



by
Banpot Napompeth
Executive Director
National Biological
Control Research Center (NBCRC)
Kasetsart University
Bangkok, Thailand
The leucaena psyllid, Heteroosylla cubana Crawford (Homoptera:Psyllidae), was not known as a serious pest of leucaena, Leucaena leucocephala (Lam.) de Wit until the first outbreak in its native range occurred in Florida in 1983. The pest status of H. cubana was suddenly recognized worldwide when it was discovered in Hawaii in 1984 and caused devastating defoliation to leucaena plantings beyond expectation. From Hawaii, H. cubana began invading exotic leucaena plantings in the Asia-Pacific region, including Australia during the latter half of the 1980s and early 1990s, causing extensive damage with resultant economic, ecological and social impacts in all affected countries.
H. cubana was detected on the islands of Reunion and Mauritius in 1991. It has now spread across eastern Africa including Kenya, Tanzania, Uganda, and Burundi. It is expected to spread quickly to the west coast of the Africa and have widespread impacts wherever leucaenas are important components of agroforestry programmes.
Problems encountered with the leucaena psyllid in the Asia-Pacific Region and the management strategies adopted there may serve as a model to help reduce the impact of this insect in Africa. Both the policy and research recommendations based on the Asia-Pacific experience are given with pest management tactics emphasizing the utilization of resistant and/or tolerant leucaena and introduction of natural enemies being utilized in the Asia-Pacific Region. These include the predator Curinus coeruleus Mulsant (Coleoptera: Coccinellidae) and the parasitoides Psyllaephagus vaseeni Noyes (Hymenoptera: Encyrtidae) and Tamarix leucaenae Boucek (Hymenoptera: Eupelmidae). Use of chemical pesticides are not recommended except for special situations such as protection of seedlings and nurseries.
There is also an urgent need to initiate a regional and/or international cooperative and collaborative leucaena psyllid research and management programmes to deal with leucaena psyllid problems. Existing expertise in the Asia-Pacific Region can assist in attempts to implement integrated pest management programmes for this insect in Africa.
*****
Leucaena, Leucaena leucocephala Lam. (de Wit), is a fast growing leguminous tree with many uses. This tree is native to Central America but has been widely established throughout the tropics. The promotion and advocation of varieties of "giant" leucaena as multi-purpose tree species (MPTS) in agroforestry programmes during the 1970s and 1980s had been very successful in the Asia-Pacific Region. However during the latter half of the 1980s a tiny insect, the leucaena psyllid, Heteropsylla cubana Crawford (Homoptera: Psyllidae), swept across the region and caused devastating damage and economic loss resulting in the stagnation of further development of leucaena as an MPTS. Although first described from Cuba in 1914, the leucaena psyllid has remained relatively unknown and was not regarded as a serious pest of leucaenas until an outbreak occurred in Florida, USA in 1983. This was immediately followed by its discovery in Hawaii, USA in 1984 and the subsequent chain invasion of the Asia-Pacific Region through the mid and late 1980s. The psyllid was detected in Kenya in August 1992 (Reynolds and Bimbuzi 1992) as predicted earlier by Napompeth (1990). Its impact could have a significant effect on the future viability of this MPTS in various African countries.
The invasion of leucaena psyllid in the Asia-Pacific Region has become a critical issue to MPTS projects underway in the Region to such an extent that it warranted two major international workshops; a Workshop on Biological and Genetic Control Strategies for the Leucaena Psyllid held 3-7 November, 1986 in Molokai, Hawaii, USA (NFTA 1987) and an International Workshop on Leucaena Psyllid Management held 16-21 January, 1989 in Bogor, Indonesia (Napompeth and MacDicken 1990). In addition, several seminars, workshops and conferences were organized independently at the national level in affected countries such as Indonesia, The Philippines and Thailand. On a regional basis and under the auspices of the Forestry/Fuelwood Research and Development (F/FRED) Project, Winrock International Institute for Agricultural Development in Bangkok, a Psyllid Advisory Team was formed together with the Regional Research Program for Leucaena Psyllid Control in 1987. A regional coordinator for the program was chosen with respective representation by national coordinators from Australia, Indonesia, Malaysia, Philippines, Taiwan, Thailand and the United States.
The objective of this paper is to provide a comprehensive review of the leucaena psyllid in the Asia-Pacific Region, its impact in the region and its implications when introduced into Africa together with proposed management strategies based on the experience encountered in the Asia-Pacific Region.
Leucaena is a genus of Central American shrubs and trees which contains about 10 species. This genus is in the family Leguminoseae and, like most other legumes, can form a mutually beneficial symbiosis with soil bacteria of the genus Rhizobium. Through this symbiotic relationship, nitrogen fixation, the transformation of atmospheric nitrogen into compounds which are available to plants as soil nutrients is accomplished.
The most widely exploited member of this genus is L. leucocephala. Commonly known as "leucaena", this species has been widely used in forestry and agroforestry planting programmes. In addition to leucaena, this tree is known by many common names including ipil-ipil, lepile, bayani (Philippines), lamtoro (Indonesia), guaje, yaje, uaxin (Latin America) koa haole (Hawaii), hediondilla (Puerto Rico), and tangatan (Guam). Some strains of leucaena are many branched shrubs which average 5 m in height at maturity while others are single stemmed trees which can grow up to 20 m in height. This species is believed to have originated in Central America and was spread widely through the region by the Maya and Zapotec civilizations. The name "Oaxaca", for Mexico's fifth largest state and a prominent city, is derived from the pre-Columbian word "uaxin" meaning "the place where the leucaena grow."
In 1565, after the conquest of Mexico, the Spanish conquistadors organized trade with the Philippines. Sometime during the 250 years that this trade continued, leucaena reached the Philippines, probably carried as forage. A low growing "common" form of leucaena became firmly established in the Philippines, Guam and other Spanish possessions in the Pacific. Local people soon learned that the tree made good firewood and later plantation owners found that crops such as coffee, cocoa, cinchola, pepper, vanilla and other shade loving crops did well beneath an understorey of leucaena. As a result, the tree was introduced into plantations in the Netherlands East Indies (now Indonesia), Papua New Guinea, Malaysia and other Asia countries. During the nineteenth century it was taken to Hawaii, Fiji, northern Australia, India, East and West Africa and the islands of the Caribbean. It is now pan-tropical in distribution.
The leucaena which became so widely distributed descended from a small seed source known as the "Acapulco" and, more recently, the "Hawaiian" type. The wealth of other leucaena germ plasm scattered throughout Central America remained uncollected and virtually unrecorded until recently.
Of all of the tropical legumes, leucaena probably offers the widest assortment of uses. Through its many varieties, leucaena can produce nutritious forage, firewood, timber and a rich organic fertilizer. Its many uses include revegetation of tropical hill sides which have been deforested and degraded, windbreaks, firebreaks, shade and ornamentation. Individual leucaena trees have yielded extraordinary amounts of wood, among the highest ever recorded. In addition, the plant is responsible for some of the highest weight gains measured in cattle (National Research Council 1984).
The leucaena psyllid, Heteropsylla cubana Crawford (Homoptera: Psyllidae) is a tiny homopterous insect, measuring about 2.0 mm in length. It is native to Central and South America which is also the natural range of Leucaena leucocephala and other species in the genus Leucaena. The insect was first described by Crawford (1914) as Heteropsylla cubana and also by Sulc in the same year (Burkhardt 1986). Since Crawford's description has taxonomic priority, Sulc's R. incisa became H. incisa (Sulc) and was later considered a synonym. Therefore H. cubana remains the valid name for this species. A short taxonomic description and key to the identification of some Heteropsylla spp, including H. cubana was given by Caldwell and Martorell (1951). After its invasion of the Pacific Island countries and Taiwan, Yang and Fang (1986) attempted to redescribe and illustrate the adults and fifth instar nymphs based on specimens collected in Taiwan.
The most recent descriptions and keys to the identification of the genus Heteropsylla, including H. cubana, with a complete taxonomic account, host plant preferences and geographic distribution is given by Muddiman et al (1992).
From the egg stage, H. cubana undergoes five nymphal instars to reach the adult stage. Eggs are laid singly but in dense clusters lodged between the folds of developing leaflets of young leucaena shoot tips. After hatching, the early instar nymphs feed gregariously. In the later instars they begin to feed in a solitary manner as the leaflets unfold. Eggs, nymphs and adults are often found together on the shoot terminals. The life cycle of H. cubana varies somewhat from one location to another. On the average, the total life cycle, from egg to adult, takes from 10 - 20 days with several overlapping generations per year.
Some limited, preliminary investigations on the population biology, especially life tables of H. cubana, have been carried out to obtain more meaningful population statistics and parameters in terms of biological attributes. Such studies were made in Indonesia by Rauf et al (1990) and in Thailand by Napompeth and Maneeratana (1990) but not in other countries affected by this insect in the Asia-Pacific Region. Although the net reproductive rate of increase (Ro) differed greatly (51.35 in Indonesia, 139.18 in Thailand) other biological attributes obtained were similar, e.g., the cohort generation time (Tc) was 14.92 in Indonesia and 17.95 days in Thailand. The intrinsic rate of increase (rm) was 0.264 in Indonesia and the capacity of increase (rc) was 0.275 in Thailand. The finite rate of increase was 1.305 and 1.361 in Indonesia and Thailand respectively. While the population doubling time was not calculated in Indonesian studies, in Thailand it was 2.52 days. A population doubling time of 2.52 days could be a convincing argument to explain the rapid development and buildup of the psyllid population and consequently the sudden damage inflicted to host plants.
Burkhardt (1986) associated H. cubana with legumes of the genera Leucaena, Mimosa, and Piptadenia in its natural range. In a test using nine leguminous species in Hawaii, Nakahara et al (1987) successfully reared H. cubana only on leucaena and monkeypod, Albizia saman. Among the recognized species of Leucaena, some showed differential degrees of resistance, tolerance or susceptibility to the leucaena psyllid (Pan 1987, Sorensson and Brewbaker 1987). L. collinsii, L. esculenta, L. pallida and L. retusa showed evident resistance to the psyllid in Hawaii with similar observations being made in Taiwan. Some varieties of L. leucocephala were reported to be highly resistant to the psyllid in one location but were more susceptible in another locality (Sorensson and Brewbaker 1987). In the study on the resistance of some Leucaena spp to the psyllid in Australia, Bray and Woodroffe (1988) found that all four leucaena species tested were susceptible but L. collinsii showed the least damage. In Mexico, H. cubana was also reported from L. pulverulenta by McClay (1990) and according to CABI (1992), H. cubana also attacks L. salvadorensis. In addition to L. leucocephala, L. pulverulenta and L. salvadorensis, Muddiman et al (1992) reported L. trichodes and L. diversifolia as hosts.
It is of biological control importance to note that a closely related species, H. spinulosa, which is also native to Central and South America, has been purposely introduced to Australia and Western Samoa via Australia for control of a noxious weed, Mimosa invisa (Muddiman et al 1992).
Both nymphs and adults feed on leucaena terminal shoots by sucking plant sap. Young flowers are also infested but less frequently. The older leaves are in most cases not attacked. Feeding results in terminal shoots which may become desiccated and consequently, growth is stunted. High psyllid populations produce copious amounts of honeydew which gives rise to the development of sticky black sooty mould coating terminal shoots and leaflets. In the case of heavy infestations, complete defoliation is not uncommon. Although plants can recover later in most cases, death of damaged plants has been known to occur.
Infestation of young terminal shoot tips begins with a few gravid females laying scattered eggs between leaf folds. With increasing numbers of females, heavy egg laying on the shoot tips could turn them yellow in colour due to the large numbers of eggs deposited on young shoot tips or leaflets. After the infestation tapers off or declines, the new shoots and leaves become somewhat distorted, deformed and necrotic. There is no data available to determine if this is merely a reaction to feeding injury or if the leucaena psyllid is the vector of a plant pathogen.
In many areas, leucaena psyllid exhibits seasonal abundance with populations peaking during the cooler part of the year. In areas north of the equator, this occurs between late September and early March. During warmer periods, numbers of psyllids are often at very low levels and difficult to detect. Exceptions to this pattern are the existence of "ecological pockets or refuges" where populations remain at higher numbers throughout the year and can be detected at any time.
Prior to April 1984, when H. cubana was first reported on leucaena in Hawaii, negligible attention was given to this insect. Specimens of H. cubana sent to the British Natural History Museum in London from the Caribbean countries date back to 1 927 from Bermuda, 1 981 from the Dominican Republic and 1 982 from Anguilla, Antigua and Puerto Rico. The detection of H. cubana in the Asia and Pacific Region during the mid 1 980s prompted the Commonwealth Agricultural Bureau (CAB) to produce a distribution map (No 478) for this insect in June 1988 and a revision in December 1992 (CAB 1986, CABI 1988, 1992).
Shortly after the report of H. cubana in Florida late in 1 983 and its discovery in Hawaii on April 26, 1 984, infestations were detected in Guam, Saipan, Cook Island, Western Samoa, Tahiti, Tonga, Fiji, Solomon Islands, New Caledonia, Papua New Guinea, Christmas Island, Okinawa, Taiwan and the Philippines in 1985. In 1986, infestations were found in Indonesia, Malaysia, Singapore, Vietnam, Cambodia, Laos, Hong Kong, Macao, southern China, Thailand and southern Myanmar. In 1987 additional infestations were found in upper Myanmar, Bangladesh, Nepal, Andaman Islands and Sri Lanka. In 1988 the insect was detected in southern India and in 1991, western India, Reunion and Mauritius. By 1992, infestations reached the African continent with populations detected in Tanzania, Kenya, Uganda and Burundi. In less than 10 years, the leucaena psyllid has spread from its native range in Mexico and Central America across the Pacific Islands, to Asia and finally to Africa (NFTA 1987, Napompeth and MacDicken 1990, Hollis 1992, Van Den Beldt and Napompeth 1992) (Table 1).
As speculated by Napompeth (1990) that H. cubana would eventually disperse from the Asia Pacific Region to Africa, its modes of introduction and dispersal from its natural range in Central America to Hawaii, westward to the Pacific Islands, Asia and Australia and finally across the Indian Ocean to Africa still remain a puzzle. According to Waage (1990), the meteorological calculations and methods developed to study the dispersal of the rice brown plant hopper indicate that its dispersal to Hawaii and subsequently from Hawaii to Guam and Samoa was almost certainly aircraft assisted. Dispersal through southeast Asia, Australia and into Sri Lanka and India was probably due to being carried on monsoon winds. He concluded that dispersal of H. cubana by monsoon winds into Africa would be difficult.
PROGRESSIVE INVASION AND DISPERSAL OF LEUCAENA PSYLLID
BETWEEN 1983 AND 1993
|
YEAR |
COUNTRIES INVADED |
|
1983 |
Florida, USA |
|
1984 |
Hawaii, USA |
|
1985 |
Guam, Saipan, Cook Island |
|
1986 |
Hong Kong, Macao, southern China |
|
1987 |
Upper Myanmar, Bangladesh, Nepal |
|
1988 |
Southern India |
|
1991 |
Western India, Reunion, Mauritius |
|
1992 |
Tanzania, Kenya, Uganda, Burundi |
It seems ironic that this tiny creature could circumnavigate the globe, moving westward from its native range and ultimately reaching its natural range in the western hemisphere. A question is also raised as to whether its dispersal was human assisted through movement of plant materials. This is not considered likely because movement of leucaena material is normally carried out through seeds and vegetative material has not been transported.
In Hawaii, L. leucocephala is considered by some people as a weed. The annual loss due to the sugar industry of these islands due to invasion of fields by leucaena is approximately $US 878,000 (Funasaki et al 1990). The plant is also considered to be a desirable plant in vacant lowlands and mountain slopes for prevention of soil erosion (Neal 1965). L. leucocephala is also utilized as fodder for cattle by ranchers and its flowers are an important source of nectar for honeybees. Considering both positive and negative values of this tree, a programme of classic biological control was advocated and undertaken as the only pest management tactic in Hawaii.
In Indonesia, the loss caused by H. cubana to L. leucocephala shade trees during 1986-88 in both agriculture and forestry sectors was estimated at Rp 4.5 billion ($US 2.8 million) (Mangoendihardjo et al 1990). The loss is mainly in estate crops such as coffee, vanilla and round cardamon with about a 55% decrease in production. L. leucocephala is considered to be economically important in Indonesia with an area of 1.2 million ha planted for a variety of uses such as shade trees for cocoa, tea, coffee and vanilla, as a fodder crop, for soil reclamation, charcoal and fuel wood, green manure, timber and leaf meals as well as a vegetable for human consumption. According to Oka (1990), during 1986, the economic loss was estimated at more than $US 316 million. It was predicted that if no control actions were taken, the estimated loss suffered by estate crops, animal production and forestry sectors would be Rp 1.4 trillion, Rp 1.2 trillion and Rp 42 billion respectively over a five year period leading to a total loss of Rp 2.64 trillion (rp 2 100 = $US 1.00).
In Australia, leucaena is used almost exclusively for grazing by beef cattle and most plantings (approximately 20 000 ha) are in central Queensland (Bray et al 1990). The plant is also found in the Northern Territory and Western Australia. The psyllid infestation in northern and southern Queensland could reduce leucaena production by at least 55%. Significant damage is found in the higher rainfall coastal areas but in the drier region of inland Queensland the psyllid is not considered a serious problem for people growing this tree. In this area, agricultural extension authorities continue to recommend planting of leucaena for fodder.
