by
C. Biney, D. Calamari, H. Naeve, T.W. Maembe, B. Nyakageni and M.A.H. Saad
The awareness of the harm caused by pollutants to natural environments has led the political and legislative authorities of industrially-developed countries to introduce or review regulations to protect the environment. However, in recent years, all over the world and particularly in many countries in Africa, there has been a remarkable population growth, accompanied by an intense urbanization, an increase of industrial activities and a higher exploitation of cultivable land. These transformations have brought about a huge increase in the quantity of discharges and a wide diversification in the types of pollutants that reach river waters and have undesirable effects on fish and on the potential for fishery exploitation.
A few reviews exist on the state of pollution of African inland waters; see for example ‘The Ecology and Utilization of African Inland Waters’ (Symoens et al., 1981) and ‘Future Hazard from Pesticide Uses with Special Reference to West Africa and Southeast Asia’ (Balk and Koeman, 1984). In the framework of the activities of the Committee for Inland Fisheries of Africa (CIFA), two reports were prepared on the state of pollution of African inland waters (Alabaster, 1981; Calamari, 1985). Eleven countries were considered: Burundi, Malawi, Sudan, Kenya, Tanzania and Zambia in the first survey; Mali, Côte d'Ivoire, Ghana, Nigeria and Cameroon in the second. The documents report on sources of water pollution, on relevant scientific research and on the legislation enforced in each country. Both reports concluded that pollution problems do exist, though in different degree, in the various countries. The need for improved legislation and expertise was recognized, since local authorities were in general well aware of the importance of the problem and willing to improve their action in the matter of water pollution control.
The purpose of this report is to examine and summarize what kind of research activities are needed to allow the adoption of regulatory measures based on scientific knowledge, for the effective control and prevention of aquatic pollution. In particular, it provides a strategy on how to deal with toxic substances, such as pesticides and heavy metals. Other types of discharges such as organic matter with high biological oxygen demand (BOD) and/or chemical oxygen demand (COD), suspended matter and nutrients, call for different consideration and a specific strategy of their own, as is the case with bacteriological pollution. It is, however, strongly recommended that control of these three types of pollution be well coordinated. An analysis is made here of how scientific knowledge is applied elsewhere, and how scientifically based regulatory actions may improve present methods of pollution control in Africa. Although socio-economic aspects are not taken into consideration in this report, it is clear that the environment is a resource for which the social price of degradation cannot be easily quantified. The cost of degradation in environmental quality can be very high, especially for future generations.
Among the prerequisites for decision-making, the following are important: political willingness, cultural attitudes and administrative capability. Consequently, there is a need to create public awareness of the deleterious effects of pollution and the dangers of incorrect application of chemical substances, particularly pesticides. In this connection, the potential role of non-scientific organizations in the preparatory phase of water pollution control activities is recognized. This report, however, concentrates on the role of scientific research in pollution control.
Water pollutants may be classified in many different ways, for instance according to chemical characteristics, physical state, environmental compartments in which they are discharged or found, sources, types of effects and target organisms which can be affected (Calamari and Chiaudani, 1984).
This paper concentrates on micro-pollutants, those elements or substances that are discharged as a result of human activities but which are present naturally in water bodies; any increase caused by man with respect to the quantities or concentrations present in unpolluted environments may have deleterious effects on organisms or ecosystems or modify biogeochemical cycles. This concurs with the concept of ‘specific pollutant’, defined by the Water Management Group of the Organisation for Economic Co-operation and Development (OECD) as a substance which, introduced into the environment essentially as a result of human activities, under given conditions reduces the quality of water due to its toxic effects on human beings and aquatic life. It differentiates ‘specific pollutants’ from the classic parameters such as BOD, COD and suspended matter. ‘Specific pollutants’ are also defined by other organizations as ‘trace pollutants’, ‘micro-pollutants’, or ‘recalcitrant pollutants’. About 1 500 commonly detected molecules have been listed as such by the Water Research Centre in the United Kingdom (Holdgate, 1979) in the Index of Solubility, Toxicity and Biodegradability. About 30 elements, molecules or classes of substances have been included in the list of limits proposed for effluents in the Italian Law No. 319 for the prevention of water pollution (Marchetti et al., 1973). Further, the Environmental Protection Agency (EPA) of the United States has set up quality criteria for water for about 40 elements or substances (Train, 1979). There is now a tendency in many countries to increase the number of substances to be regulated or kept under control.
