Variabilité génétique d'Acacia albida (synonyme Faidherbia albida) en essais de provenances au Burkina Faso. In proceedings of the workshop “Physiologie des arbres et arbustes en zones arides et semi-arides, Nancy, Avril 1990.
DANTHU, P. & PRAT, D. 1991 Study of the genetic variability by means of isoenzymes in Faidherbia albida; preliminary results. In Biochemical markers in the population genetics of forest trees, S. Fineschi M.E. Malvoti, F. Cannata and H.H. Hattemer editors. SPB Academic Publishing by The Hague.
DE FRAMOND, H. 1990 Development of Genetic Resources of Multipurpose Trees in Sudano-Sahelian Africa. Forest Genetic Resources Information 18: 21–27.
FAO 1974 Third Session of the FAO Panel of Experts in Forest Gene Resources. Held in Rome, Italy, 6–10 May 1974. 90 p.
FAGG, C.W. and BARNES, R.D. Africa acacias: study and acquisition of the genetic resources. ODA research scheme R.4348 Final Report, Oxford Forestry Institute, 170 pp.
JOLY, H.I. 1991 Population genetics of Acacia albida (syn. Faidherbia albida). Bull. Int. Group Study of Mimosoideae, 19:86–95.
JOLY, H.I. 1991 Acacia albida: genetic aspects. In the proceedings of the International Workshop on Faidherbia albida in West Africa held in Niamey 22–26 April 1991 (in press).
JOLY, H.I., ZEH-NLO, M., DANTHU, P. & AYGALENT, C. 1992 Population genetics of an African Acacia: Acacia albida. Genetic diversity of populations from West Africa. Aust. J. Bot. 40:59–73.
JOLY, H.I. & AYGALENT, C. 1992 Breeding system of Acacia albida (syn. Faidherbia albida). Preliminary results. In “Population genetics and gene conservation in forest trees”, IUFRO symposium, Carcans Maubuisson, 24–28 August 1992.
NEI, M.F. 1977 Statistics and analysis of gene diversity in subdivided populations. Ann. Human Genet. 41:225–233.
RITLAND, K. 1990 A series of FORTRAN computer programs for estimating plant mating systems. J. Heredity, 81:235–237.
RITLAND, K. & JAIN, S. 1981 A model for the estimation of outcrossing rate and gene frequencies using an independent loci. Heredity, 47:35–52.
SNIEZKO, R.A. & STEWART, H.T.L. 1989 Range-wide provenance variation in growth and nutrition of Acacia albida seedlings propagated in Zimbabwe. Forest Ecology and Management 27:179–197.
SURLES, S.E., HAMRICK, J.L. & BONGARTEN, B.C. 1990 Mating systems in open-pollinated families of black locust (Robinia pseudoacacia). Silvae Genet. 39:35–40.
TYBIRK, K. & JORGENSEN, A. 1991 Floral biology and pollination of some African acacias and Faidherbia albida. In the proceedings of the A.E.T.A.T. meeting held in Malawi 1991 (in press).
WICKENS, G.E. 1969 A study of Acacia albida Del. (Mimosoideae). Kew Bull. 23:181–202.
WRIGHT, S. 1965 The interpretation of population structure by F-statistics with special regard to systems of mating. Evolution 19:395–420.
ZEH-NLO, M. & JOLY, H.I. 1991 Observations préliminaires sur la phénologie d'Acacia albida: Etude d'une population du nord Cameroun. In the proceedings of the International Workshop on Faidherbia albida in West Africa held in Niamey 22–26 April 1991 (in press).