In the Philippines, Sanchez (1990) reported that three years after the invasion of leucaena psyllids, the income of the leaf meal gatherers and charcoal producers was reduced and damage to the other sectors of the economy continued.
In Malaysia, H. cubana poses a serious threat to the development, expansion and utilization of leucaena in that country (Lim et al 1990). It is reported that defoliation from 95-100% was observed among older trees and the psyllid infestation was greater in older trees than in younger trees. Leucaena forage has considerable potential for the production of animal feed and of special importance is the potential utilization of leucaena in leaf meal industries. Currently, Malaysia is importing over 48 000 tons of leaf meal at an estimated cost of over M$ 20 million for pig and poultry feeds.
In Taiwan, leucaena is utilized mainly by the pulp and paper industry. The psyllid infestation, which began in 1985 led to serious damage in 1986 - 87. This resulted in a replacement of some leucaena plantations with Eucalyptus camaldulensis and E. grandis (Pan 1990). However, since 1988, the psyllid population began to decrease and leucaena recovered and began to grow quite well again.
In Thailand, the economic status of leucaena is difficult to assess because of the presence of naturalized or "common" leucaenas which are the result of earlier introductions which have escaped cultivation. In some cases these are considered to be weeds. They are also heavily infested by the leucaena psyllid. The recent introduction of "giant" leucaena during the 1970s was to support various agroforestry practices, fodder, nitrogen fixation and other uses. However, the total area of leucaena of all types can not be accurately estimated. Although leucaenas could become weeds under several situations, the benefit of leucaenas outweigh their detriments, especially in the production of leaf meals for export.
The impacts of leucaena psyllid in Thailand and its economic status have been moderate to severe. People gathering young shoot tips and young pods for sale as human food have complained about the defoliation caused by the psyllid on leucaena that grows wild along roadsides or is grown for "edible" hedges. Leucaenas planted for fodder and commercial plantations for leaf meal production could not meet the demand of the industries during the severe infestation which occurred between 1986 and 1990. Many leucaena plantations were either abandoned or ploughed up and replaced by other crops. During the peak of psyllid infestation in the country, farmers and the authorities responsible began to panic and resorted to using chemical insecticides to control the infestation. As expected, the results were not encouraging and proven to be uneconomical, impractical and ecologically undesirable.
According to Gunasena et al (1990), the greatest impact of leucaena psyllid in Sri Lanka has been to the livestock industry. Farmers in the dry zone of the country planted leucaena along fences for feeding dairy cattle, buffaloes and goats and for fuelwood for domestic use. It is also used in agroforestry systems such as alley cropping and avenue cropping, as shade for annual and some perennial crops, for compost for vegetables and horticultural crops. However, no estimates of the area of leucaena plantings is available for the country nor is there an estimate of the economic impact of leucaena psyllid.
In India, Veeresh (1990) reported that the cultivation of leucaena in India began in 1972 and now occupies an area of 10 000 ha in Karnataka State alone. Within six months of its entry into India, the psyllid invaded the entire area under leucaena. The Karnataka Plantation Company had planned to extend the area of leucaena to over 4 000 ha in 1988 but abandoned the idea for fear of loosing the plantations and instead, destroyed their nursery of over 200 000 seedlings.
There are no substantial reports of economic, ecological or social impacts from other affected countries in the Asia-Pacific region such as the Pacific Island countries, Okinawa, southern China, Laos, Vietnam, Cambodia, Myanmar, Bangladesh and Nepal.
In a review of economic damage caused by leucaena psyllid in Southeast Asia- and Australia, Heydon and Affonso (1991) concluded that the leucaena psyllid is a serious pest of Leucaena leucocephala in all countries studied. It has limited the exploitation of leucaena as a potential forage crop in Malaysia and to a lesser extent in Australia. In Indonesia and the Philippines, leucaena has provided the key to the development of more intensive, stable and sustainable farming systems for smallholder. In the commercial sector, leucaena psyllid was one of the factors that contributed to the decline of the dendro-thermal power programme in the Philippines. This insect has also had a significant impact on exports of leaf meal from Thailand and the Philippines. In Indonesia, considerable economic damage has been sustained by that country's cocoa, coffee and vanilla produces as a result of defoliation of (eucaenas planted to provide shade for these crops.
There seems to be an overall concern that while chemical insecticides may provide effective short term relief from damage, they are not desirable both from an ecological and economic standpoint except for special conditions such as nurseries, seed production areas or young seedlings as recommended during the NFTA meeting in Molokai, Hawaii, USA (NFTA 1987).
Pest management strategies developed and adopted for leucaena psyllid control in the Asia-Pacific Region are highly diversified. Common to all are the difficulties in attempts to establish economic threshold levels (ETLs) and economic injury levels (EILs) which will be acceptable and practical for various vegetation types and ecological situations ranging from the escaped or "weedy" leucaenas to small holdings, shade trees, fodder plantings, pasture legumes, grazing lands, alley cropping or plantations for fuel wood and other wood products. In many situations, no single pest management strategy or tactic would lead to a satisfactory level of population suppression. An integrated pest management approach (IPM) is convincingly essential. However, under certain situations, from the practical and economic point of view, a single pest management tactic such as biological control or genetic control using resistant and (or) tolerant varieties or species of leucaena could be effective.
An example of timely use of biological control agents for control of leucaena psyllid is the Hawaiian Islands, USA. Soon after the detection of the psyllid in Hawaii in April 1984, an exploratory entomologist from the Hawaiian Department of Agriculture (HDA), who was at the time stationed in Trinidad and Tobago, was directed to search for potential natural enemies of leucaena psyllid for possible introduction into Hawaii.
While the search for potential exotic natural enemies was underway, natural enemies of the psyllid already present in Hawaii were also evaluated. Several species of predators were found feeding on nymphs and adults of the leucaena psyllid in Hawaii including the coccinellids, Curinus coeruleus Mulsant and Olla v-nigrum (Mulsant) (= O. abdominalis) (Funaski et al 1990).
C. coeruleus was originally introduced into Hawaii from Mexico in 1922 for the control of the coconut mealybug, Nipacoccus nipae Maskell (Homoptera: Pseudococcidae). Subsequent to its introduction and establishment, C. coeruleus became rare and was hardly encountered until the arrival of the leucaena psyllid. It has since become the most abundant species of the family Coccinellidae in Hawaii and is an important predator of the leucaena psyllid.
O. v-nigrum was also introduced from Mexico into Hawaii in 1908 for the biological control of scale insects. The O. v-nigrum population has also been sparse until the arrival of leucaena psyllid when its population was found to increase significantly and actively feeding on psyllids. It was not as abundant as C. coeruleus, however. In spite of being considered general feeders, both coccinellids have played an important role as "indigenous" natural enemies suppressing the populations of H. cubana significantly during the early years of its presence in Hawaii.
As a result of the exploration for natural enemies of the leucaena psyllid in Trinidad and Tobago initiated in 1984, an encyrtid nymphal parasite, Psyllaephagus yaseeni Noyes ( = Psyllaephagus sp. near rotundiformis (Howard) (Hymenoptera: Encyrtidae) from Tobago, a eupelmid nymphal parasite, Tamarixa leucaenae Bouek (Hymenoptera: Eupelmidae) ( = Tetratstichus triozae Burks), and a coccinellid, Cycloneda conjugata Mulsant (Coleoptera: Coccinellidae), in Trinidad were obtained and introduced into Hawaii (Nakahara et al 1987). Of these natural enemies, T. leucaenae failed to propagate under quarantine conditions in Hawaii and C. conjugata was found not to be adequately specific and its culture was eventually destroyed, leaving only P. yaseeni. This species was further screened, field tested and finally approved for field release in June 1987 to become firmly and widely established in Hawaii (Funaski et al 1987).
The three biological control agents of the leucaena psyllid available in Hawaii have since been introduced to other psyllid infested areas in the Asia-Pacific Region. C. coeruleus was introduced from Hawaii and became established in several Pacific Island countries including Guam, Samoa, Saipan, the Philippines, Indonesia and Papua New Guinea during 1986 (Waterhouse and Norris 1987). In Thailand, the first introduction of C. coeruleus was made from Saipan in April 1987 and subsequently from Hawaii during the same year. It has become firmly established and widely distributed in all areas of introduction and has been found feeding heavily on leucaena psyllid. O. v-nigrum has been used to a much lesser extent. This species was first introduced from Hawaii to Tahiti and from Tahiti to Tonga and New Caledonia (Chazeau et al 1989). O. v-nigrum was also introduced into Thailand in April 1 989 (Napompeth 1990). In Tonga, O. v-nigrum was found widespread at low populations feeding on nymphs and adults of leucaena psyllid during June 1 993 by personal observations. The psyllid damage was insignificant.
In Thailand, C. coeruleus has become widespread following extended field releases carried out all over the country. O. v-nigrum, on the other hand, has been released and is established but tends to be localized in its distribution. C. coeruleus was introduced into Vietnam in April 1988, to India in October 1988, to Myanmar in November 1988, to Nepal in March 1 989 and to New Caledonia in November 1 991. It has been widely established in India (Veeresh 1990) and New Caledonia (J. Chazeau, personal communication). It has also been recovered in countries adjoining Thailand including Laos, Cambodia and Malaysia where it has not been intentionally introduced.
The encyrtid nymphal parasitoid, P. yaseeni, was introduced from Hawaii to Thailand from October 1987 through September 1988 (Winotai 1989). It has been mass reared on dwarfed leucaena plants infested with psyllids in an insectary for field release. Releases were made using mummified leucaena psyllid nymphs (mummies) in a plastic cup attached to the small stem of a leucaena plant. P. yaseeni has become firmly established and widespread in all areas. During the peak psyllid season from October to March, parasite densities were much higher than those observed in Hawaii (R. Burkhardt, personal communication).
From Thailand, this parasitoid was introduced into Indonesia in 1990 where it has become firmly established after its field releases. P. yaseeni mummies could be observed year round in Indonesia. It was also introduced from Thailand to New Caledonia in December 1991. P. yaseeni was also reported from the Philippines although no introductions have been made there. It has been speculated that the parasitoid apparently reached these islands by becoming airborne like its host (C. Baltazar, personal communication). However this is not considered likely because otherwise P. yaseeni should also be present in other psyllid infested countries in the Pacific including Australia.
Similar to C. coeruleus, the parasitoid P. yaseeni has been recovered in countries which adjoin Thailand and where it has not been introduced, indicating its ability to disperse widely. It can be concluded, therefore, that both C. coeruleus and P. yaseeni could become established and widespread throughout continental southeastern Asia through introduction of these natural enemies in Thailand.
While biological control activities were being carried out in several Asian and Pacific Island countries using the available natural enemies, C. coeruleus, O. v-nigrum and P. yaseeni from Hawaii and Thailand, the CAB International Institute of Biological Control (IIBC) began to search for additional natural enemies of leucaena psyllid in the insect's natural range in Central and South America in 1988 with support from the U.S. Agency for International Development (USAID), the Canadian International Development Research Centre (IDRC) and the Australian Center for International Agricultural Research (ACIAR) (Waage 1990). This work resulted in the acquisition of 18 species of Coccinellidae, 11 insect predators from other families, 3 species of spiders, and 6 species of parasitoides together with a species of entomogenous fungus. In addition, P. yaseeni, T. leucaenae and C. coeruleus were found to be associated with leucaena psyllid in similar searches made in Mexico (McClay 1990).
T. leucaena was introduced from Trinidad via the UK to Thailand by IIBC in December 1991. A total of 75 adults and 65 mummies were received. Of these, 27 adults remained alive and 1 2 adults emerged from the mummies. After exposing them to psyllid nymphs in quarantine, only one parasitized nymph was obtained and the entire culture eventually perished. As a result, no further attempt was made to utilize this species in Thailand.
According to IIBC (1992), cultures of T. leucaenae and P. yaseeni have been maintained to supply Asian countries in 1993. However, nine shipments containing ca 5 000 individuals of both parasitoides were sent to IIBC, UK for forwarding to China, Malaysia and Nepal from Trinidad and Tobago. There is no further report to indicate if these were introduced and established. It is believed that T. leucaenae may have some advantages over P. yaseeni under certain conditions and may compliment P. yaseeni through differences in habitat or phenology (P. Baker, personal communication).
A number of entomopathogens have been reported from several psyllid infested countries in Asia. The more dominant ones are Conidiobolus coronatus (Constantin) Butko, Entomophthora sp. and Hirsutella thompsonii Fischer in Thailand (Napompeth 1990), Entomophthora sp., Paecilomyces farinosus, Hirsutella citriformis and Fusarium sp from the Philippines (Villacarlos et al 1990) and Beauvaria bassiana, Metarhizum anisoplae var. anisoplae and Paecilomyces javanicus from Taiwan (Yao et al 1990). Under suitable climatic conditions many of these fungi were found infecting populations of the leucaena psyllid resulting in heavy mortality. Unfortunately, none of these fungi have been cultured and developed under laboratory conditions and cannot be presently utilized as microbial control agents.
The only practical cultural control tactic currently available for managing leucaena psyllid is the planting of alternative MPTS which are not damaged by this insect. However, such an approach is not very realistic because alternative species may not provide the wide range of benefits which leucaena can provide. Other MPTS which have been recommended as alternatives to leucaena include Gliricidia spp, Calliandra spp and others. However none of these are able to equal leucaena in terms of performance.
Various workers have reported that among the individual species of Leucaena spp and over 50 interspecific hybrids recognized, L. collinsi, L. esculenta and L. pallida seem to be resistant or tolerant to damage by leucaena psyllid. Some of these have been crossed with L. leucocephala. A series of leucaena psyllid trials have been organized by the Nitrogen Fixing Tree Association (NFTA) and its collaborators in many Asian countries. Progress has been relatively slow and inherently time consuming.
A number of studies on psyllid tolerance or resistance have been conducted in many countries affected by this insect. The following paragraphs summarize the results of several of these studies.
In West Timor, Indonesia, three trials were carried out to measure the adaptation and psyllid resistance of several varieties of leucaena and other tree legumes. All varieties of L. leucocephala were severely damaged. L. pallida K376 and L. diversiflora K784 were well adapted and psyllid resistant. Other selections of L. pallida, L. diversifolia and L. collinsi appear promising. Other tree legumes were not attacked by the psyllid (Mella et al 1990).
In the Philippines, 14 Leucaena collections from the NFTA and one local collection (K28) were evaluated for psyllid tolerance and resistance. Ratings of psyllid counts and resultant damage indicate that L. diversifolia K784 and K785 are resistant to psyllid attack. K785 appears to have better vegetative growth than K784 (Crizaldo 1990)
An evaluation of the resistance of several species of Leucaena was undertaken in Sri Lanka in cooperation with NFTA. Based on preliminary data, the species were categorized as follows (Gunasena et al 1990):
RESISTANT
L. pallida K376, L. collinsi, Hybrid KX2, L. diversifolia # 46568.
MODERATELY RESISTANT
Hybrid KX1, L. diversifolia K785, L. esculenta.
SUSCEPTIBLE
L. leucocephala K636, L. diversifolia K156, L. diversifolia #33820.
HIGHLY SUSCEPTIBLE
L. leucocephala K8, Hybrid KX3.
The Thailand Institute of Scientific and Technical Research in Bangkok conducted a trial to assess psyllid resistance in 1987. Eight varieties were tested including 5 of L. leucocephala (K8, K28, K500, K527, K636), 2 of L. diversifolia (K156, K784) and 1 L. leucocephala x L. diversifolia hybrid (K743). Among all of the varieties tested, L. diversifolia K784 and K156 showed the highest tolerance to psyllids. However, the L. leucocephala varieties coppiced rapidly after cutting and recovered quickly from defoliation (Buranasilpin et al 1990).
According to work by Brewbaker et al (1990), the genus Leucaena contains a high degree of variability which remains largely unexploited. Many of the new hybrids such as L. leucocephala x L. pallida have shown psyllid resistance and may be useful for timber and other uses. Other hybrids show promise for timber, fodder and gum production but are yet untested.
The rapid invasion of the Asia-Pacific Region by the leucaena psyllid, despite a vast expanse of ocean indicates that this insect has a combination of extremely efficient dispersal mechanisms and an excellent ability to avoid interception at international points of entry. Based on experiences in the Asia-Pacific Regions, attempts to limit the rate of spread through increased plant inspection and quarantine procedures in an area where countries are not separated by large bodies of water is, very likely, an exercise in futility.
Undeniably, a variety of chemical pesticides are effective against leucaena psyllid and could be used as a short term emergency control measure. However, a series of constraints inherent to pesticides makes there long term use impractical. These include potential undesirable effects on non-target organisms, risk of exposure of humans to toxic pesticides, high cost and high probability of populations of the target insect developing resistance to the pesticides. In addition, it is logistically impractical to treat the variety of conditions under which leucaena grows, which range from populations which have escaped cultivation to intensively managed plantings such as shade trees , alley cropping or various plantation systems. It is generally agreed that pesticides should be used only for protection of young seedlings or nurseries.