The most commonly accepted definition of marine pollution is that given by GESAMP (IMO/FAO/Unesco-IOC/WMO/WHO/IAEA/UN/UNEP Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection); this definition could, mutatis mutandis, also apply to fresh waters: “The introduction by man, directly or indirectly, of substances or energy into the marine environment (including estuaries), resulting in such deleterious effects as harm to living resources, hazards to human health, hindrance to marine activities including fishing, impairment of quality for use of sea water and reduction of amenities”.
Pollution can limit possible uses of water and aquatic resources, and water quality criteria have been formulated therefore as a function of the various uses. Four main uses are taken into consideration here: drinking water supply, agricultural use, bathing and amenity, and aquatic life. Since for potable water the hygienic and sanitary aspects require a specific scientific and regulatory approach, and agricultural and aesthetic uses are generally less demanding, quality criteria for aquatic life are generally considered as the most important. In Africa, where a majority of water bodies is subject to multiple use, the application of the more protective criteria would be even more justified.
In recent years there has been consolidation of the view that an aquatic ecosystem in which structures and functions are not disturbed possesses a quality which is immediately suitable, or suitable after simple treatment, for a variety of uses. This, together with the concept that every recipient body has a certain capacity to assimilate contaminants, has been the basis for the establishment of quality criteria for aquatic life by different international and national organizations (see EEC, 1978; Alabaster and Lloyd, 1982; Train, 1979; Marchetti et al., 1973). On this basis, it is assumed that, for each pollutant, there is a margin of safety between the zero level, or the natural concentration, and the concentration at which undesirable disturbances can be observed. This margin can be identified and used quantitatively for establishing water quality criteria.
In general, water quality criteria are defined through the preparation of critical reviews of the scientific information available and by specifying on experimental evidence concentrations not to be exceeded. According to the European Inland Fisheries Advisory Commission (EIFAC), water quality criteria for freshwater fish should “ideally permit all stages in the life cycle to be successfully completed and, in addition, should not produce conditions in a river water which would either taint the flesh of the fish or cause them to avoid a stretch of river where they would otherwise be present, or give rise to accumulation of deleterious substances in fish to such a degree that they are potentially harmful when consumed. Indirect factors like those affecting fish-food organisms must also be considered should they prove to be important”.
The scientific reports of EIFAC are usually prepared by working groups that discuss the chemistry of the pollutant in water, lethal action on fish, sub-lethal effects, type of toxic action, factors which influence lethal levels, field observations in polluted waters and data regarding toxicity to algae and invertebrates. Reports are concluded with a tentative quality criterion for aquatic life. Other bodies or organizations work in an analogous way. The scientific community has the responsibility to produce the basic data by experimental research and to provide the expertise necessary to assess the data and establish, by deduction, a sufficiently reliable quality criterion.
The terms ‘standard’, ‘objective’ and ‘criterion’ are frequently used indiscriminately, but the majority of research workers and people responsible for water quality management follow the definitions given by Warren (1971):
The term ‘standard’ applies to any definite rule, principle or measure established by authority. The fact that it has been established by authority makes a standard somewhat rigid, official or quasi-legal; but this does not necessarily mean that the standard is fair, equitable or based on sound scientific knowledge, for it may have been established somewhat arbitrarily on the basis of inadequate technical data tempered by a cautious factor of safety. Where scientific data are sparse, such arbitrary standards may be justified.
The word ‘objective’ represents an aim or a goal toward which to strive and it may designate an ideal condition. Most certainly, however, it does not imply strict adherence nor rigid enforcement by an agency or health department. It is gaining favour among engineers on boards and commissions that strive to achieve water pollution control by persuasive methods and cooperative action.
A ‘criterion’ designates a condition defined by means of a critical review of scientific information and suitable to conserve structures and functions in the ecosystems. Unlike a standard, it carries no connotation of authority other than that of fairness or equity, nor does it imply an ideal condition.
To obtain the desired quality criterion or objective, two different approaches have been followed in different countries.
The first approach does not take into account the type and use of the water body receiving the contaminant but considers only the concentration of pollutants in the effluents. Uniform quality standards are set for any type of discharge (rigid effluent standards). These standards must necessarily be very restrictive in order to be effective since they have to protect even the most critical systems with a single regulation. As a result, the application of this criterion in many cases may lead to the requirement of a higher level of control than is necessary in the situation considered and hence to excessive and unnecessary costs, while more permissible limits may lead to a reduction in the level of protection. In fact, this procedure includes, by definition, neither the quantity of pollutant discharged over a period of time nor the number of discharges to the same receptor, and it does not take into account the receptive capacity of the water body. The main advantage lies in its ease of administrative management.