At the time when leucaena psyllid first arrived in the Asia-Pacific Region, little was known about the population dynamics of this insect or the factors which regulate its abundance. Waage (1990) reported that H. cubana was highly seasonal in its occurrence and usually extremely rare in its natural range in the Caribbean Islands, Central and South America. Clay (1990) reported that populations of H. cubana, although abundant and widespread in Mexico, were never heavy enough to defoliate trees and were rare during the wet summer season. In Thailand, populations increased at the end of the wet season and beginning of the dry season, between September to October and peaked during the cooler months between November and March. In Hawaii, where the temperature is somewhat cooler, leucaena psyllids can be encountered during the entire year. Patterns of psyllid abundance and decline were also observed in Laos and Vietnam.
In Thailand, during the periods of peak leucaena psyllid abundance from September-October to March-April, infestations can be seen country wide. During periods of warmer weather infestation levels tend to decline except for population "pockets" which are found in cooler microclimates or high elevation sites.
In countries near the equator, such as Malaysia and Indonesia (Java), psyllids can be detected all year round but at times at relatively low population densities.
South of the equator, Bray et al (1990), reporting from Australia, indicate that psyllid populations fluctuate in all areas of Western Australia, the Northern Territory and Queensland. Personal observations by the author in the Northern Territory of Australia, Papua New Guinea, Fiji and Tonga revealed sparing populations of leucaena psyllid during June and July which was also the cooler season.
Based on these reports and observations, it is most likely that the leucaena psyllid exhibits preferences for certain temperature ranges provided that there is ample food available. Such temperature preferences could help explain the peaking of psyllid populations during the cooler months of the year. This is accompanied by a flushing of leucaena foliage following the wet monsoon season. The existence of population pockets of leucaena psyllids in cooler microclimates and higher elevations further supports the hypothesis that the insect has a definite optimum temperature range.
A gradual reduction in the abundance of leucaena psyllid could be witnessed in Thailand from its original invasion in September 1986 to the early part of 1 994. as described earlier by Van Den Beldt and Napompeth (1992), these declining psyllid populations have caused progressively lower levels of damage in Thailand. The factors responsible for this decline remain to be investigated. Such patterns of population decline have also been observed in other countries in the Asia-Pacific Region. It is speculated that if such a trend continues, leucaena psyllid will no longer be a serious problem within a few years time. However, the economic loss during a period of ten years of heavy psyllid infestation may not be tolerable from an economic point of view.
The decline of leucaena psyllid populations in Asia and the Pacific island countries cannot be explained scientifically. It is not known what the existing mortality factors are but most certainly some biological or density dependent factors are at work. The indigenous natural enemies, regardless of their generality or specificity, could be partially responsible for this decline. It was reported from several countries that the high populations of leucaena psyllid are often infected with entomogenous fungi at the epizootic level. Indigenous predators, such as various species of Coccinellidae, Hemiptera, spiders and other natural enemies, are almost always found associated with psyllid populations. The role of introduced natural enemies, particularly C. coeruleus and P. yaseeni, could also be factors contributing to the decline of leucaena psyllid populations. In all situations, it is most certain that these factors have harmoniously and continually reduced populations of leucaena psyllid in the Asia-Pacific Region to their present low levels.
No attempt will be made in this review to discuss other aspects of the population dynamics of this insect, especially those which are largely of academic interest and significance. The investigation of various population statistics and parameters has been carried out to some extent in Hawaii, Indonesia, the Philippines and Thailand covering studies on biological attributes, construction and analysis of life tables of the psyllid and its key natural enemies. Information on these studies can be found detailed in Napompeth and MacDicken (1990).
Napompeth (1990) and Waage (1990) have predicted that leucaena psyllid would eventually invade Africa. The psyllid was reported from Reunion and Mauritius in 1991, probably spreading from the Indian subcontinent. Infestations have since been reported from Kenya, Tanzania, Burundi and Uganda in 1 992 (Reynolds and Bimbuzi 1 992). Having now established a firm foot hold in Africa, the insect could reach the west coast of the continent in just a few years.
Leucaenas have been promoted and cultivated for various purposes in Africa, using the same narrowly based lines or varieties used in the Asia-Pacific Region. Therefore leucaena psyllid can be expected to have serious economic and social consequences for at least a decade following its initial establishment. This prediction is based on patterns of psyllid population dynamics and resultant damage in the Asia-Pacific Region.
Concerns have been felt and technical assistance has been requested by the African countries affected thus far. The Asia-Pacific experience should provide Africa with what can be expected from the invasion of leucaena psyllid in terms of damage and economic impacts. A review of the economic impacts of leucaena damage in Southeast Asia and Australia reported by Heydon and Affonso (1991) should provide adequate indication on the effects this insect might have on African agroforestry programmes. Affected the most would be the smallholders who utilize leucaena for various purpose at the subsistence level. Equally affected would be the various MPTS programmes which incorporate and utilize leucaenas.
There is without a doubt, a better opportunity for the rapid dispersal of leucaena psyllid on the African continent because it consists of a single land mass instead of a series of islands separated by relatively large expanses of water. In Africa, the key factors which will influence the spread of the psyllid are prevailing winds patterns and the availability of suitable host material. Economic and social impacts of psyllid damage on a country by country basis, will depend on the relative importance of leucaena in national agroforestry programmes and the purposes for which leucaena plantings are established. Comprehensive analysis of the magnitude and purposes of the existing leucaena resource and near term plans for the expansion of that resource should form the basis for an integrated pest management programme for this insect.
In a review of psyllid damage to leucaena plantings in Thailand, Malaysia, the Philippines, Indonesia and Australia for the Australian International Development Assistance Bureau (AIDAB), Heydon and Affonso (1991) concluded that pesticides could provide an effective means of control against the psyllid but are neither economically feasible nor ecologically desirable, with the exception of seedlings and nurseries. Biological control, using introduced natural enemies offers clear economic advantages with respect to the pest management tactics available. A need to strengthen the capacity to understand biological control on a regional basis was also advocated. They also concluded that the leucaena used in planting programmes has come from a narrow genetic base with respect to tolerance to cold temperatures and drought, pod production, wood durability and susceptibility to leucaena psyllid. Thus continuing support is required for a coordinated evaluation of genetically broad based leucaena material on a range of site conditions in a number of countries where this tree is an important agroforestry species and for establishment of seed production capacities for production of high quality genetic material. Because of its long term nature, the development of resistant leucaena varieties must be carried out in parallel with other pest management tactics which will provide short term relief from damage.
To help counter the recent introduction of leucaena psyllid in Africa, Van Den Beldt and Napompeth (1992) advocated the introduction and use of resistant leucaena varieties, which are already being tested in Africa, and other resistant leucaena hybrids from Asia and the Pacific. They recommend that this be done in conjunction with the introduction of biological control agents which have been successfully established in Asia and the Pacific.
Many of the recommendations of the International Workshop on Management of the Leucaena Psyllid held in Bogor, Indonesia, 16-21 January, 1989 (Napompeth and MacDicken 1990) are considered to be valid for African conditions. They include both policy and research recommendations. It must be recognized, however, that implementation of these recommendations is constrained by the availability of monetary resources, technical skills and commitment on the part of the affected countries to address the problem.
The more pertinent recommendations of this international workshop which could be adapted to African conditions in order to ensure the continued viability of leucaena as a MPTS include:
- Make full use of regional and international cooperation to manage the leucaena psyllid and minimize losses to the leucaena resource.
- Biological control and use of leucaena varieties which are both genetically broad based and resistant or tolerant to the leucaena psyllid should be the primary tactics used for management of this insect.
- Emphasis should be on the use and continued species/provenance selection and testing of leucaenas which are resistant or tolerant to leucaena psyllid rather than replacement of leucaena with alternative MPTS.
- Networks for research, monitoring and technology transfer on leucaena should be initiated and strengthened.
- Funding for management of leucaena psyllid should be sought first from national sources, either government agencies or private institutes and then from international sources.
- Linkages among international donors and development agencies to improve research and development of leucaena and other MPTS should be promoted.
- Local tree breeders should be encouraged to test and select trees which show apparent resistance to leucaena psyllid and collect seed for use in cooperative trials. They are also encouraged to select and test resistant trees found in plantations.
- Leucaena seed orchards for production of seeds of psyllid resistant composites should be established in as many locations as possible.
- Development of a biological control capacity should be emphasized as one of the main components of an integrated pest management programme for this insect.
- Initiate studies to identify the indigenous natural enemies of leucaena psyllid and the factors which regulate their abundance.
- Where acceptable, utilize the exotic natural enemies which are presently available including the coccinellid predator, Curinus coeruleus and the parasitoides Psyllaephagus yaseeni and Tamarixa leucaenae.
- Monitor the psyllid populations and the establishment and effectiveness of introduced natural enemies wherever they are used.
- The introduction and release of biological control agents should be coordinated on a regional basis in order to avoid any possible conflicts of interest.
Last but not least is the recommendation of Van Den Beldt and Napompeth (1992) for initiating a coordinated research and management programme for leucaena psyllid for Africa. The development of such a programme would benefit from the involvement of technical experts from the Asia-Pacific Region.
Bray, R.A. and T.D. Woodroffe, 1988. Resistance of some Leucaena species to the leucaena psyllid. Tropical Grasslands 22(1):11-16
Bray, R.A., M.H. Julien and P.M. Room, 1990. Leucaena psyllid in Australia - the current situation. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 8-11.
Brewbaker, J.L., C.T. Sorensson and R.W. Wheeler, 1990. New tree crops from interspecific Leucaena hybrids. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 105-110.
Buranasilpin, P., J. Watanakul, P. Athipunyakom, S. Tunapanich and S. Pattanavibul, 1990. Leucaena spp resistance to psyllid, Heteropsylla cubana Crawford in Thailand. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 94-98.
Burkhardt, D., 1986. Nomenclatural note on Heteropsylla cubana Crawford (Homoptera: Psylloidea), a new pest in Pacific Countries.. Revue Suisse Zool 93(4): 1023-1024.
CAB (Commonwealth Agricultural Bureau), 1986. Distribution maps of pests. No 478, Heteropsylla incisa (Sulc), UK.
CABI, 1988. Distribution maps of pests. No. 478 (Revised), Heteropsylla cubana Crawford ( = H. incisa (Sulc)). UK.
Caldwell, J.S. and L.F. Martorell, 1951. A brief review of the Psyllidae of Puerto Rico (Homoptera). Annals Entomological Society of America 44:603-613.
Chazeau, J.S., E. Bouye and L. Bonnet de Larbogne, 1989. Lutte biologique contre le psylle Heteropsylla cubana, ravaguer du faux-mimosa Leucaena leucocephala en Nouvelle Caledonie. ORSTOM Institut Francais de Recherche Scientique pour le Developpment en Cooperation, Centre de Noumia. 82 pp.
Ciesla, W.M., 1993. Recent introductions of forest insects and their effects: A worldwide overview. Proceedings Conferência Regional da Vespa da Madeira, Sirex noctilio, na America do Sul, 23-27 November, Florianópolis, SC, Brazil, EMBRAPA, Curitiba, pp 9-21.Crawford, D.L., 1914. A monograph of the jumping plant lice or Psyllidae of the new world. Bulletin U.S. National Museum 85, 185 pp.
Crizaldo, E.N., 1990. Evaluation of leucaena cultivars for psyllid tolerance. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 73-81.
Funasaki, G.Y., P.Y. Lai and L. Nakahara, 1990. Status of natural enemies of Heteropsylla cubana Crawford (Homoptera: Psyllidae) in Hawaii in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 153-158.
Gunasena, H.P.M., I.P. Wickramasinghe and H.M.G.S.B. Hitinayake, 1990. Status of the management of leucaena psyllid Heteropsylla cubana in Sri Lanka. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 43-44.
Gunasena, H.P.M., M.A.S.K. Ranasinghe, I.P. Wickramasinghe and H.M.G.S.B. Hitinayake, 1990. Evaluation of Leucaena germplasm for psyllid resistance. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 82-89
Heydon, D. and M. Affonso, 1991. Economic review of psyllid damage on leucaena in Southeast Asia and Australia. A report prepared for the Australian International Development Assistance Bureau (AIDAB) CAB International Development Services, Wallingford, Oxon, U.K., 129 pp.
Hollis, D., 1992. Leucaena psyllid, Heteropsylla cubana Crawford, newly recorded on Mauritius and Reunion. FAO Plant Protection Bulletin 40 (1-2):49-50.
IIBC (CAB International Institute of Biological Control), 1992. Annual report 1992. Wallingford, Oxon, U.K. pp 25-26.
Lim, G.S., C.L. Tan and C.C. Wong, 1990. Studies on leucaena psyllid in Malaysia. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 28-39.
McClay, A.S., 1990. Distribution of leucaena psyllid and its natural enemies in Mexico: Implications for biological control. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 139-143.
Mangoendihardjo, S., F.X. Wagiman, A. Sulthoni and Subyanto, 1990. Economic impact of leucaena psyllid infestation on estate crops and teak forest plantation. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 184-188.
Mella, P., M. Zaingo and M. Janing, 1990. Resistance of leucaena and other tree legumes to Heteropsylla cubana in West Timor, Indonesia. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 56-61.
Muddiman, S.B., I.D. Hodkinson and D. Hollis, 1992. Legume-feeding psyllids of the genus Heteropsylla (Homoptera: Psylloidea). Bulletin of Entomological Research 82:73-117.
Nakahara, L., W. Nagamine, S. Matayoshi and B. Kumashiro, 1987. Heteropsylla cubana and its predators. Leucaena Research Report 7(2):39-44.
Napompeth, B, 1990. Leucaena psyllid problems in Asia and the Pacific. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 1-11.
Napompeth, B. and K.G. MacDicken, eds., 1990. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, 208 pp.
Napompeth, B. and T. Maneerat, 1990. Biological and partial ecological life tables of Heteropsylla cubana Crawford and its predator, Curinus coeruleus Mulsant, in Thailand. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 130-1 38.
National Research Council, 1984. Leucaena: Promising forage and tree crop for the tropics. National Academy Press, Washington DC, 1 1 5 pp.
Neal, M.C., 1965. In gardens of Hawaii. B.P. Bishop Museum Special Publication 50:411-412.
NFTA (Nitrogen Fixing Tree Association), 1987. Proceedings of a workshop on the biological and genetic control strategies for the leucaena psyllid. D. Worthington and J.L. Brewbaker, eds., Leucaena Research Reports (Special Issue) 7(2), Waimanalo, Hawaii, 109 pp.
Oka, I.N., 1990. Progress and future activities of the leucaena psyllid research programmes in Indonesia. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 25-27.
Pan, F.J., 1987. Psyllid resistance of leucaena species in Taiwan. Leucaena Research report 7(2):35-38.
Pan, F.J., 1990. Leucaena psyllid in Republic of China - a country report. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 12-15.
Reynolds, L. and S. Bimbuzi, 1992. Leucaena psyllid arrives in Kenya. Agroforestry Today, July-September, p 2.
Rauf, A., P. Hidayat, N. Maryana and I.W. Winasa, 1990. Biology and demography of Heteropsylla cubana Crawford (Homoptera: Psyllidae) in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 114-118.
Sanchez, F.F., 1990. Leucaena psyllid in the Philippines - a country report. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 40-42.
Sorensen, C.T. and J.L. Brewbaker, 1987. Psyllid resistance of leucaena hybrids and species. Leucaena Research report 7(2): 29-31.
Van Den Beldt, R.J. and B. Napompeth, 1992. Leucaena psyllid comes to Africa. Agroforestry Today 4(4): 11-12.
Veeresh, G.K., 1990. The status of leucaena psyllid, Heteropsylla cubana Crawford in India. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 14-16.
Villacarlos, L.T., R.V. Pagnilawan and R.P. Robin, 1990. Factors affecting leucaena psyllid populations in Leyte, Philippines. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 122-129.
Waage, J., 1990. Exploration for biological control agents of leucaena psyllid in tropical America. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 144-152.
Waterhouse, D.F. and K.R. Norris, 1987. Biological control, Pacific Prospects. Australian Center for International Agricultural Research (ACIAR), Inkata Press, Melbourne. 454 pp.
Winotai, A., 1989. Biological control of leucaena psyllid, Heteropsylla cubana Crawford (Homoptera: Psyllidae), in Thailand. Ph.D. Thesis, Kasetsart University, Bangkok. 128 pp.
Yang, C.T. and S.J. Fang, 1986. A serious pest, Heteropsylla cubana Crawford, of Pacific Islands (Homoptera: Psylloidea: Pauropsyllidae). J. Taiwan Museum 39(1 )-.59-61.
Yao, A.L., Y.S. Hwang and W.Y. Wang, 1990. Natural enemies and biological control of Heteropsylla cubana on Leucaena leucocephala in Taiwan. in B. Napompeth and K.G. MacDicken, eds. Leucaena psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, p 162.
by
M.R. Rao1
International Centre
for Research in Agroforestry (ICRAF)
P.O. Box 30677, Nairobi, Kenya
Since its appearance on the Kenya and Tanzania coast in August 1992, the leucaena psyllid, Heteropsylla cubana, has spread to all countries in the eastern and southern Africa. It has caused extensive damage to Leucaena leucocephala, which is widely used in agroforestry and planted by many farmers for fodder and poles in these countries. Psyllid can easily be controlled by any systemic and broad spectrum insecticide such as dimethoate, cypermethrine, cyhalothrine and carbofuran. Chemical sprays are effective for 2 to 3 weeks. Sprays of 5% neem seed powder extract were not effective when the insect population was high. Insecticides can be applied by foliar sprays in the case of hedges and small trees and by brushing or dropping 2 to 3 drops of chemical over 3 to 4 superficial stem cuts (per tree) in the case of big trees. Insecticide use is neither economical nor practical for small farmers in Africa. Moreover, they pose health and environmental hazards and their use prevents the build up of the psyllid's natural enemies. Chemicals should only be considered only as a short term solution. The long term strategy should be the development of psyllid resistant leucaenas and biological control through introduction of parasites and predators.
*****
Leucaena leucocephala has extensively been used in agroforestry in Africa in the last two decades. It is adapted widely to the humid and sub-humid regions of eastern and central Africa, the southern African plateau, the humid lowlands of West Africa and even semi-arid regions wherever adequate moisture is available. It can be used as barrier (or contour) hedgerows for soil conservation, fodder banks for dairy cattle, hedgerow or rotational hedgerow intercropping for soil fertility improvement or woodlots and boundary plantings for fuelwood or poles. The importance of leucaena can be gauged from the World Bank estimation that it can meet 48% of the fuelwood needs of Kenya in the year 2,000 (57.3 million m3) by planting on 50% of the potentially suitable land in the country (Stone et al 1991). Although no proper estimate of the area planted to leucaena is available in the country, it is recognized that leucaena is planted for fodder wherever dairy cattle are reared by zero-grazing system. Pockets of leucaena plantings exist mostly along the coast, the central highlands and western Kenya.
Leucaena psyllid arrived on the coast of Tanzania and Kenya in July-August 1992 and it was noted for the first time in Mtwapa Regional Research Station in August 1992 (Table 1). Since then it has spread rapidly, infesting all the leucaena planted along the coast. It arrived at the Machakos Research Station by January 1993 and reached western Kenya
TABLE 1
SPREAD OF LEUCAENA PSYLLID IN AFRICA
|
YEAR |
LOCATION |
REFERENCE |
|
November 1991 |
Mauritius, Reunion |
Hollis 1991 |
|
March 1992 |
Madagascar |
Reynolds and Bimbuzi 1993 |
|
July/August 1992 |
Kenya & Tanzania Coast |
Hill 1992, Reynolds & |
|
January 1993 |
Machakos, Kenya |
ICRAF 1994 |
|
June-August 1993 |
Kampala/Kabale, Uganda |
D. Peden ICRAF1 |
|
June 1993 |
Bujumbura, Burundi |
Akyeampong ICRAF1 |
|
March 1994 |
Central & Eastern Rwanda |
A. Niang ICRAF1 |
|
November 1993 |
Zomba, Malawi |
J. Maghembe ICRAF1 |
|
May 1994 |
Ethiopia |
Hassen 19941 |
|
June 1994 |
Chipata, Zambia |
F. Kwesiga, ICRAF1 |
|
September 1994 |
Mozambique |
Unconfirmed |
by March/April that year, severely affecting the leucaena plantings along the way. It is now known that psyllid has spread all over East Africa and many countries in southern Africa (Table 1). We have no reports yet of its appearance in the central and west African states but it is only a matter of time before its eventual occurrence there. The leucaenas which have been grown in Africa are mostly the Hawaiian giants which come from a narrow genetic base and are highly susceptible to psyllid attack. If potential benefits of leucaena are to be realised, there is an urgent need to take up steps for combating the psyllid.
The psyllid causes severe and extensive damage to leucaena quite rapidly. For example, the lush and vigorously growing hedges in January 1993 at Machakos, following the extended rains of the previous "short rains" (Oct 92 - Jan 93), were completely defoliated by June 1993. Similar observations were also made at the Regional Research Stations at Mtwapa and Embu (L. Reynolds, ILCA and Mick O'Neill, ICRAF, personal communications). Psyllid was active throughout 1993 and first half of 1994 building up huge populations. The unprotected hedges on farms and the station were completely defoliated, leaving nothing to harvest. Psyllid populations fluctuate from season to season and the extent of damage also varies depending on the management (Van Den Beldt and Napompeth, 1992). The populations were observed to be slightly low during rainy periods, probably due to washing off of the insects by rains. The build up increased towards the end of rains resulting in heavy damage. The populations decreased again in the very cool and dry periods (July-September) when there was little growth of new shoots. Thus the extent of damage at present is somewhat less than that was noted in 1993 and hopefully it may reduce further in the coming seasons. This is exactly what had happened in Asia, the damage was less severe after its arrival there in mid eighties (Van Den Beldt and Napompeth, 1992).
Damage was generally higher on pruned hedges than on unpruned trees. This may be related mostly to the greater number of young shoots that emerge on coppiced hedges than on trees. Detailed observations on population dynamics have not been taken up but such studies in different leucaena-based systems would help to understand the effect of climatic and management factors for the development of psyllid control strategies. A few lady bird beetles were noted feeding on the psyllid but no parasitoides were found so far in Kenya. Since leucaena is widely used in agroforestry systems it is important to know whether the associated crops in the systems affect the psyllid populations.
The different options available for the control of leucaena psyllid are:
Many papers in this workshop may be describing the potential and progress made so far on biological control and host plant resistance in other parts of the world. However, little progress has been made in identifying psyllid tolerant leucaenas and use of biological control in Africa. We hope that some concrete action programme will emerge from this workshop that will greatly help in developing an integrated management strategy for leucaena psyllid. This paper reviews the chemical methods employed for psyllid control and their relevance in Africa.
Like all other sap sucking insects, such as aphids and thrips, the psyllid can be controlled easily by a wide range of systemic and broad spectrum insecticides. Organo-halogens, organo-phosphates, carbamates and pyrethroides have been tested. For example, foliar applications and stem injections of carbosulfan, isoprocarb and monocrotophos were found to control the psyllid up to a period of one month (Chazeau 1987). Dimethoate was widely used, and was found to be effective in checking the psyllid damage (Thomas and Liebregts 1986, Heydon and Alfonso 1990). Other chemicals frequently employed in Southeast Asia, India and Australia were azodrin, carbaryl, carbosulfan, dicrotophos, bifanthrin and cyhalothrine.
In an experiment conducted at 4 sites in Australia and Indonesia, 0.04% dimethoate spray applied at 4 week intervals increased yield by more than 100% at both sites in Australia where the level of infestation was high, but by only 40% at one of the two sites in Indonesia where the extent of infestation was low (Palmer et al 1 989). In the early years of psyllid infestation in India, 0.07 to 0.1 % endosulfan was used at 14 day intervals with good success (Singh and Bhandari 1988). In an experiment at Mudigere, India, 0.05% spray of quinalphos, phosalene or endosulfan was found to reduce significantly the eggs up to 15 days and nymphs up to 30 days after spraying compared to untreated checks. Nymphal population treated with monocrotophos showed similar levels as the check at 30 days after spraying (Thimmaiah et al 1991). In the Philippines, foliar sprays of carbaryl, applaud, isoprocarp and applaud combined with isoprocarp gave immediate control 3 days after spraying. Stem injections of carbosulfan and monocrotophos showed effectiveness up to 7 days later. All these chemicals including the fungal pathogen Beauveria bassiana were effective against psyllid for about 30 days, except for carbofuran and carbaryl. Carbaryl held the population to a low level only for a week (Barrion et al 1987).
Chemicals can be applied on young trees and cropped hedges by low or high volume sprays. Older trees can also be sprayed but they should be treated with care by a foot sprayer or boom sprays. Alternatively the chemical could be applied with a fine brush or dropping 2-3 drops with a pipette on three to four superficial cuts made on stem about 0.5 to 1.0 m above the ground (Oka and Bahagiawati 1988, Table 2). The residual effect of pesticides depends on the chemical and intensity of insect infestation. The effect of sprays may last 2 to 3 weeks but the residual action of chemicals applied through stem injections can last longer.
PESTICIDES AND APPLICATION METHODS FOR LEUCAENA PSYLLID
CONTROL IN INDONESIA.
|
INSECTICIDE |
DOSE |
APPLICATION |
INTERVAL |
|
Sihalotrin |
0.1 % formulation |
For young shoots that emerge after pruning, stem diameter <5cm, application by spraying |
1 Week |
|
Acephate |
33.3% formulation 5ml/plant |
Stem diameter >5cm application by making superficial cut |
2 weeks |
|
Dimethoate |
Concentrate |
Stem diameter >5cm, application by making two reversed superficial cuts |
4 weeks |
Source: Oka and Bahagiawati 1988.
Leucaena in the long-term experiments was sprayed with commonly available chemicals, when it was realised that it might be subjected to stand mortality under severe psyllid attack. At Embu, weekly sprays of dimethoate 40 EC (Rogor E) @ 40 ml per 20 litres water, cypermethrine 5 EC (Ambush) @ 100 ml per 20 litres water or lambda cyhalothrine 17.5 EC (Karate) @ 100 ml per 20 litres water were given, which completely protected leucaena from the psyllid and allowed its normal growth. In contrast, the unprotected hedges failed to produce any harvestable yield (Dr. Mick O'Neill, ICRAF Senior Agroforester, personal communication). The chemicals were changed every week in a rotation to prevent the potential pest resistance that might develop with the continuous use of any one chemical.
A neem based insecticide, JawanR, which is marketed in India, and neem seed extract were tested at ICRAF's Machakos Station in the early stage of psyllid infestation (May-September 1993). The logic was that botanical pesticides are environmental friendly and may be economical to use on tree crops such as leucaena compared with synthetic pesticides. Weekly sprays of Jawan @ 5 ml per litre kept the leucaena hedges free from the psyllid for some time. However, neem seed extract (5% spray) and Jawan failed to protect leucaena in a subsequent trial (November 1993 to June 1994) on the station, when the psyllid population was extremely high. Sap of Melia azedarach and tobacco extracts were also suggested against psyllid but botanical pesticides in general may have limited effect when the infestation is severe.
The potential benefits of spraying leucaena fodder banks was studied at Machakos in a small replicated trial from November 1993 to July 1994 spread over three pruning cycles. The hedges were protected by sprays of dimethoate (@ 3 ml per litre and 500 litres/ha) or cypermethrine (@ 10 ml per litre) at 2 week interval. The protected leucaena yielded 2 to 4½ times higher biomass than the unprotected leucaena (Table 3). At the current market price, each spraying costs about US$30. With an estimated value of Kshs 0.7/kg of leucaena fodder (on dry weight basis), returns for investment on chemical control would be 1:1.35 which is not at all attractive to farmers. The intensive level of protection given in the above studies was perhaps not necessary at the farm level. This was evident from a satisfactory control obtained with dimethoate sprays at 1.5 ml per litre and 2 to 3 week interval in an adjacent field-scale alley cropping trial on the station during the same time. This level of protection would be economical because of reduced chemical cost for each treatment and fewer number of sprays. Nevertheless, considering the frequency of applications required, chemical control of psyllid appears to be a risky proposition from the point of livestock health and safety to people.
Chemical control is impractical in forest plantations. It is also inappropriate on leucaena plantings for soil conservation and soil fertility improvement because of uncertain crop yield response to compensate the input costs. Nevertheless, chemical control is worth considering on leucaena shade trees for coffee, tea, vanilla and cocoa, fodder banks, seed orchards and nursery seedlings. However, care must be taken while spraying pesticides on fodder banks with respect to appropriate dosage and interval between the last spraying and livestock feeding of the material. Spraying should be stopped at least 3 to 4 weeks before the commencement of feeding.
EFFECT OF INSECTICIDE SPRAY ON
LEUCAENA BIOMASS (T/HA) PRODUCTION
UNDER SEVERE INFESTATION BY LEUCAENA PSYLLID,
MACHAKOS, KENYA
|
Treatments |
Pruning Cycles |
|||||
|
1 |
2 |
3 |
||||
|
Nov 16, 93 - Jan 14, 94 |
Feb 19 - April 8, 94 |
Apr 22 - Jul 15, 94 |
||||
|
Leaves |
Twigs |
Leaves |
Twigs |
Total Biomass |
||
|
Sprayed |
3.08 |
2.14 |
2.40 |
1.61 |
2.61 |
|
|
Unsprayed |
0.65 |
0.68 |
1.29 |
0.92 |
0.85 |
|
|
SED |
0.46 |
0.31 |
0.13 |
0.20 |
0.33 |
|
The high fecundity of psyllid and perenniality of leucaena do not make chemical control an easy task. Reduction of this pest by chemicals is difficult to achieve because leucaena is managed in different ways on-farms (trees, hedges etc) and that complete coverage of tree canopies with chemical sprays is difficult to achieve. The other disadvantages of chemical control include health hazards to the user, environment pollution in the entire process of manufacturing and use of chemicals, elimination of natural populations of parasites and predators, high cost and inaccessibility to small farmers in developing countries.
The economical, safe and effective strategy for mitigating the psyllid is only the combined use of psyllid resistant leucaena plant material and biological control. The parasites and predators may move along with the pest but they take long time to reach the new destinations; so deliberate attempts should be made to introduce these effective biological control agents into Africa to help combat the problem rapidly. In this respect the experience gained in the Asia-Pacific Region should be capitalised upon.
Barrion, AT., R.M. Aguda and J.A. Litsinger, 1987. The natural enemies and chemical control of the leucaena psyllid, Heteropsylla cubana Crawford (Hemiptera: Psyllidae), in the Philippines. Leucaena Research Reports 7(2):45-49.
Chazeau, J., 1987. Le psylle du faux-mimosa en Asie du Sud-Est et dans la Pacifique: état du probleme et perspectives de lutte (Leucaena leucocephala (Lam.) de Wit -Heteropsylla cubana Crawford). Revue Élev. Med. Vet. Nouv. Calédonie 9:23-27.
ICRAF (International Centre for Research in Agroforestry), 1994. Annual Report 1993 pp 89-92, ICRAF, P 0 Box 30677, Nairobi, Kenya.Heydon, D. and M. Alfonso, 1990. Economic review of psyllid damage on leucaena in Southeast Asia and Australia. A report prepared for the Australian International Development Assistance Bureau, CAB International Development Services, Wallingfold, Oxon, U.K. pp 129.
Hill, G., 1992. Report of a visit to Mauritius and Reunion, 23-29 June 1992; to study infestations of leucaena psyllid on Leucaena leucocephala. IIBC, Kenya Station, Muguga, Kenya, pp. 6.
Hollis, D., 1992. Leucaena psyllid, Heteropsylla cubana Crawford newly recorded in Mauritius and Reunion. FAO Plant Protection Bulletin 40 (1-2): 49-50.
Oka, I.N. and A.M. Bahagiawati, 1988. Comprehensive programme towards integrated control of leucaena psyllid, a new insect pest of leucaena trees in Indonesia. IARD Journal 10(1):10-30.
Palmer, B. R.A. Bray, T.M. Ibrahim, and M.G. Fulloon, 1989. The effect of leucaena
psyllid on the yield of Leucaena leucocephala cv. Cunningham at four sites in the tropics. Tropical Grasslands 23:105-107.
Reynolds, L. and S. Bimbuzi, 1 992. Leucaena psyllid arrives in Kenya. Agroforestry Today 4(3):2.
Singh, P. and R.S. Bhandari, 1988. The arrival of leucaena psyllid in India. Leucaena Research Reports 20.
Stone, S.W., S.C. Kyle and J.M. Conrad, 1991. What role for Leucaena leucocephala in meeting fuelwood demand in Kenya. Leucaena Research Reports 12:121-123.
Thimmaiah, G., V.V. Belavadi, and C. Parvathi, 1991. Chemical control of subabul psyllid. Curr. Sci. 20:187-189.
Thomas, P.O. and W. Liebregts, 1 986. Depredation of Leucaena leucocephala by psyllids in Western Samoa. Leucaena Research Reports 7:21-23.
Van Den Beldt, R.J. and B. Napompeth, 1992. Leucaena psyllid comes to Africa. Agroforestry Today. 4(4): 11-12.
1Presented by F. Owino, Senior Forester, ICRAF, Nairobi, Kenya.
by
J. Tassin and M.
Hermet
CIRAD-Forêt,
Ligne Paradis, 97410, St-Pierre, Réunion
and
S. Quilici
CIRAD-FLHOR, BP 180,. 97455 St-Pierre, Réunion
Leucaena was introduced to Réunion more than a century ago (Jacob de Cordemoy 1893) and is now very common. It is widely used for forage and windbreaks but recognised as a serious weed in the western part of the country. Since 1991 (Vandeschricke et al. 1992), the leucaena psyllid, Heteropsylla cubana (Crawford), has become a pest of leucaena in Réunion. An integrated pest management research programm was set up by CIRAD in collaboration with local partners (DAF, SPV, FDGEC, ONF) and biological control experiments were initiated. Resistance to leucaena psyllid was observed on Leucaena diversifolia Buitenzorg hedgerows at Trois-Bassins CIRAD station, in a highly infested area, and more attention was given to it during the first months of 1994.
This report provides six-month data on the resistance of this cultivar for which psyllid infestation levels have been compared with L. diversifolia K-1 56 and four L. leucocephala entries. Resistance is defined as lower levels of insects on the plants and lower levels of damage (Wheeler et al. 1989, Luego and Josephine 1990).
The study focused on leucaena hedgerows which were planted between March and May 1991 at the Trois-Bassins CIRAD station before detection of the psyllid. Site data for the study area are summarized in Table 1.
Two seed lots of L. diversifolia Buitenzorg were obtained from ISABU, Burundi. This cultivar is an Indonesian variety which was present in the Bujumbura gardens, Murongwe and Gisozi (Brandelard and Ncahimigo 1989). K156 was obtained from NFTA and two Mexican varieties (not specified) were obtained from OFI. Four L. leucocephala entries (not specified) were provided by CIRAD-Forêt. All these entries may be considered as controls for L. diversifolia Buitenzorg resistance evaluation (Table 2). All seeds were scarified in boiling water and were not inoculated.