The second approach limits the quantity of the pollutant according to the characteristics of the receptor (river, lake, coastal waters) and of the pollutant (toxicity, persistence, bioaccumulation). This approach is favoured from many points of view because it requires, on a case-by-case basis, a treatment level which is suitable to the receptive capacities and the use for which the water is intended (flexible effluent standards). This procedure, therefore, is considered as the most economical overall, offering the highest reliability as regards the protection of the environment.
It might be concluded that the second approach would naturally be adopted in taking regulatory actions on effluents, but basic information is often needed on a number of elements:
The receptive capacity of the water body. This implies a thorough knowledge of the type of receptor, its hydrological balance, with particular reference to the critical flow, dilution capacity, oxidizing capacity, chemistry, biological structure, thermal variations, relationship with ground water, and the existence of other sources of pollution and any other factor that can influence the maintenance of a safe concentration of the discharged pollutant.
The development of the sources of pollution over time. This involves information on population characteristics and future trends of urban and industrial development, which has to be considered in accordance with the local situation and, obviously, within the framework of general economic planning.
In many countries, therefore, an intermediate approach has been followed so as to allow for adaptation of the effluent standards to a variety of receptors and the groups of pollutants. It's also allows limits to be established in other compartments of the ecosystem rather than in water (e.g., in organisms or sediments).
For situations or countries where chemical analyses required to enforce effluent standards are not feasible, the practice of applying effluent toxicity standards has developed. By means of a relatively simple toxicity test (in general with fish) discharges or effluents are permitted or forbidden according to their toxicity. This practice is used particularly when chemical characterization of the effluents is difficult or when mixtures of chemicals have to be tested. An interesting review of experience in this approach in U.S.A. and Canada is given by Tebo (1986).
Recently, there has been a tendency to consider each case individually, to differentiate the approaches according to the type of pollutants, and, consequently, to establish different criteria for receptors with different characteristics.
Other approaches have been used, for example for mercury and phosphorus. For mercury, limits have been set for concentrations in fish or in sediments, since scientific findings do not allow to establish a quality criterion for water. For phosphorus, the theoretical discharges are relatively easy to calculate (quantify) and, from mass balance calculations, for lakes a water quality criterion of 10 μg/l has been set, in contrast to rivers, where the possibility of eutrophication is very limited.
A common simple approach is to classify potentially harmful substances in a ‘black’ and a ‘grey’ list; the ‘black list’ concerns substances not to be discharged at all, while the ‘grey list’ includes those substances which may be discharged within certain limits and under controlled conditions.
An adaptation of these approaches to water pollution control to suit African conditions (geographical and administrative) could be made.
An important use of pesticides in Africa lies in the public and animal health sectors to curb, if not to eradicate, endemic diseases such as malaria, onchocerciasis, schistosomiasis and trypanosomiasis through the control of their insect or mollusc hosts. It is of interest to analyze this specific use of pesticides in relation to (i) the possible impact on fishery resources, (ii) the measures taken to control undesirable effects and (iii) the protection of the environment.
One case for which information is available is that of the Onchocerciasis Control Programme (OCP). The brief summary below is mainly based on ‘Ten years of onchocerciasis control in West Africa’ (WHO, 1985) and on the review of Lévêque (1989) on aquatic biological monitoring. Other sources of information are the reports of the OCP Ecological Group.
4.1 The problem and the environment
The female of the black fly, Simulium damnosum, is the vector of dermal filariasis (caused by the parasitic worm Onchocerca volvulus), which can result in blindness. The illness is prevalent along rivers, whence the name ‘river blindness’, as the larvae of these flies are aquatic and occur only in fast-flowing waters. In 1974, UNDP, FAO, World Bank and WHO launched a campaign for the control of the larval stages of the vector. About 18 000 km of rivers were treated weekly in several countries of West Africa. The rivers are of the savanna type, with a flood period (July–December) and a dry period (January–March/April) during which some of them dry up completely.
Before this programme was launched, little was known on the ecology of these rivers. A catalogue of aquatic insects and fish was therefore compiled, several studies were made of the river biology and an extensive monitoring programme was carried out prior to the treatments with pesticides.
4.2 Criteria applicable for the selection of larvicides
Several hundred insecticides were tested by OCP for their effectiveness against Simulium; for application in the programme, it was decided that any new larvicide should meet the following criteria:
The acute effects of a candidate pesticide, in the formulation and dose rate as appropriate for its use against Simulium, should not include reduction in the number of invertebrate species to a level at which their survival in a given locality would be endangered.