The four Leucaena leucocephala entries were planted in 10 m long, three row plots, with 1,50 m between rows and 0.25 m between plants within the row (120 trees/plot). The five L. diversifolia entries were planted for hedged parcelling around crops as two or three replicated contour line hedgerows, about 20 m long, with 20-30 m between rows
SITE DATA FOR LEUCAENA PSYLLID RESISTANCE
EVALUATION, TROIS-BASSINS CIRAD
STATION, RÉUNION
|
PARAMETER |
VALUES |
|
Trial location |
Trois Basins (Cocâtre) |
|
Latitude |
21° 06' 3" |
|
Longitude |
55° 19' 1 " |
|
Topography |
8-20% slope |
|
Elevation |
990 m |
|
Mean annual rainfall |
1380 mm |
|
Mean annual temperature |
16° |
|
Soils |
Andisols (Andepts) |
|
Soil pH |
5.6 |
LEUCAENA SPECIES AND VARIETIES INCLUDED IN
RESISTANCE EVALUATION
|
LEUCAENA SPECIES AND VARIETY |
SEED SOURCE |
|
L. diversifolia Buitenzorg (1) |
Gitega, Burundi |
|
L. diversifolia Buitenzorg (2) |
Moso, Burundi |
|
L. diversifolia ( 1 ) |
Corral Falso, Vera Cruz, Mexico |
|
L. diversifolia (2) |
Jalapa, Vera Cruz, Mexico |
|
L. diversifolia K-156 |
Vera Cruz, Mexico |
|
L. leucocephala (1) |
Marouna, Cameroun |
|
L. leucocephala (2) |
Bhavnagar, India |
|
L. leucocephala (3) |
Davao, Philippines |
|
L. leucocephala (4) |
El Salvador |
and 0.25 m spacing within the row. The plantations have been managed as hedges for fodder production by cutting trees back after six months of growth to 50 cm above ground level and subsequently cut back to a height of 50 cm every six months.
The following measurements were taken:
PSYLLID COUNT AND DAMAGE - Psyllid counts for nymphs were scored for each entry twice a month (from 27 April 94 to 6 June 94) from samples of ten 10 cm long young sprouts taken from different trees. Psyllids were then spread and fixed on a paper sheet for automatic counting using image analysis. Damage was briefly described twice a month between 27 April 94 and 30 September 94.
CLIMATOLOGICAL DATA - Monthly rainfall and maximum and minimum temperatures were recorded during the whole study.
Nymph populations for the various leucaena species/varieties for the period from 27 April 94 to 7 June 94 are given in Table 3. Climatological data are reported in Table 4.
NUMBERS OF LEUCAENA PSYLLID NYMPHS ON VARIOUS
LEUCAENA
SPECIES AND VARIETIES
(Insects/gm
of dry leucaena foliage)
|
SPECIES/VARIETY |
27 APRIL |
11 MAY |
24 MAY |
06 JUNE |
|
L. diversifolia Buitenzorg (1) |
0 |
0 |
0 |
0 |
|
L. diversifolia Buitenzorg (2) |
0 |
0 |
0 |
0 |
|
L. diversifolia (1) |
603 |
0 |
1029 |
870 |
|
L. diversifolia (2) |
1522 |
602 |
1838 |
1337 |
|
L. diversifolia K 156 |
1371 |
0 |
323 |
294 |
|
L. leucocephala (1) |
650 |
1308 |
2733 |
2150 |
|
L. leucocephala (2) |
5545 |
5458 |
6194 |
6129 |
|
L. leucocephala (3) |
441 |
1133 |
2391 |
1875 |
|
L. leucocephala (4) |
1998 |
3170 |
2811 |
2952 |
METEOROLOGICAL DATA
FOR THE LEUCAENA RESISTANCE STUDY
March - June 1994
|
MONTH |
RAINFALL (MM) |
MAXIMUM TEMPERATURE |
MINIMUM TEMPERATURE |
|
March |
289.0 |
24.9 |
18.2 |
|
April |
43.5 |
24.8 |
16.7 |
|
May |
4.5 |
23.6 |
17.2 |
|
June |
22.0 |
20.7 |
13.8 |
Highest levels of psyllid populations were recorded at the end of May, which seems to be correlated with lower rainfall while high atmospheric humidity was maintained. L. leucocephala varieties were heavily attacked. Damage levels and psyllid populations were less severe for the L. diversifolia varieties but only L. diversifolia Buitenzorg was entirely psyllid free during the complete period of the study (until 30 September 94).
The high psyllid populations observed on L. leucocephala and some L. diversifolia entries confirmed that the Trois-Bassins CIRAD station was a highly infested area during this study. L. diversifolia Buitenzorg was exposed to high psyllid pressure but remained free of infestations and damage. However, these observations are only the first results on this evaluation of the apparent resistance of L. diversifolia Buitenzorg to leucaena psyllid resistance and cannot be considered as definitive. A similar trial with a randomized complete block experimental design will be established at Trois-Bassins CIRAD station in the near future. There is also need to collect data on growth performances, forage value and productivity of L. diversifolia Buitenzorg.
Brandelard, P. and 0. Ncahimigo, 1989. Les arbres fourragers au Burundi. In, Séminaire National L'agroforesterie au Burundi, Bujumbura, Burundi 28-31, March 1989, pp 203-220.
Jacob de Cordemoy, E., 1893. Flore de I'île de la Réunion (Phanérogames, Cryptogames vasculaires, Muscinées). Librairie des Sciences Naturelles, Paul Klincksieck, Paris, 574 pp.
Luego, Josephine N., 1990. Evaluation of twelve leucaena species for psyllid (Heteropsylla cubana Crawford) resistance.
Wheeler R.A., G.K. Uchida, J.W. Beardsley, and J.L. Brewbaker, 1990. A twelve week study of leucaena psyllid (Heteropsylla cubana Crawford) on seven leucaena varieties in Hawaii. In Proceedings, Leucaena psyllid: Problems and management. Bogor, Indonesia, pp 99-104.
Vandeschricke, F., S. Quilici, J. Gauvin and Y. Roederer, 1992. Le psylle du Leucaena à la Réunion. Bois et Forêts des Tropiques 234: 47-59.
by
S. Quilici, A.
Francki and B. Montagneux
CIRAD-FLHOR, BP 180, 97455, St-Pierre, Réunion.
and
J. Tassin
CIRAD-Forêt, Ligne Paradis,
97410, St-Pierre, Réunion
On 15 December 1991, we detected on the CIRAD-FLHOR Station of Bassin Plat the presence of a new pest of striking abundance on the windbreaks of Leucaena leucocephala. Samples were immediately sent to Dr Hollis (B.M.N.H.) who identified the insect as the Leucaena psyllid, Heteropsylla cubana (Vandeschricke et al. 1992). Soon after this first detection, it appeared that this windborne pest had established itself in numerous sites of the island. A few meetings were organised with local partners (DAF, SPV, FDGDEC, CIRAD-Forêt, ONF), and, considering the local importance of leucaena for various uses, the decision was taken to initiate research on tolerant varieties of leucaena and possibilities for biological control.
In this latter field, some research began in early 1992, within the Entomology Laboratory of CIRAD FLHOR Réunion. Our attention focused primarily on two exotic Coccinellidae which had proven promising in various countries of south-east Asia and some Pacific islands; Curinus coeruleus and Olla v-nigrum (Vandeschricke et al. 1992). In the meantime, a survey of psyllid abundance and relative importance of indigenous natural enemies was also initiated. This paper reports on the establishment of Olla v. nigrum in Réunion.
Both species of exotic predators were introduced following usual procedures. C. coeruleus was obtained from Thailand on 09 February 1992, thanks to Dr R. Nachapong, and O. v-nigrum from New Caledonia, thanks to Dr Chazeau, on 27 March 1992. A small-scale rearing of both species was developed in the laboratory, the predators being fed with leucaena psyllids collected daily on the Station grounds. After a few generations reared for quarantine purposes, regular inoculative releases of both species were done mostly in two localities near Saint-Pierre, in the south of the island; Bassin Plat (altitude: 1 50 m) and Bassin Martin (altitude: 300 m) (Fig 1). Both sites belong to the lowlands of the leeward side of the island, and have a tropical climate with an average rainfall of 1500 mm.
Figure 1 - Inoculation release sites for Olla v-nigrum and Curinus coeruleus in Réunion -1992 to 1993.
During the first phase of the program, both species were released mostly as larvae. A total of 1357 O. v-nigrum, 603 in 1992 and 754 in 1993 (Table 1), and 2092 C. coeruleus, 562 in 1992 and 1530 in 1993 were released (Table 2).
Though casual observations on the nature and abundance of indigenous natural enemies were done during 1992 and the first half of 1993, a regular survey was only initiated in October 1993. For this purpose, 10 sites were selected in various areas of the island, with 3 of them monitored monthly and 7 every three months. During each visit, an estimation is done on the abundance of flushes of leucaena foliage, psyllid populations, and natural enemies. For the sites monitored monthly, samples are collected with a D-Vac vacuum, in order to identify the indigenous natural enemies and quantify their relative abundance.
INOCULATION
RELEASES OF OLLA V-NIGRUM IN RÉUNION
1992-1993
|
YEAR |
SITE |
MONTH |
NUMBER RELEASED (LARVAE) |
|
1992 |
Bassin Plat
Bassin Martin |
July November |
50 265 |
|
1993 |
Bassin Plat |
June |
20 |
INOCULATION RELEASES OF CURINUS
COERULEUS IN RÉUNION
1992-1993
|
YEAR |
SITE |
MONTH |
NUMBER RELEASED |
|
|
LARVAE | ADULTS |
|||
|
1992 |
Bassin Plat |
June |
301 |
26 |
|
1993 |
Bassin Plat |
June; |
355 |
|
The survey on indigenous natural enemies, which is still underway, showed the absence of any parasitoid but the presence of numerous predators. The dominant groups are Heteroptera, with numerous species, and Coccinellidae. Among this latter family, Exochomus laeviusculus appears to be widespread in all the areas where the psyllid occurs and apparently increased notably its populations following the arrival of H. cubana. Other predators are found such as Thysanoptera and Chrysopidae.
Though individuals of C. coeruleus were regularly observed at the release sites soon after the releases, no establishment of this species occurred. On the contrary, a massive establishment of O. v-nigrum was observed on the Bassin Plat site from November 1993 on. During this period preceding the heavy rainfall of austral summer (usually from December to April), an impressive abundance of all instars (eggs, larvae, pupae and adults) was present on the leucaena windbreaks of the CIRAD-FLHOR Station of Bassin Plat. From this moment on, the establishment of O. v-nigrum was considered effective. Rearing in the laboratory was stopped and numerous dispersal releases were done by regularly collecting individuals of different instars and transferring them to other release sites in the north, west and south of the island (Fig 2). A total of 6417 predators were dispersed in this way; (1930 in 1993 and 4487 in 1994 (Table 3).
During the first half of 1994, casual observations indicated that O. v-nigrum was present in some localities in the highlands of the west, where no releases were made (Les Colimacons), and the south (Le Tampon). A survey conducted in July-August 1994 in order to establish the distribution of this species, showed that it was largely established in the highlands of the west (altitudes of 700-800 m), in the highlands of Saint-Louis in the south-west, in various sites of the south (Le Tampon, Petitelle), in the north (Gillot, near Saint-Denis) and in the east (Cambourg, near Saint-Benoit) (Fig 3). In the west, although O. v-nigrum was also detected in some areas at medium altitudes (Bellemene, 400 m), it seems probable that the lowlands, with a hot and dry climate, are not favourable for this predator. However, complementary releases are currently underway, in collaboration with FDGDEC (Fédération des Groupements de Défense contre les Ennemis des Cultures) to try and enlarge the distribution to a few areas where the predator is not yet present, such as the lowlands of the west.
Several months later, we also observed in Bassin Plat the occurrence of high levels of parasitism on the nymphs of the established population of O. v-nigrum. At least three species are involved, the dominant ones being Tetrastichus sp. (Eulophidae) and Cheiloneurus cyanonotus (Encyrtidae) (det by G. Delvare). Studies are currently underway on the precise status of these parasitoids, their distribution and biology.
From the initial establishment site in Bassin Plat, we could observe a rapid natural dispersion of O. v-nigrum. The excellent dispersal ability of this predator is well-known. The species now has a large distribution area in the island, two years after the first releases. This is similar to what was observed in New-Caledonia following the introduction of this ladybird beetle (Chazeau, pers. com.).
We are however not yet able to assess the real impact of the species on the populations of the psyllid. During the first phase of establishment in Bassin Plat, where very high numbers of ladybirds were observed, they obviously contributed to the decrease in psyllid populations that were rather low in this period. It is probable that the heavy rainfall of austral summer of 93-94 was unfavourable to the predator. Further, the recently discovered parasitoids will probably limit the future effectiveness of O. v-nigrum.
DISPERSION RELEASES OF OLLA
V-NIGRUM, RÉUNION
1993-1994
|
YEAR |
SITE |
MONTH |
NUMBER RELEASED |
|
|
LARVAE | ADULTS |
|||
|
1993 |
St Denis |
November |
800 |
|
|
Etang-Salé |
November |
700 |
||
|
Entre Duex |
November |
200 |
||
|
St
Denis |
November |
200 |
||
|
Bassin Plat |
December |
30 |
||
|
1994 |
Bassin Plat |
January |
160 |
|
|
April |
153 |
|||
| May | 334 | |||
|
Etang-Salé |
May |
100 |
200 |
|
|
June |
80 |
150 |
||
|
St
Denis |
May |
50 |
100 |
|
|
June |
150 |
|||
|
August |
40 |
78 |
||
|
Bassin Martin |
May |
68 |
||
|
June |
92 |
|||
|
Tampon |
May |
50 |
||
|
June |
151 |
|||
|
Ligne Paradis |
May |
20 |
||
|
June |
175 |
40 |
||
|
July |
56 |
|||
|
Ligne des Bambous |
May |
10 |
25 |
|
|
June |
80 |
|||
|
July |
75 |
|||
|
Cilaos |
August |
100 |
20 |
|
Figure 2 - Dispersion release sites for Olla v-nigrum, Réunion - 1 993-94.
The biological characteristics of O. v-nigrum are very interesting and include good trophic adaptation to the leucaena psyllid, good voracity and fecundity and a development time much shorter than C. coeruleus (Chazeau 1991). Clearly, the species won't solve the leucaena psyllid problem by itself but, in conjunction with other biotic factors such as the influence of indigenous predators, establishment of other natural enemies etc, it may have a significant impact in the regulation of psyllid populations.
Figure 3 - Survey of Olla v-nigrum establishment in Réunion (July-August 1994)
Following the successful establishment of O. v-nigrum in a large distribution area within the island, our efforts will now focus, in collaboration with the FDGDEC, in trying to establish C. coeruleus by releasing this species in areas where releases were not attempted earlier, such as the east side of the island. If necessary, in the future, attempts to introduce one or both parasitoids of the psyllid, Tamarixia leucaenae and Psyllaephagus yaseeni will also be made.
Other studies on leucaena varieties tolerant to H. cubana or substitute species (eg Calliandra calothyrsus, are currently in progress at CIRAD-Foret in Réunion. Together with the results of biocontrol operations, they should contribute in the near future to the establishment of integrated control strategy against the leucaena psyllid on Réunion Island.
Chazeau, J., 1991. Cycle de développement et table de vie d'Olla v-nigrum (Col.: Coccinellidae), ennemi naturel d'Heteropsylla cubana (Horn: Psyllidae) introduit en Nouvelle-Calédonie. Entomophaga, 36:275-285.
Vandeschricke F., S. Quilici, J. Gauvin et Y. Roederer, 1992. Le psylle du Leucaena a la Réunion. Bois et Forêts des Tropiques, 234:47-59.
by
S.S. Madoffe Sokoine
University of
Agriculture
Department of Forest
Biology
P 0 Box 3010, Morogoro, Tanzania
and
A. Massawe
Tanzania Forestry
Research Institute
Silviculture Research
Centre
P.O. Box 95, Lushoto, Tanzania
The periodicity of infestation and damage by leucaena psyllid to Leucaena leucocephala was observed on a monthly basis for about one year in the Mafiga and Cairo agroforestry trials. Serious damage to trees coincided with the dry period of the year and development of new shoots from the pruned trees. Mortality was recorded on some pruned trees in Gairo. Infestation declined gradually and tree growth revived towards the onset of the rainy period.
Large numbers of Coccinellidae, Dragon flies and Hymenopterous species in association with the heavily infested shoots/branches were observed. This may indicated existence of indigenous natural enemies of leucaena psyllid in Tanzania.
* * * * *
The leucaena psyllid, Heteropsylla cubana (Homoptera: Psyllidae), is a native of Central America and feeds almost exclusively on Leucaena leucocephala and, to a lesser extent on closely related mimosoid leguminous trees. Leucaena trees were free from significant pest damage in areas where it was introduced for more than 300 years until in 1984 when the leucaena psyllid was found on leucaena plantings in Hawaii. In its area of origin, leucaena psyllid is not known to cause significant damage to the trees and its populations appear to be held at low levels by natural enemies (USAID 1991, Hydon and Affonso 1991).
From Hawaii, the pest spread rapidly westwards through the Pacific region, reaching Taiwan, Australia, Japan, India and Southeast Asia between 1986 and 1988. Between the years 1991 and 1992 the insect spread to Mauritius, Reunion, Madagascar, Kenya, Tanzania, Uganda and Burundi (Hollis 1992, Van Den Beldt and Napompeth 1992, Reynolds and Bimbuzi 1992). Judging by its recent rapid dispersal, it can be predicted that it will spread rapidly throughout sub-saharan Africa in the near future.
In Tanzania, trials with Leucaena leucocephala were first made in the early 1950s, however, it was not until the mid 1970's when agroforestry research with this species started (Lulandala 1978). In spite of the importance of the tree, (Lulandala and Hall 1987, 1990, Maghembe and Redhead 1981) the current status of the psyllid infestation has not yet been given a serious attention. The extent of damage, the loss it causes to estate crops, animal production and forestry sectors is little or not known and this could be a dilemma to leucaena growers and users. There is no quantitative data on its periodicity and impact to the growth of L. leucocephala and related mimosoids in Tanzania. The effect of indigenous natural enemies is also not known. Since data is lacking for the entire country, appropriate pest management tactics are difficult to develop.
This study was therefore carried out in the Mafiga and Gairo agroforestry trials with two principle objectives:
The information so obtained could be used to develop a basis for pest management control measures.
Field observations were made in the Gairo and Mafiga Agroforestry trials. In these experiments, Leucaena trees and Faidherbia albida (amongst other tree species) were planted in hedgerows and interplanted with agricultural crops. The trials were established in 1978 in Mafiga and 1989 in Gairo. The experimental design for Mafiga has earlier been reported by Lulandala (1979) and Lulandala and Hall (1990). In Gairo, two out of nine existing trials; Leucaena leucocephala for fuelwood and L. leucocephala for fodder production, were used for this study.
MAFIGA - The Mafiga agroforestry estate lies at an elevation of 520 meters above mean sea level the southeastern foot of the Mindu Hills. The total annual rainfall varies between 500 and 1200 mm with a mean of 860 mm. The rainfall pattern is bimodal with short rains from October to December and long rains from March to May (Table 1). Temperatures are high with a somewhat cooler season from May to August. Monthly means of daily temperature maxima vary from 2°C to 34°C and minima from 14°C to 23°C. Generally high wind speeds coincide with the dry seasons. Soils are fine, loamy sands which have developed from alluvial deposits.
GAIRO - The Gairo agroforestry research area lies at an elevation of 1200 meters. Rainfall is unevenly distributed, with one rainy season starting in November and ending in May. The mean annual rainfall (1982 - 1987) is 500 mm. Mean monthly rainfall, temperatures and wind speeds are not available. Soils are predominantly loamy.
ONE YEAR CLIMATIC DATA FOR MAFIGA, MOROGORO, TANZANIA
(August 1993-August 1994)
|
YEAR |
RAINFALL |
MEAN TEMPERATURE |
MEAN WIND SPEED (MILES/DAY) |
|
|
MAX | MIN |
|||
|
1993 |
||||
|
August |
3.2 |
27.2 |
16.0 |
81.4 |
|
September |
2.1 |
29.4 |
16.4 |
105.2 |
|
October |
45.8 |
30.7 |
17.6 |
107.5 |
|
November |
37.4 |
19.5 |
17.5 |
102.2 |
|
December |
0.8 |
33.9 |
21.1 |
44.4 |
|
1994 |
||||
|
January |
54.0 |
32.9 |
21.7 |
161.4 |
|
February |
134.6 |
32.0 |
20.2 |
95.1 |
|
March |
77.4 |
31.0 |
20.5 |
56.3 |
|
April |
168.8 |
29.0 |
19.5 |
52.0 |
|
May |
88.2 |
27.6 |
18.6 |
167.2 |
|
June |
8.2 |
27.6 |
14.3 |
53.1 |
|
July |
37.0 |
27.5 |
15.2 |
68.2 |
|
August |
18.7 |
28.1 |
15.8 |
79.1 |
The periodicity of attack and damage (defoliation, shoot die-back and mortality) caused by the leucaena psyllid on L. leucocephala was systematically observed and recorded along the borders and adjacent to longest middle rows of both pruned and unpruned plots on monthly basis for eleven months; December 1993 - October 1994. In both pruned and unpruned plots, 72 trees were randomly selected in the Gairo trial and 100 hundred trees in the Mafiga trial. The intensity of damage was coded according to four subjective damage classes as follows:
| 1. | No attack: | No psyllid observed and most leaves/twigs looked healthy. |
| 2. | Mild attack: | A few psyllid observed and some black soot had started appearing on leaves/twigs. |
| 3. | Moderate attack: | Several psyllids observed and much more black soot present on the leaves/twigs. |
Invertebrates associated with the psyllid were also observed and some were collected for identification. Presence of leucaena psyllid was also observed in adjacent plots of Faidherbia albida.
The subjective damage ratings for individual trees were pooled to procure the mean damage levels.
The severity of attack by leucaena psyllid intensified soon after the rains in June through October. During the December to May rainy season, minor attack occurred with almost no attack in March, April and May (Tables 2 and 3). These observations indicate an annual cycle in the pattern of psyllid population abundance. Leucaena psyllid exhibits seasonal abundance (Waage 1990) with populations peaking during the cooler and drier parts of the year (Napompeth 1994, Mangoendihordjo et al 1990). As expected, higher wind speed in Mafiga during this period could probably account for high psyllid infestation. It has been postulated that wind velocity during dry seasons escalates the psyllid problem because it aids dispersal of the insect (Mangoendihordjo et al 1990). On the other hand most insects appear to be washed away during periods of heavy rainfall. It appears that trees are also less stressed during this period, an effect which could assist to produce more defensive chemicals (Rhodes 1979).
In the sample plots, nearly all trees were attacked with only minor differences in the degree of damage and period of the year. In both sites, records show that the frequency and time of pruning was analogous. Young flushes of foliage from pruned trees suffered more severe damage than unpruned mature trees, particularly in Gairo. Die-back was a common occurrence in both sites. In extreme cases, entire plants were killed at Gairo.
The differences in the magnitude of attack experienced on the two sites could partly be explained by the differences in rainfall, soil conditions and possibly by differences in levels of indigenous natural enemies. Low rains and relatively poor leucaena growing sites in Gairo could contribute to some stresses in the leucaena crop which consequently creates a favourable habitat for psyllid attack. Gregarious hymenopterous insects, coccinellids, dragon flies, heteropterous insects and snails were found on branches/trees infested with large numbers of psyllids, particularly in the Gairo site. Identification of these organisms was not done due to lack of experts within Tanzania.
The economic impact of leucaena defoliation by the psyllid was not evaluated in this study, however, studies from Indonesia indicate that psyllid infestation has serious effects on leucaena trees, associated crops and animal production (Mangoendihardjo et al 1990). In Tanzania, farmers in Lushoto, where L. leucocephala is widely used in agroforestry have registered their concerns on the future of this important tree (Mngulwi 1994 per. comm.). Many people in the Kondoa District, who were forced to keep their herds of cattle under zero grazing following a successful feeding with leucaena fodder are now facing a dilemma on the future of their herds after the psyllid catastrophe. On the other hand, there is an indication that Faidherbia albida (Mimosoidae) which is another important multi-purpose tree, is also attacked by the same psyllid in Morogoro. Who knows which other mimosoids which are widely grown within the county will also be attacked.
The intensity of damage caused by the psyllid to leucaena needs special attention. The most important relationships between the pest and their hosts that are essential to the development of appropriate control measures are not well known in Tanzania. Use of natural enemies, particularly of native origin, development of highly resistant leucaena strains and use provenances best suited to sites peculiar to Tanzania have all been suggested as components of an integrated pest management (IPM) system for this insect. Use of chemicals should be discouraged since the foliage is widely used as a source of nutritious fodder for livestock.
MONTHLY CHANGES IN SEVERITY OF ATTACK OF LEUCAENA TREES
BY LEUCAENA PSYLLID IN THE MAFIGA AGROFORESTRY PLOTS
J A S O N D J F M A M J
|
No attack |
- XXX--XXX |
|
Mild attack |
- XXXXXXXX - |
|
Moderate attack |
- |
|
Severe attack |
XXXXXXXX - - XX |
MONTHLY CHANGES IN SEVERITY OF ATTACK OF LEUCAENA TREES
BY LEUCAENA PSYLLID IN THE GAIRO AGROFORESTRY PLOTS
J A S O N D J F M A M J
|
No attack |
- XXXXXX |
|
Mild attack |
- XXXXX |
|
Moderate attack |
XX - XX XX |
|
Severe attack |
XXXXXXXX |
Funnasaki G.Y., Lai, Po-Yung and L. Nakahara 1990. Status of natural enemies of Heteropsylla cubana Crawford (Homoptera: Psyllidae) in Hawaii. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 153-158.
Heydon, D., and M. Affonso. 1990. Economic review of psyllid damage on leucaena in Southeast Asia and Australia. A report prepared for the Australian International Development Assistance Bureau (AIDAB), CAB International Development Services, Wallingford, Oxford, U.K., 129 pp.
Hollis, D. 1992. Leucaena psyllid, Heteropsylla cubana Crawford, newly recorded on Mauritius and Reunion. FAO Plant Protection Bulletin 40:4250.
Lulandala, L.L.L. 1 979. The establishment techniques of Leucaena leucocephala: Effects of different treatments on the germination, survival and growth performance. MSc. Diss. UDSM, Tanzania.
Lulandala, L.L.L. and J.B. Hall. 1990. Nutrient removals in harvesting of leucaena hedgerows at Mafiga, Morogoro, Tanzania. Forest Ecology and Management, 35:207-216.
Maghembe, J.A. and J.F. Redhead, 1981. Agroforestry: Preliminary results of intercropping Acacia, Eucalyptus and Leucaena. In: B.J. Ndunguru and C.L. Keswani (eds.) IDRC/UDSM.
Mangoendihardjo, S., F.X. Wagiman, J.A. Trisyono and M. Sujono. 1990a. Seasonal abundance of leucaena psyllid populations in Yogyakarta, Indonesia. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 111-113.
Mangoendihardjo, S., F.X. Wagiman, A. Sulthoni and Subyanto. 1990b. Economic impact of leucaena psyllid infestation on estate crops and teak forest plantation. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 184-188.
Napompeth, B. I990. Leucaena psyllid problems in Asia and Pacific. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 1-11.
Napompeth, B., 1994. Leucaena psyllid in the Asia Pacific Region: Implications for its management in Africa. FAO RAPA Publication 1994/13, Bangkok. 27 pp.
Piliai, S.R.M. and K.C. Gopi., 1993. Progress of leucaena psyllid (Heteropsylla cubana Crawford) in peninsular India. In IUFRO Symposium on impact of diseases and insect pests in Tropical Forests, Peechi, Kerala, India.
Rhodes, D.F., 1979. Evaluation of plant chemical defense against herbivores. In G.A. Rosenthal and D.H. Janzen eds., Herbivores: Their interaction with secondary plant metabolites. Academic Press, New York, pp 3-54.
Reynolds, L. and S. Bimbuzi, 1992. Leucaena psyllid arrives in Kenya. Agroforestry Today 4:2.
USAID, 1991. Environment Assessment of the classical biological control of the leucaena psyllid in Indonesia, Laos, Malaysia, Nepal, Philippines and Thailand. USAID Report, Washington DC.
Van Den Beldt, J. and B. Napompeth, 1992. Leucaena psyllid comes to Africa. Agroforestry Today 4:1 2.
Veeresh, G.K., 1990. The status of leucaena psyllid, Heteropsylla cubana, Crawford in India. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 14-16.
Villacarlos L.T., R.V. Pagnilawan and R.P. Robin, 1990. Factors affecting leucaena psyllid population in Leyte, Philippines. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 122-129.
Waage, J., 1990. Exploration for biological control agents of leucaena psyllid in tropical America. In Napompeth, B. and K.G. MacDicken, eds. Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand pp. 144-152.
by
G.D. Tribe
Plant Protection
Research Institute, Ryan Road, Rosebank,
Cape Town 7700, Republic of South Africa.
Leucaena leucocephala is known to have been in South Africa at least since early in the century and is regarded as a valuable tree. Yet its ability to form dense thickets in certain areas and its large seed bank indicates its potential as a weed problem. The proposed release of a seed-feeding predator, Acanthoscelidus macrophthalmus (Brucidae) from Mexico will serve to curb the invasiveness of the tree without impairing any of its useful attributes. Seed required for propagation can be protected from the brucid by simple means. The leucaena psyllid, Heteropsylla cubana, is not yet present in South Africa but is expected within the next few years. A second biological control programme will then become necessary to control the psyllid.
* * * * *
The genus Leucaena has been in South Africa for more than 70 years and cannot be regarded as invasive when compared with such exotics as the Australian Acacia species (Kruger 1991). Environmental constraints, especially climatic restrictions, are regarded by Fenn (1987) and Underwood (1986) as arguments against the invasiveness of L. leucocephala in South Africa. The poor competitive ability of seedlings and the fact that exceptional yields can only be expected under irrigation or where the rainfall exceeds 800 mm p.a. (Kruger 1991) would restrict any invasive potential only to areas of optimum growth for L. leucocephala. Only two areas in South Africa were identified by Underwood (1986) where Leucaena colonized areas beyond the original area of planting and in both cases these were at an altitude below 300 m and a rainfall in excess of 900 mm per annum.
But vigorous growth of L. leucocephala does occur on disturbed land where little competition exists (Kruger 1991). Weed scientists are particularly concerned about the vast quantities of viable seed produced by L. leucocephala and the steady densification of the leucaena thickets (Neser 1994). For this reason leucaena is regarded by the weed scientists as a potential weed problem whose invasiveness should be curbed before it is propagated more widely (Neser 1994).
A seed-feeding bruchid has been imported into South Africa to combat the potential invasiveness of leucaena but has yet to be released. The arrival of the Leucaena psyllid into South Africa will necessitate the implementation of a biological control programme to counter its destructiveness. Thus a unique situation may occur where two biological control programmes concerning the same plant species may eventually be running simultaneously; one to protect the tree from the psyllid and the other to curb its invasive potential.
The value of leucaena in South Africa is undisputed and all efforts will be made to protect it against the leucaena psyllid when it arrives in South Africa. Where the controversy lies is in whether the tree is to be regarded as potentially invasive or not. Should leucaena become invasive in South Africa this is likely to occur along the eastern escarpment and coastal regions where the mean annual rainfall is above 800 mm. This would be a belt starting from a narrow base in the George area and widening with increasing latitude into the Drakensberg mountains of Natal and the eastern Transvaal.
The proposal by the weed scientists is to import a specific seed predator which will curb the potential invasiveness of the tree without affecting its value in any way (Neser 1994). The brucid Acanthoscelidus macrophthalmus collected by Dr Neser in Mexico in 1989 is just such a specific seed predator. Since 1989 the brucid has been undergoing host specificity tests in quarantine in Pretoria (Neser 1994). The effect of this brucid will be to reduce the seed bank formation and the consequential ability of the tree to form dense thickets. Where seeds are required for propagation purposes, bunches of near mature pods can be covered with a sleeve to protect them from insect attack (Neser 1994).
Further parasites could also be introduced to reduce the invasiveness of the trees by preventing the formation of pods altogether, which could be doubly advantageous in the sense that more of the plants resources may be channelled to vegetative growth, rather than to seeds (Neser, pers. comm.) Curbing the potential invasiveness of leucaena at this early stage would make general planting of the tree more acceptable.
Such specific seed-feeding predators could benefit other countries such as the Taiwanese islands where leucaena has colonized much of the area (Söhnge 1994). Any enquiries about the brucid seed predator can be directed to Dr. Stefan Neser of the Plant Protection Research Institute, Private Bag X134, Pretoria 0001. [Fax: (021) 329-3278].
Fenn, T.J., 1987. Leucaena - friend or foe? Department of Development Aid, P.O. Box 384, Pretoria, 0001.
Kruger, A.J. and D. Grossman, 1991. The potential of the genus Leucaena in relation to its biology and possible environmental risks. In Koen, J.H. (ed), African Agroforestry: Emphasis on Southern Africa. A collaborative report of the Forest Biome Group, pp 46 -56.
Neser, S., 1994. The Leucaena controversy. Plant Protection News 36: 8.
Söhnge, H., 1994. The greening of China ................. and of Taiwan. Arbor 1(4): 6-7.
Underwood, M., 1986. Leucaena leucocephala - a reconnaissance survey of its occurrence in South Africa; with some comments in the broad context of the existing literature. Agricultural and Rural Development Research Institute, Fort Hare, Ciskei.
by
C.K.P.O. Ogol
Zoology Department,
Kenyatta University
P.O. Box 43844, Nairobi, Kenya
The leucaena psyllid, Heteropsylla cubana Crawford, was first reported on the Kenya coast in August 1992. Without its major natural enemies to limit its population growth, this pest has since spread throughout the country wherever leucaena is grown and seriously threatens the continuous cultivation of this valuable multipurpose tree species.
Like with most exotic pest species, it was expected that H. cubana populations would be attacked by local or indigenous generalist natural enemies (predators, pathogens and/or parasitoids). Thus, an attempt was made to find and identify these natural enemies (with the exception of pathogens) at Mtwapa on the Kenya coast and Machakos in eastern Kenya.