The pesticide should not give rise to the regional disappearance of any invertebrate species; the temporary (seasonal) local disappearance of some invertebrate species at the breeding sites of Simulium may have to be accepted.
The pesticide should not cause a long-term (i.e. extending beyond the next season) imbalance under normal conditions of application, i.e. marked shifts in the relative abundance of species should not occur.
The use of the pesticide should have neither direct impact on fish nor any effect on their life cycle.
Compounds likely to accumulate in the food web should be avoided.
In selecting pesticides for Simulium control in an area, account should be taken of human activities which, either by themselves or in combination with the vector control operations, might cause adverse effects on the environment.
4.3 Pollutant load
Temephos (Abate), a degradable organophosphorous insecticide with very low toxicity on mammals and fish, was the only molecule used in the first phase of the programme (until 1979). Unfortunately, the development of resistance in insect populations led to the use of chlorphoxim, another organophosphorous insecticide with a higher toxicity to non-target fauna. In the meantime, a dispersible concentrate of the biological insecticide Bacillus thuringiensis, serotype H-14 (Teknar), began to be used successfully and it is increasingly employed (see Table I). However, B. thuringiensis H-14 is not effective during high-water conditions, and chemical insecticides have still to be used. In 1985, limited use of permethrin, an artificial pyrethroid, has been made in a restricted area with the consent of the Ecological Group.
Table I
Insecticide consumption (expressed in litres of marketed preparation)
| Larvicide | 1975 | 1976 | 1977 | 1978 | 1979 | 1980 | 1981 | 1982 | 1983 |
| Abate C200 | 75631 | 129947 | 155615 | 215879 | 263377 | 184517 | 130000 | 162750 | 74807 |
| Chlorphoxim | 5713 | 70000 | 6699 | 35796 | |||||
| Teknar | 416 | 1500 | 232986 | 310000 |
4.4 The aquatic monitoring programme
An extensive biological monitoring programme was set up at a number of stations in order to check on the possible short- and long-term effects on the communities of the treated rivers.
Overcoming a great number of scientific and practical problems, the monitoring programme on benthic populations and fish has operated since 1975, using national teams of scientists, locally available manpower and facilities. Short-term research was performed also on specific problems and at different ecological levels (phytoplankton, zooplankton). The invertebrate populations were studied by means of: (i) drift net sampling, (ii) Surber sampling (on rocky substrates in low-water periods), and (iii) fixed and floating artificial substrates. The state of fish populations was studied mainly by monitoring catch per unit of effort, variation in species composition, coefficient of condition and fecundity. A great number of publications on the results of the biological monitoring is available, and all are listed in the review by Lévêque (1989) already cited.
During ten years of monitoring, appreciable changes have occurred in aquatic insect populations, e.g., the disappearance of some Simulidae, the rarefaction of other groups and the proliferation of Chironomidae; it is, however, difficult to attribute all the changes observed to pesticide treatments since hydrological fluctuations could also have played an important role. The results of the fish monitoring activities have shown that treatments have little or no impact and that no major changes occur in the fish catch or in the coefficient of condition and fecundity. It can be concluded that temephos has no discernible long-term effects on fish populations, and that some changes observed following the use of other insecticides are of only limited extent; moreover, also in this case, hydrological fluctuations may be responsible. On a long-term basis, the ability of insect populations from non-treated zones to recolonize the treated areas has been demonstrated.
Lévêque (1989) concludes that the results obtained after many years' treatment indicate that the larvicides employed had little effect on the non-target fauna. Although the first applications of temephos and chlorphoxim had fairly strong impact on invertebrate communities in the short term, it would seem that these conditions cease to exist fairly quickly after a year or less of successive applications. In operational conditions, the treated rivers seem to have fairly strong resilience and at any rate a great capacity for recovery. The situation is still improving with the reduction in the number of rivers treated, resulting from the success of the vector control, and with the increasing utilization of B. thuringiensis H-14, a pesticide exceptionally safe for non-target fauna.
Nevertheless, future activities, which include extension and improvement of biological monitoring, are envisaged. As a result of difficulties often encountered with routine chemical monitoring in Africa, only a few analyses of fish samples were made. Temephos was found to be present in fish muscle but levels sharply decreased a few days after the treatment. A new pilot project on chemical monitoring of fish is under consideration in relation to the use of new pesticides within OCP.