A study was established in an experimental trial with seven treatments and five replications located at the Kenya Agricultural Research Institute (KARI) field station at Mtwapa on the Kenya coast. During each second week of the months of June to September 1994, 2160 leucaena plants were observed for any predators feeding on leuceana psyllid. All such predators were preserved in 70% alcohol. these were then taken to the Department of Invertebrate Zoology of the National Museums of Kenya for identification. Similar observations were made during September 1994 on 450 leucaena plants located at the International Centre for Research in Agroforestry (ICRAF) station at Machakos in eastern Kenya.
Concurrently, a terminal shoot with three standard open leaves and containing infestations of leucaena psyllid, was carefully cut from 300 and 100 randomly selected leucaena plants at Mtwapa and Machakos respectively. The leaves were placed individually in glass vials which were then plugged with cotton. These were then observed over a period of 10 days for the emergence of any parasitoids.
In order to monitor the population fluctuation of H. cubana, at four week intervals after pruning of leucaena trees (eg the second week of May 1994), 10 plants were randomly selected from each of the treatment plots. A terminal shoot with three open leaves was carefully cut from each plant and placed in a plastic vial containing 70% alcohol. In the laboratory, the contents of each vial were transferred into a petri dish and the number of psyllid nymphs and eggs was counted under a stereo microscope. This was repeated every four weeks until September when there were no suitable "standard" shoots to sample as a result of cumulative psyllid damage.
Eight species of Coccinelids were found feeding on the leucaena psyllid. Six of these were found at Mtwapa and two at Machakos. None were common to both sites (Table 1). In addition, various unidentified species of spiders (Aranae) were found at both sites.
Of the known species of Coccinelids, Chielomenes lunata, C. sulphurea and Miraspis striata have all been found to be predators of the cassava mealybug, Phenococcus manhoti, in central and western Africa (Neuenschwander et al 1987) while Chilocorcus distigma has been reported as a predator of the sugarcane scale, Aulacaspis tagalensis, in Kenya (KARI 1986). This is probably an indicator of the generalist nature of these predators.
A superimposition of the coccinelid population data over the psyllid population data (Fig 1) shows that the predator population first rises in response to a rising prey population and falls in response to a falling prey population. This is in agreement with the model of Southwood (1975) where the overall response of predators to prey population density is sigmoid.
INDIGENOUS PREDATORS OF HETEROPSYLLA CUBANA IN KENYA
|
PREDATOR |
LOCATION |
Micraspis striata F |
Mtwapa |
Chilocorcus distigma Klug |
Mtwapa |
Cheilomenes sulphurea (Oliv) |
Machakos |
Cheilomenes lunata F |
Machakos |
Coccinellidae sp 1 |
Machakos |
Coccinellidae sp 2 |
Mtwapa |
Coccinellidae sp 3 |
Mtwapa |
Coccinellidae sp 4 |
Mtwapa |
Aranae |
Mtwapa/Machakos |
No parasitoids were recovered from the field samples of H. cubana. Other than from the countries of origin (Mexico, Central America and the Caribbean), surveys for natural enemies have failed to detect local parasitoids which attack leucaena psyllid.
In a survey carried out between October 1985 and July 1986 of the indigenous natural enemies of the citrus psyllid, Trioza erytrea, the vector of citrus greening disease in Kenya (Ogol, Unpublished final report on the ICIPE -FAO project on biological control of the vectors of citrus greening disease in Kenya, 1986), five parasitoids and two hyperparasites were found. The parasites include Tamarixia sp, near T. dryi, Waterson, Tamarixia sp 2, T. sicarius Silvestri (Hymenoptera: Eulophidae), Aphanogamus triozae Dessart
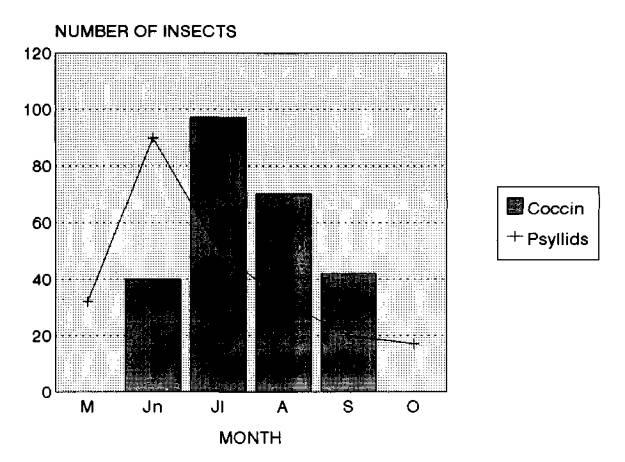
Figure 1 - Coccinellid/leucaena psyllid population fluctuation. (Psyllid populations are expressed as number per shoot and coccinellids are expressed as numbers per 2160 plants.
(Hymenoptera: Ceraphronidae) and Dilyta sp (Hymenoptera: Encyrtidae). S. cassatus is a known hyperparasite of various psyllids in eastern and southern Africa. It should be noted however that the citrus psyllid does not occur along the Kenya coast, including Mtwapa, where this study was conducted.
No surveys were carried out for insect pathogens of H. cubana because of the complexity of such studies with regards to equipment and expertise. Nevertheless, fungal entomopathogens of the leucaena psyllid have been found elsewhere. These include Entomophthora sp, Hirsutella thomsoni Fisher and Conidiobolus coronatus (Constantin) Butko, in Thailand (Napompeth 1990), Hirsutella citriformis, Fusarium sp, Entomophthora sp and Paecilomyces farinosus from the Philippines and Beauvaria bassiana, Metarhizum anisopilae var anisopilae and Paecilomyces javanicus from Taiwan (Yao et al 1990).
In Kenya, Entomophthora sp and Hirsutella sp have been isolated from the cassava green spider mite, Mononychellus sp (Tetranicidae) while Beauvaria bassiana has been isolated from lepidopteran pests (Bartkowski et al 1987).
Bartikowski, J., M.O. Odindo and W.A. Otieno, 1987. Some fungal pathogens of the cassava green spider mite, Mononychellus spp (Tetranichidae) in Kenya. Insect Science and its Application, 9: 457-459.
Kenya Agricultural Research Institute, 1986. Entomology and biological control. in Record of Research, Annual Report 1982, KARI, Nairobi, Kenya, pp 107-116.
Napompeth, B., 1990. Leucaena psyllid problems in Asia and the Pacific. in Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 1-11.
Neuenschwander, P., R.D. Hennessey and H.R. Herren, 1987. Food web of insects associated with the cassava mealybug, Phenococcus manihoti Matile Ferrere (Hemiptera: Pseudococcidae), and its introduced parasitoid, Epinocarsis lopezi (DeSantis) Hymenoptera: Encyrtidae) in Africa. Bulletin Entomological Research 77:177-187.
Southwood, T.R.E., 1975. The dynamics of insect populations. in Insect Science and Society, Academic Press, New York, pp 151-199.
Villacarlos, L.T., R.V. Pagnilawan and R.R. Robin, 1990. Factors affecting leucaena psyllid populations in Leyte, Philippines. in Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 122-129.
Yao, A.L., Y.S. Hwang and W.Y. Wang, 1990. Natural enemies and biological control of Heteropsylla cubana on Leucaena leucocephala in Taiwan. in Leucaena Psyllid: Problems and Management. Winrock International/IDRC/NFTA/F-FRED, Bangkok, Thailand, pp 162.
by
Roger Day, Garry Hill, Sean Murphy and
Brigitte Nyambo
International
Institute of Biological Control,
Nairobi Kenya and
Silwood Park, UK
Classical biological control is one of two major strategies appropriate for controlling leucaena psyllid. In Asia and the Pacific, two coccinellid predators, Curinus coeruleus Mulsant and Olla v-nigrum (Mulsant)) and two hymenopterous parasitoids, Psyllaephagus vaseeni Noyes and Tamarixia leucaenae Boucek, have been used in biological control programmes. Various considerations in selecting candidates for possible introduction to Africa are reviewed. These include the impact of natural enemies in the pest's area of origin, the biology of the four main candidates, an assessment of previous biological control attempts against leucaena psyllid and other psyllids, host specificity and multiple introductions, and an assessment of the potential risks involved. Following a review of the available information, it is suggested that the two specific parasitoids, P. vaseeni and T. leucaenae, be introduced and evaluated. The need to import further agents should be considered after assessing the impact of these and indigenous natural enemies on H. cubana populations.
* * * * *
Biological control is widely recognised as one of the two preferred strategies for controlling the leucaena psyllid, Heteropsylla cubana Crawford (Homoptera: Psyllidae), (Heydon & Affonso 1989, Napompeth 1990a, Napompeth 1994, USAID 1991), the other being host plant resistance. As the psyllid is exotic to Africa, classical biological control, the introduction of exotic natural enemies, is the most appropriate form of biocontrol. This has been implemented in many countries in Asia and the Pacific, and there are now plans for its implementation in Africa.
As several different natural enemies have been used in biological control programmes for H. cubana, and many more have been identified in the pest's area of origin, decisions must be made on which, if any, natural enemies should be imported to Africa. Consensus amongst affected countries on the strategy to be pursued would be desirable, particularly as introduced agents are likely to spread across borders.
In this paper we first review the known natural enemies of leucaena psyllid, particularly those in its area of origin. We summarise the biology of four species that have been used in previous biological control programmes, and which are therefore being highlighted as candidates for introduction into Africa.
The various factors that are taken into consideration when selecting candidate agents for biological control programmes are discussed with particular reference to the four most likely candidates. Finally we propose a strategy for the introduction of biological control agents of Leucaena psyllid into Africa.
Heteropsylla cubana originates in the tropical American region (Muddiman et al 1992), where Leucaena leucocephala (Lam.) de Wit also originates (Brewbaker 1987). As part of the biological control programme for H. cubana in Asia, the International Institute of Biological Control (IIBC) and collaborating institutes made surveys and collections of natural enemies of H. cubana in 13 countries in the region: Trinidad & Tobago, Barbados, Dominican Republic, Haiti, Cuba, Jamaica, Mexico, Belize, Guatemala, Honduras, Costa Rica, Colombia and Guyana (Baker et al. 1993).
Predatory beetles collected in the survey are shown in Table 1, and other predatory taxa in Table 2. Coccinellids are the predominant group of predators, and include Curinus coeruleus Mulsant (Coleoptera: Coccinellidae) and Olla v-nigrum (Mulsant) (Coleoptera: Coccinellidae), two exotic species found preying on H. cubana in Hawaii and since used as biological control agents, although they were originally introduced to Hawaii to control other pests.
Of the parasitoids recovered (Table 3), Psyllaephagus yaseeni Noyes (Hymenoptera: Encyrtidae) was a new species (Noyes 1990), previously referred to as P. sp. nr rotundiformis. Tamarixia leucaenae Boucek (Hymenoptera: Eulophidae) was also a new species described from the surveys (Boucek 1988). It is likely that this is the species collected by the Hawaii Department of Agriculture in 1985, and recorded as Tamarixia triozae ( = Tetrastichus triozae) (Dr. R. Burkhardt, pers. comm., Waage 1990).
The other Tamarixia species recovered during surveys may be hyperparasitic. It is not known whether the encyrtid, Sectiliclava sp., attacks H. cubana in Mexico, though some species of this genus are known to be parasitoids of adult psyllids. Further work is required before the parasitoid complex on H. cubana can be described fully.
During the surveys only one fungus (Entomophthora sp.) was found attacking H. cubana, though several have since been found in other parts of the world.
In all areas where H. cubana has been studied in the field, generalist predators of various types have been found preying on it. The predators include coccinellids, mantids, several families of predatory Hemiptera, Neuroptera, staphylinids, syrphids, asilids, vespids, formicids, Odonata and Araneae. Amongst the coccinellids, Menochilus spp., frequent predators of aphids, are reported from several countries in Asia.
In Hawaii an interesting situation has occurred, where the coccinellid Curinus coeruleus has become abundant following the appearance of H. cubana (Funasaki et al 1990, Napompeth 1994). This species was imported to Hawaii along with other predators and parasitoids in 1922 (Fullaway 1923) for control of coconut mealybug, Nipaecoccus nipae (Maskell), (Clausen 1978). Although it established, any effect it had on mealybug populations was overshadowed by the introduction of an encyrtid parasitoid, Pseudaphycus utilis, which reduced populations to very low levels in a year.
PREDATORY COLEOPTERA ASSOCIATED WITH HETEROPSYLLA CUBANA ON LEUCAENA LEUCOCEPHALA. (FROM BAKER ET AL 1993).
|
Family |
Countries |
|
Coccinellidae |
|
|
Curinus coeruleus |
Trinidad & Tobago, Colombia |
|
Cycloneda sanguinea |
Mexico, Haiti |
|
Olla v-nigrum |
Mexico, Trinidad & Tobago |
|
Olla sp. nr. v-nigrum |
Trinidad & Tobago |
|
Hippodamia convergens |
Mexico |
|
Coleomegilla maculata |
Mexico, Cuba |
|
Brachiacantha laevis |
Mexico |
|
Procula douei |
Jamaica |
|
Chilocorus cacti |
Jamaica |
|
Exochomus jamaicensis |
Jamaica |
|
Hyperaspis sp. ?distinguenda |
Trinidad & Tobago |
|
Hyperaspis sp. |
Trinidad & Tobago |
|
Coccinellina emarginata |
Trinidad & Tobago |
|
Chnoodes terminals |
Trinidad & Tobago |
|
Nephus sp. nr. flavifrons |
Trinidad & Tobago |
|
Nephus spp. |
Trinidad & Tobago, Mexico |
|
Diomus spp. |
Trinidad & Tobago, Jamaica |
|
Scymnus sp. |
Jamaica |
|
Staphylinidae |
|
|
Euwira sp. |
Mexico |
The coccinellid Olla v-nigrum (= O. abdominalis) was also imported to Hawaii from Mexico earlier this century, for the control of scale insects (Napompeth 1994). This species has also become more abundant since the arrival of H. cubana, though not to the same extent as C. coeruleus (Funasaki et at 1990).
Pan (1990) reported a parasitic wasp attacking H. cubana in Taiwan, but no further details were given. In many other countries, surveys for natural enemies have failed to find local parasitoids attacking leucaena psyllid. This is not surprising for the following reason. Parasitoids that attack more than one host species often attack only closely related species. But as Heteropsylla is a New World genus (Muddiman et al 1992), there are no close relatives of H. cubana indigenous to Asia, the Pacific or Africa which might have supported such parasitoids prior to the arrival of H. cubana.
OTHER PREDATORY TAXA
ASSOCIATED WITH
H. CUBANA ON L. LEUCOCEPHALA. (FROM BAKER ET AL
1993).
|
Family Species |
Country |
|
Sryphidae |
|
|
Allograpta exotica (Weidemann) |
Trinidad & Tobago |
|
Gen. & sp. indet. |
Mexico |
|
Chrysopidae |
|
|
Ceraeochrysa sp. |
Trinidad & Tobago |
|
Gen. & sp. indet. |
Mexico |
|
Miridae |
|
|
Neurocolpus sp. |
Mexico |
|
Rhinacloa forticornis Reuter |
Mexico |
|
Genus & sp. indet. |
Trinidad and Tobago |
|
Anthocoridae |
|
|
Orius ?tristicolor (White) |
Mexico |
|
Phymatidae |
|
|
Phymata parva Handlirch |
Mexico |
|
Reduviidae |
|
|
Sinea undulata Uhler |
Mexico |
|
Araneidae |
|
|
Araneus theisi (Walckenaer) |
Mexico |
|
Thomisidae |
|
|
Misumenops sp. |
Mexico |
|
Salticidae |
|
|
Gen. & sp. indet. |
Mexico |
PARASITOIDS ASSOCIATED WITH H. CUBANA ON L
LEUCOCEPHALA
(FROM
BAKER ET AL 1993).
|
Family |
Countries |
Comments |
|
Encyrtidae |
||
|
Psyllaephagus yaseeni |
Trinidad, Mexico, Cuba, Haiti, |
P |
|
Jamaica, Puerto Rico1, Barbados? |
||
|
Sectiliclava sp. ?nov. |
Mexico |
P |
|
Eulophidae |
||
|
Tamarixia leucaenae2 |
Trinidad, Mexico, Barbados, Florida1 |
P |
|
Tamarixla sp. indet. |
Cuba, Jamaica, Haiti |
P |
|
Tamarixia sp. A |
Puerto Rico1 , Mexico |
H? |
|
Signiphoridae |
||
|
Signiphora ?unifasciata |
||
|
Ashmead |
Mexico, Puerto Rico1 |
H |
P = Primary parasitoid, H = hyperparasitoid
Pathogenic fungi have been found attacking H. cubana in several countries. Napompeth (1990b) reported Conidiobolus coronatus and Entomophthora sp. to be the most abundant in Thailand, and Mangoendihardjo, Wagiman, Trisyono & Sujono (1990) recorded 60-80% mortality to H. cubana in the rainy season in Indonesia, caused by an unidentified fungus. In the Philippines Fusarium sp. and Entomophthora sp. are found (Villacarlos et al 1990), but epizootics have only been observed in humid areas, though at those sites pathogens are thought to be a major mortality factor. Beauvaria bassiana, Metarhizium anisopliae, and Paecilomyces javanicus have been recorded in Taiwan (Yao et al 1990).