It appears that, in view of the intensive campaign of treatment and the possibility of Simulium developing resistance, efforts to detect undesirable effects have to continue, since the margins of safety between tolerable levels of chemicals and those provoking dramatic modifications in the environment have not yet been identified and are probably narrow.
4.5 Relevance of the programme to the work of CIFA
This brief description of the OCP environmental activities has demonstrated the feasibility and usefulness of different types of biological monitoring of African rivers. Vast experience already exists in some countries, by which a number of other countries can profit. Several methods have undergone large-scale testing under African conditions and have given good results. Local personnel have been adequately trained at individual level, and several institutes exist in which comprehensive experience has been gained. However, this is not the general situation for all countries involved in OCP, where the ability to perform biological monitoring is unequal.
Within the framework of the UNEP Regional Seas Programme, four Action Plans covering African countries have been signed: the Action Plans for the Mediterranean, the Red Sea and Gulf of Aden, West and Central Africa and the East African Region (UNEP, 1982). Three Action Plans (Figure 1) comprise projects on marine pollution monitoring and research with components on analyses of metals and organochlorines in biota, oil pollution monitoring on beaches and in coastal waters, and bacteriological quality control of bathing waters (UNEP, 1983, 1985, 1988). MED POL (Mediterranean) has operated since 1975, WACAF 2 (West and Central Africa) since 1983, while EAF 6 (East Africa) is at the planning stage. Although these projects deal with marine and coastal pollution, the structures created, including analytical facilities and capabilities in a number of research centres, are undoubtedly of interest and relevance to any pollution research projects to be established under CIFA.
Programmes and projects in the freshwater sector also are being executed by the UN system, e.g., the ONCHO Programme (Section 4) and the WHO/UNEP GEMS-Water Project, a world-wide programme aiming at monitoring of freshwater systems, improving the validity and comparability of water quality data and assessing trends of water pollution.
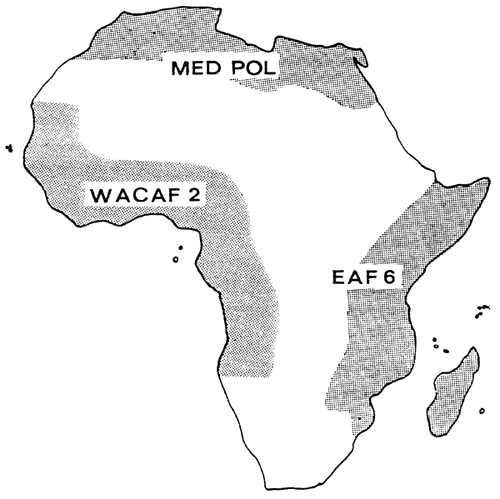
Figure 1. Coverage of relevant UNEP Action Plans in Africa
Part of the scientific community endeavour to elaborate more precise quality criteria, a ‘traditional’ approach. For a higher degree of precision of the criteria, these scientists take into account, for instance, the chemical and biological transformations which might occur, and study the statistical distribution over time. Work is also being done to develop criteria for substances which had not been considered previously. This conventional approach has good reasons to exist, as demonstrated by the critical reviews of Sprague (1976) and Thurston (1979). They underline that for a great number of pollutants no criteria exist and that only about 50% of the criteria proposed can be considered acceptable or would be acceptable if somewhat modified; the rest are inadequate and need correction. They also stress the need for more information on identified pollutants, i.e. the chemical behaviour of the molecule, environmental fate, toxicity of the principal forms and possible transformation and bioaccumulation.
Another issue that needs experimental study concerns mixtures of toxicants; divergent positions have been taken by two competent organizations. In Water Quality Criteria, 1972 (NAS/NAE, 1973), the U.S. EPA maintains that, in the presence of a mixture of pollutants at a No Observable Effect Concentration (NOEC), it is necessary to lower the water quality criteria, i.e. to reduce to a fraction, the acceptable level for each of the pollutants present. The EIFAC Working Party on Water Quality Criteria for European Freshwater Fish, after extensive review of the literature on mixtures of toxicants, concludes instead that experimental evidence suggests that the proposed quality criteria for individual toxic substances could be applied also in cases where several pollutants are present (EIFAC, 1980; Alabaster and Lloyd, 1982). However, reviewing the subject in 1986, the EIFAC Working Party concluded on the basis of recent research on organic chemicals that low concentrations at no-effect level of such substances may still exert a harmful effect when present in mixtures (EIFAC, 1987; Howells, 1994). Pending further research, it is prudent to assume that the effects of chemicals in mixtures are additive at very low concentrations. Thus individual water quality criteria may have to be slightly reduced if other pollutants are present in significant amounts. This shows that even ‘traditional’ approaches serve to fill gaps in knowledge or at least to clarify controversial points.
‘Non-traditional’ research follows two main currents: one aims at forecasting, as far as possible, the effect and the fate of pollutants in ecosystems, the other at the definition, in quantitative terms, of biogeochemical cycles. Both tend towards producing information necessary for control measures prior to discharge of pollutants.
Several substances have been banned recently because of their marked effects on the environment and on human health. There is now pressure to evaluate the risks inherent in new chemical substances in advance of their production on a commercial scale. A similar approach has been proposed for substances already in use and produced in very large quantities for which insufficient information exists. Different screening methods have been developed for such evaluations. On the basis of a wide debate (see, for example, the Chemical Testing Programme, OECD) agreement has been reached on chemical properties to be measured and the toxicological tests to be performed to define the degree of hazard of the various substances (Integrated Rating System (IRS); Schmidt-Bleek et al., 1982).
Another approach is the application of the Quantitative Structure-Activity Relationships (QSAR), proposed by Hansch (1969, 1973). Recent experimental work indicates the possibility of attaining a high degree of predictability, with substantial agreement between expected and observed toxicity levels within relatively homogeneous classes of organic chemicals. In some cases the hydrophobic characteristics, expressed by the logarithm of the n-octanol/water partition coefficient (log P), were sufficient to give high correlation with the toxic activity of the molecules. Good results have been obtained for several industrial organic chemicals (Veith et al., 1983; Konemann, 1981; Hermens et al., 1984; Calamari et al., 1983). Electronic characteristics (pKa) are a determinant factor in the toxicity of several amines (Calamari et al., 1980). The toxic activity of phenols, chlorophenols, chloro- and alkylanilines is adequately described by a biparametric equation (Hermens et al., 1984; Saarikoski et al., 1986). In other cases, log P alone, although significantly correlated with the toxic effect, was not sufficient to give good predictability. For example, a study by Vighi and Calamari (1985) on organotin compounds showed good predictability only within the different subclasses (mono-, di-, tri- and tetrasubstitutes). Better results, with very high predictability, have been obtained with a biparametric equation, introducing electronic or steric parameters as pKa or molecular connectivity indices (1X and 1Xv). The usefulness of molecular connectivity in structure activity relationships has also been demonstrated for several other compounds (Schultz et al., 1982; Sabljic, 1983).
Basak et al. (1984) found quantitative correlations between toxicity and structure of several organic esters with a multi- parametric relationship which included a lipophilic (log P), an electronic (1Xv) and a steric parameter (CIC).
From the few examples quoted and the ample literature available, Konemann and Calamari (1983) stated that:
QSAR have been developed for several classes of chemicals and allow, within these classes, good estimates of toxicity of chemicals.
Most QSAR have been calculated for a limited number of chemicals. Expert judgement is necessary to indicate the boundaries of the validity of QSAR.
If used in a proper way QSAR can be very helpful for selecting priority chemicals, if experimental data are not available.
Until recently, the application of the QSAR only to homogeneous groups of chemical substances was a limitation. A wider application of this approach will provide more accurate and scientifically reliable lists of dangerous substances.
The second ‘non-traditional’ approach aims at the definition in quantitative terms of the biogeochemical cycle of the various molecules or elements.
Simulation models and tests in partially-controlled ecosystems quantitatively define the biogeochemical cycles in more accurate form than earlier work on pesticides (Metcalf et al., 1971); they are certainly more practical than the gigantic detailed models constructed for some pollutants, e.g., DDT (Randers, 1973). Such research aims at determining a Predicted Environmental Concentration (PEC) on the basis of a few physico-chemical parameters. The degree of affinity of chemicals to the fundamental environmental compartments (water, air, soil, biota) is assessed on the basis of four physico-chemical properties: S - water solubility; H - Henry's constant; Koc - soil absorption coefficient; Kow - n-octanol/water partition coefficient. Values for these parameters can be measured experimentally or calculated by means of a property correlation equation (Kenaga and Goring, 1980). On the basis of this simple approach calculations can be made using models such as the fugacity model of Mackay and Paterson (1981) of the Predicted Environmental Distribution (PED). More complex models based on the same principles are the Quantitative Water, Air, Sediment Interaction model (QWASI) (Mackay et al., 1983, 1983a) and the Exposure Analysis Modelling System (EXAMS), developed by EPA (Burns et al., 1981).
However, the PED is of limited help in predicting the final concentration of a molecule in a given environment, since a molecule in contact with the environment could have different fates according to its characteristics and those of the environment. Basic processes affecting the molecule are photolysis, photo-oxidation, redox and other types of chemical reactions with other molecules present in the environment, and biodegradation and metabolism.
Few attempts have been made to specify data on organic chemicals needed to predict their environmental fate; however, some information is available (see, for example, Haque (1980) and Hutzinger (1980, 1982)). More data are available on biodegradability, and the persistence of a number of substances can be predicted, although methods for studying biodegradation are still controversial (Gerike and Fisher, 1979, 1981). Metabolism, although important for organisms, plays in general a minor role in the quantitative transformation of environmental contaminants. When data are available, a transformation matrix can be prepared and, using the time of persistence, the PEC can be calculated. In this context, physical dispersion and the characteristics of receiving waters have to be taken into account.
Utilizing these new approaches, it is possible to formulate a scheme of environmental management to take account of the assimilative capacity (absorption, transformation and storage) of each environmental compartment. At legislative level, the new approach is acknowledged in laws on new chemical substances such as the Toxic Substances Control Act in U.S.A. (U.S. EPA, 1978) and the 6th Amendment to Directive 831/79 on Dangerous Substances in the European Economic Community (EEC, 1979). The current view is that pollution prevention is preferred to post hoc control. A policy of prevention implies that toxicological characteristics of molecules are known before a chemical is marketed. Consequently, the prediction of damage is a key issue in scientific and regulatory activities and most of the ecotoxicological studies are directed to its improvement.
In an editorial in ‘Environmental Toxicology and Chemistry’, Kimerle (1986) raised the provocative question: ‘Has the water quality concept outlived its usefulness?’. Since the fifties this concept has been the scientific basis for any water quality control. During the seventies, when a shift from control to prevention took place, it was realized that control of conventional pollutants alone was an inadequate strategy. Moreover, with the improvement of waste treatment technology, a higher reduction of toxic inputs was envisaged. Three additional reasons lead towards a different strategy in pollution control: (i) the need to protect the whole environment and not just one or the other of the compartments (water, air, etc.) or an identified target species; (ii) the huge quantity of information necessary to formulate water quality criteria, and (iii) the great number of chemical substances actually used by man as well as the variety of chemical groups to which these substances belong.
Simply as an example, the EIFAC Working Party on Water Quality Criteria in more than twenty years has produced only 15 reports and the U.S. EPA ‘Red Book’ contained only 53 criteria in 1976. On the other hand, in the European Economic Community about 10 000 chemicals are widely used. To overcome this problem, EPA proposed in 1983 a simplified method of deriving a water quality criterion (Stephan et al., 1983); the method required 16 acute tests on different animal species, 3 chronic tests, 2 tests on plants and one bioconcentration study. This proved a too complex procedure to speed the preparation of all criteria documents necessary for an appropriate water pollution control.
One can assume that no exposure means no need for toxicity data. This simple concept highlights the importance of exposure, and it was realized that a toxicological criterion is only part of the pollution control strategy. Obviously, it is not necessary to conduct very complex toxicological studies when, at a first estimate, the predicted concentration of a substance in the environment will be very low or several orders of magnitude less than the acute toxic concentration.
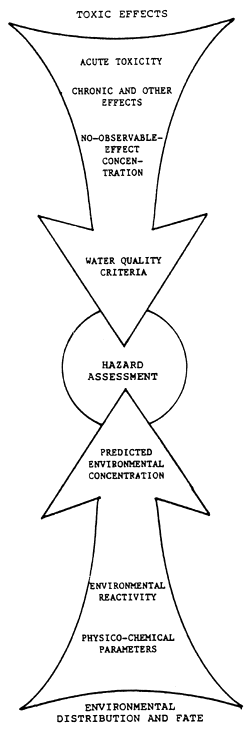
Figure 2. Scheme of the relationships existing among exposure, effects and risk assessment
The Risk Assessment approach (Fig. 2, Calamari, 1984) has become popular in the scientific community and with administrators involved in regulatory and control activities. In-depth toxicological investigations are necessary only when the predicted environmental concentration is not far from the No Observable Effect Concentration (NOEC) derived from relatively simple toxicological tests or even data on acute toxicity. The value of this approach lies in the possibility of managing a higher number of chemicals for which there are limited toxicological data.
An outline of Risk Assessment is given by Bro-Rasmussen and Christiansen (1984). The prediction of the environmental concentration of a substance starts with the determination of a few basic parameters such as physico-chemical characteristics and production and/or emission data to ascertain the distribution and mobility of the chemical in the environment. Then the persistence is evaluated from data on chemical or biological degradation in the various compartments. The toxicological studies to follow will provide acute and chronic toxicity data; studies of microecosystems need only be conducted when the data on potential exposure show there is cause for concern.
In general, it is easier to obtain information on production and use and data on physico-chemical characteristics than toxicological data, so simple calculations or scenarios can be of great help in pollution control management. However, the problem is not always simple and at least 30 types of approaches to Risk Assessment have been proposed (Hushon, 1982).
Under African conditions, where basic ecotoxicological data are frequently scarce or even nonexistent, Risk Assessment could provide a preliminary step. For example, for a pesticide, data on quantities entering into a country and the extent of the area treated, are relatively easy to obtain; the basic physico-chemical characteristics can be taken from handbooks or obtained from the manufacturers. Then the mass balance and the theoretical concentration can be calculated for each water body from available data. Acute toxicity data from the literature can provide the basis for an approximate toxicological evaluation. By comparing the PEC in water, sediments or fish with acute toxicity data, one can decide if direct research on the chemical is necessary, and if reduction in the quantities applied or alternative options (other chemical to be used) are needed in order to protect the environment.
For water pollution management in the African continent, several strategies are available for limiting undesirable effects as well as for pollution prevention. Considering the limited resources available, a number of methodological approaches are proposed; they can be used in sequence, from the simple to the more complex, or in combination.
The concept that water quality criteria are the basis for any kind of water pollution control policy is certainly valid. However, data from industrialized countries with temperate ecosystems should only be applied with caution to African conditions since toxicity, persistence and accumulation rates will either be higher or lower, depending on the substance. Comparative toxicity studies are therefore needed, even if these are, for example, simple short-term tests under static conditions to assess the toxicity of a few chemical substances and effluents on one or two species of native fish (e.g., Tilapia sp. and another) or eventually a crustacean or mollusc.
The following is a proposal for a general scheme for pollution management:
Collection of basic data on all existing sources of pollution by means of national surveys, calculation of the theoretical loads.
Evaluation of the state of water bodies by means of simple chemical analyses and biological monitoring. (This could be an important task in several countries).
Evaluation of the potential damage to fisheries through review of literature data, toxicity testing with local species and, whenever possible, chemical analyses of environmental matrixes.
Establishment of safe effluent ‘standards’ and compilation of a ‘black’ and a ‘grey’ list of dangerous substances.
Determination of ‘environmental objectives’ for recipient waters using water quality criteria appropriately modified on the basis of those proposed outside Africa.
For management of polluting discharges, the following strategies can be applied:
Limitation of the effluents by means of a rigid effluent standard, both with chemical concentration limits and/or with a toxicological limit derived by simple acute toxicity tests on effluents. This does not take into account the specific characteristics of the receiving water body.
Limitation of the effluents by flexible effluent standards. In this case, the limits are calculated in order to maintain water quality criteria in a specific water body. Also in this case the limit can be defined as chemical and/or toxicological. Values and limitations of these approaches have been discussed in the previous chapters.
For some chemical substances it is scientifically unsound, and insufficient to protect the environment, to set up ‘objectives’ or ‘criteria’ for water (the classical case is mercury). In such cases it is necessary to indicate objectives or criteria for another environmental compartment or matrix (for example sediments or fish).
Sometimes, with particular environments and for certain chemical substances, a species is shown to be particularly sensitive (crustaceans to pesticides, for instance). In such cases, an ‘indicator species’-oriented management has to be preferred to the water quality criterion approach.
The classification of chemical substances in use in a country into ‘black’, ‘grey’ and ‘white’ lists can be of help, especially in the framework of a Risk Assessment approach to water quality control (i.e. the comparison of predicted environmental exposure with available toxicity data). This does not necessarily mean that the black list chemicals are to be totally banned but that they should be used only on certain conditions and under strict control (such as for PCB for certain industrial activities in Europe).
Finally, it should be noted that the control and management of water quality in the general interest of society implies the choice of priorities and objectives, the selection of criteria through which the quality must be verified in relation to fixed objectives and, finally, the definition of standards for the criteria chosen in order to pursue and achieve the goals. The establishment of the objectives is essentially an administrative decision made on the basis of social, economic and technological considerations, taking into account the divergent interests of the different users of water. The actual management of water resources thus depends on the choice of appropriate objectives, on the basis of which it is possible to advise on optimal and often simple methods for water quality management.