As with many exotic pests, on arrival in a new area H. cubana has thus become prey to a range of generalist predators and pathogenic fungi, but not to parasitoids. This contrasts markedly with the situation in tropical America, the pests area of origin, where there are several parasitoids that specialise on Heteropsylla species. One approach to classical biological control of such pests is to try to rebuild in the pest's area of invasion, the natural enemy complex found in the pest's area of origin. For H. cubana this would indicate that parasitoids should be selected for introduction.
In Africa it can be expected that, as was observed in Asia, a number of generalist predators, particularly coccinellids, will utilise the new resource. Some species of coccinellids such as Exochomus sp. are already commonly found feeding on H. cubana (personal observation).
Curinus coeruleus and O. v-nigrum are typical coccinellids, capable of feeding on a range of Homopteran prey species, as demonstrated by the fact that they were originally imported to Hawaii for the control of mealybugs and scale insects. C. coeruleus has also been imported to Bermuda for control of cedar scales Carulaspis minima and Lepidosaphes newsteadi (Clausen 1978), and in the Philippines has been found feeding on several different families of Homoptera (Villacarlos & Robin 1992). O. v-nigrum has been reported feeding on cereal aphids in Argentina (Botto et al 1979), pecan aphids in USA (Tedders 1978) and whitefly in USA on cotton and soybean (Watve & Clowe 1976).
Rauf et al (1990) studied C. coeruleus in the laboratory, and found a generation time of 51 days, with 190 eggs produced per generation. Napompeth and Maneeratana (1990) made similar studies using H.cubana and Aphis gossypii as prey items. C. coeruleus performed marginally better on H. cubana, with a net reproductive rate (R0) and generation time of 26 and 61 days respectively, compared to 17 and 64 days with A. gossypii as prey. Basic developmental biology of the predator has also been studied in the Philippines (Villacarlos & Robin 1992), India (Jalali & Singh 1992) and elsewhere in Indonesia (Hardi et al 1988).
O. v-nigrum has been less intensively studied. At 25-26°C, Chazeau et al. (1991) reported R0 of 201.4, the innate capacity of increase was 0.1 65, and the mean generation time 32.2 days. In New Caledonia O. v-nigrum is parasitised by a phorid, Phalacratophora quadrimaculata (Disney and Chazeau 1990).
T. leucaenae is a solitary ectoparasitoid of the nymphal stages of leucaena psyllid. The eggs are usually laid behind the hind coxae of the psyllid, where the feeding also occurs. Although it will attack host instars 2 to 5, there is a marked preference for instars 3 and 4. Oviposition by T. leucaenae appears to inhibit further nymphal development (Patil et al 1993), a common feature of ectoparasitoids.
P. yaseeni is a solitary endoparasitoid. It usually attacks host instars 1 and 2, which continue development until instar 5 when they mummify.
In neither species are many eggs laid on the first day after emergence, but egg laying increases thereafter, and both species host feed (Patil et al 1993). These observations indicate females are synovigenic.
In the laboratory, only 1.25% of a sample of 320 emergences of T. leucaenae were male, and cultures were maintained using unmated females, so the species is assumed to be thelytokous (Patil et al 1993). In P. yaseeni. the sex ratio was approximately equal for eggs laid in first instar hosts, but in 2nd instars 88% were male (Patil et al 1993), an interesting observation as in arrhenotokous species usually more female eggs are laid in larger hosts.
BIOLOGICAL DATA FOR P. YASEENI AND T. LEUCAENAE
AT 25°C
(FROM BAKER ET
AL. 1993, PATIL ET AL. 1993).
|
VARIABLE |
P. YASEENI |
T. LEUCAENAE |
|
Instars attacked |
1 and 2 |
3 and 4 |
|
Adult longevity (days, honey provided) |
17.9 |
10.6 |
|
Development time (egg to adult) |
21.9 |
12.8 |
|
Net reproductive rate (Ro) |
192.9 |
71.2 |
|
Mean Generation time (days) |
28.0 |
18.1 |
|
Innate capacity for increase (rm) |
0.188 |
0.236 |
|
Population doubling time (days) |
3.9 |
2.9 |
|
Finite rate of increase (female/female/day) |
1.207 |
1.264 |
Biological data for T. leucaenae and P. yaseeni are given in Table 4. Although P yaseeni is more fecund, giving a net reproductive rate of around three times that of T. leucaenae, T. leucaenae has a higher capacity for increase because it has a shorter development time, and is thelytokous.
Both parasitoids are much more host specific than the two coccinellids. Where their biology is known, all species of Psyllaephagus are primary endoparasitoids or hyperparasitoids of nymphs of Psyllidae (Noyes 1990). In host specificity tests conducted prior to its introduction to Hawaii, P. yaseeni only attacked psyllids in the genus Heteropsylla (H. huasachae Caldwell and H. fusca Crawford) (Murai 1986). Four indigenous psyllids (Trioza ohiacola Crawford, Hevaheva sp., Megatrioza sp. and Kuwayama minuta Crawford) and two other exotic species (Leptynoptera sulfurae Crawford and Psylla uncatoides (Ferris & Klyver) were all rejected as hosts.
Tamarixia was previously regarded as a synonym of Tetrastichus, which included parasitoids attacking several different orders. However, the genus has been reinstated (Graham 1987) and includes species attacking the family Psyllidae and the closely related family Triozidae (Boucek 1988). No host specificity tests of T. leucaenae have been reported, but during surveys for natural enemies in tropical America, it was only ever found on Heteropsylla spp. (H. cubana, H. huasachae, H. aurantiaca and H. boquetensis).
HOST SPECIFICITY - When several biological control agents are available, a major criterion used for choosing the best ones is their degree of host specificity. Host specific natural enemies are preferred to generalist species for two main reasons.
MULTIPLE INTRODUCTIONS AND COMPETITION - A decision that must be made in biological control programs where there are several natural enemies available is whether two or more species should be introduced. It is not uncommon for more than one species to be introduced (Keller 1984), and competition between agents has been demonstrated in such situations in theory as well as in practice (DeBach 1966). This can lead to the competitive exclusion of one species, but this is not necessarily a drawback. The important question is whether the introduction of two or more species results in less depression of the pest population than if fewer species were introduced. Evidence for negative interactions of this type is rare.
Baker at al. (1993) investigated the potential for competition between T. leucaenae and P. yaseeni. In laboratory experiments they found multiparasitism (i.e. attack by both species on the same host individual) could occur, with T. leucaenae generally being the more effective competitor. Behavioral observations suggested T. leucaenae may avoid searching on plants previously exposed to P. yaseeni. However, as Baker et al. (1993) point out, the situation in the field may be very different, with the different parasitoids occupying slightly different habitats, or favouring different host densities. Observations on the two species in the field in tropical America support this suggestion. An interesting parallel is provided by a study of the bionomics of the psyllid Trioza erytreae in South Africa (Catling 1 969). There it was found that parasitism was an important factor limiting populations and that while Tamarixia radiata was usually the main parasitoid, there were times when Psyllaephagus pulvinatus was important.
Competition is also a possibility between introduced and indigenous natural enemies. Again this is only a concern if it results in poorer control of the target pest. Competition is most likely between species occupying similar niches, and so introduction of specific parasitoids to a system where these are absent would be unlikely to result in competition between species.
There are several countries where more than one natural enemy has been introduced (intentionally or otherwise) for H. cubana control, but there are no reports of competition reducing control. However, in the absence of detailed studies of the impact of any of the natural enemies, it is perhaps unlikely that competition would have been detected even if it is occurring.
Although H. cubana sometimes becomes locally abundant in its area of origin, damage levels never reach those observed in Asia (McClay 1990). This is a common situation with exotic pests, and the explanation often given is that this is because the pest has "left behind" the natural enemy complex that controls it in its natural environment.
Detailed studies of the impact of natural enemies on H. cubana populations in its area of origin have not been conducted. In the surveys, coccinellids were sometimes locally abundant, but did not appear to be a limiting factor to H. cubana populations (Waage 1990), and in Mexico no single species was strongly associated with H. cubana, with C. coeruleus only collected once (McClay 1 990). Predatory syrphids, wasps and ants appear to be important sometimes. Parasitoids were regularly associated with the psyllids, and T. leucaenae appeared to be more widespread and cause more mortality than P. yaseeni (Baker et al 1993). Both were found on apparently recent H. cubana infestations, suggesting that they are good at locating hosts (McClay 1990). Of 22 sites where parasitoids were found (Trinidad 3, Mexico 1 7, Costa Rica 1, Honduras 1), both parasitoids occurred at 10 sites, P. yaseeni alone at 3 sites, and T. leucaena alone at 9 sites. This suggests the possibility of temporal, spatial or habitat-related differences in distribution, and would lend weight to the argument that both species should be introduced to Africa.
Baker et al. (1993) conducted a short field study on a population of H. cubana in Trinidad. They concluded that combined predation by ants, wasps and syrphids was significant, though an exclusion experiment failed to demonstrate any impact of ants alone. T. leucaenae was present, but P. yaseeni was not, and no evidence was found that the parasitoid was contributing to the unexplained fluctuations of psyllid numbers.
Evidence that natural enemies are controlling H. cubana in tropical America is thus poor, though assessing their effectiveness at low pest densities can be difficult. Thus the lack of evidence does not mean natural enemies are not important, and McClay (1990) suggested that although they clearly do not prevent quite rapid increase of H. cubana under suitable conditions, natural enemies may prevent the very high populations observed in Asia from occurring.
Previous experience with biological control of a pest is often used as the starting point for programmes in other areas. Table 5 shows which natural enemies have been imported and introduced to which countries.
The results of releasing a biological control agent can be assessed in several ways, including establishment, dispersal and impact of the natural enemy on the pest population.
DATE OF IMPORTATION
OR RELEASE OF NATURAL ENEMIES
FOR BIOLOGICAL CONTROL OF H. CUBANA1
|
COUNTRY |
C. COERULEUS |
0. V-NIGRUM |
P. YASEENI |
T. LEUCAENAE |
|
Cambodia |
?6 |
|||
|
China |
1992 |
19925 |
||
|
Guam |
1986 |
|||
|
Hawaii |
19222 |
19083 |
1985 |
19854 |
|
India |
1988 |
|||
|
Indonesia |
1986 |
1986 |
1990 |
|
|
Laos |
?6 |
|||
| Malaysia | ?6 | |||
|
Mauritius |
19924 | |||
|
Myanmar |
1988 |
|||
|
Nepal |
1989 |
1991 |
19925 |
|
|
New Caledonia |
1991 |
1987 |
1991 |
|
|
Papua New |
1986 |
?6 |
||
|
Guinea |
||||
|
Philippines |
1986 |
|||
|
Reunion |
19925 |
19925 |
||
|
Saipan |
1986 |
|||
|
Samoa |
1986 |
|||
|
Sri Lanka |
1991 |
19916 |
||
| Tahiti |
? |
|||
|
Taiwan |
||||
|
Thailand |
1987 |
1989 |
1987 |
19925 |
|
Tonga |
? |
|||
|
Vietnam |
1987 |
1 A gap in the table indicates either that the natural enemy has not been imported,
or that no information has been found. (Note that dates from different sources are
not always consistent, due to repeated imports and/or releases, and confusion
between import and release dates. Where different dates have been reported, the
earliest is given in the table.)2 Released for control of mealybugs.
3 Released for control of scale insects
4 Imported but not released due to culturing difficulties
5 Imported but no information on release
6 Arrived unassisted
? Date of import/release unknown
Unfortunately much of the information concerning the effect of the programmes against H. cubana is either anecdotal or consists of general observation. To the best of our knowledge there are no published studies in which the objective has been to demonstrate or quantify the impact of the natural enemies using manipulative methods (Luck et al 1988) or a life table approach (Bellows et al 1992).
C. COERULEUS - C. coeruleus has become established in all countries where it has been released, though not at all release sites. In Indonesia large numbers have been mass reared and released and established (Mangoendihardjo & Wagiman 1 990), but Hardi (1989) reports an experiment where the predator did not establish, and suggests high rainfall or humidity might have been responsible. Villacarlos & Robin (1992) suggest that survival is better if adults rather than larvae are released.
C. coeruleus appears to disperse slowly. Hardi (1989) reported released individuals only spread 26 metres from the release point in 45 days, and Wagiman et al(1 990) found that in 3 years the predator had spread 5 km. Funasaki et al (1 990) remark that in Hawaii, C. coeruleus is not good at moving in to new infestations, that being the reason for importing P. yaseeni. However, the species has been recovered in Laos, Cambodia and Malaysia where it has never been officially released (Napompeth 1994), suggesting it may have spread from neighbouring countries where it was released.
General observations suggest that C. coeruleus has some impact on the pest. It has been seen "feeding heavily" on H. cubana in Thailand (Napompeth 1994), and Funasaki et al say it can reduce high populations of the psyllid in Hawaii, where it became the most abundant predator about a year after the psyllid arrived.
Observations from several countries indicate that the predator is less effective in seasonal climates. In Indonesia its performance has been satisfactory where rainfall is fairly evenly distributed throughout the year, but predator survival is poor where there is a long dry spell (Oka 1990). In Philippines it did not perform well, apparently due to seasonality in most parts of the country (Sanchez 1990).
Another way to assess biological or any control efforts is to monitor damage to the crop. Wagiman et al. (1990) report a study in which C. coeruleus was released to control H. cubana in Leucaena shading coffee. An annual figure for percentage damage to the Leucaena dropped from 100% in 1986 to 90% the following year (when mass rearing and releases stopped), and to 20% in 1988 (data to August). Although monthly data for February to August 1988 show a steady increase in the number of predators, damage to Leucaena was between 39 and 48% every month. This level of control was reported to be adequate to prevent reduction in coffee production.
Van den Beldt & Napompeth (1992) conclude that C. coeruleus can contribute effective local control in the early years following invasion of H. cubana.
O. V-NIGRUM - O. v-nigrum has been used less than C. coeruleus (Table 5), probably because it did not become as abundant as C. coeruleus in Hawaii following the appearance of H. cubana. However, it has become established in Tonga, Tahiti, New Caledonia and Thailand, though its occurrence is localised in Thailand (Napompeth 1 994). In Tonga it is widespread at low densities, feeding on nymphs and adults of H. cubana, and psyllid damage was insignificant in 1993 (Napompeth 1994). It was also present in most parts of New Caledonia by mid-1989 (Chazeau et al 1991), but control was considered inadequate (Chazeau et al 1992). However, it has been reported to be controlling the pest in Tahiti (Chazeau 1987).
P. YASEENI- In Hawaii approximately 31,000 individuals of P. yaseeni were released over a year, and by the end of the releases the insect was found on all islands (Funasaki et al 1990). It has also become firmly established in Thailand and Indonesia, and in Thailand parasite densities are reportedly much higher in the peak season than those observed in Hawaii (Napompeth 1994). The parasitoid has been imported to New Caledonia, but at least one release failed to establish (Baker et al. 1993). The outcome of imports to China, Nepal and Sri Lanka is not known. P. yaseeni has been reported from the Philippines without having been released there, which may indicate that the insect can disperse long distances, but Napompeth (1994) considers this unlikely.
As with the coccinellids, no detailed observations on the impact of P. yaseeni havebeen reported. The fact that it has become common and widespread suggests it is having some impact, as given its host specificity, observed populations are almost certainly being sustained by H. cubana. Van den Beldt and Napompeth (1992) say P. yaseeni appears to be particularly effective in Asia.
T. LEUCAENAE - To the best of our knowledge, this natural enemy has never been released, although it has been imported to various countries. This is due to difficulties experienced in rearing the insect, although cultures have been maintained at IIBC in Trinidad (Baker et al 1993, Patil et al 1993), where well defined rearing procedures have been defined.
OTHER OBSERVATIONS - One of the difficulties in interpreting general observations on changes in pest abundance is that while biological control agents may be responsible, it is not possible to eliminate other explanations. For example, several authors report that C. coeruleus is not effective where there is a distinct dry season (eg. Oka 1990, Sanchez 1990), but Mangoendihardjo et al. (1990) report that populations of the psyllid may be higher in the dry season anyway, and that rain may suppress populations of H. cubana. Thus the greater abundance of C. coeruleus in the wet seasons may be correlated with lower H. cubana populations without being responsible for them.
Van den Beldt & Napompeth (1992) suggest that over a period of years there has been a successive decline in the annual peak populations of the psyllid in Thailand. Again, it is not possible to say whether this is due to the introduced natural enemies, local natural enemies or some other factor such as induced plant resistance.
Psyllids have rarely been the target of biological control programmes, but the few examples have yielded notable successes, particularly using specific parasitoids. In the 1 970's the citrus psyllids Diaphorina citri and Trioza erytreae, vectors of citrus greening disease, were controlled on Reunion island by Tamarixia radiata and Tamarixia dryi, introduced from South Africa and Asia respectively (Aubert & Quilici 1983). Trioza erytreae has not been found on Reunion for 10 years, and eradication of the pest by Tamarixia dryi is suspected (S. Quilici pers. comm.). T. dryi has also been introduced to Mauritius, where it has again controlled Trioza erytreae (A. Joomaye pers. comm.).
Tamarixia radiata was introduced from Reunion to Taiwan between 1 984 and 1 988 for control of Diaphorina citri. It has caused a substantial reduction in populations of the psyllid, with up to 100% parasitism recorded (Chien et al 1989).
There are a few cases where predators have been introduced for the biological control of psyllids. In 1973 two coccinellids, Harmonia conformis and Diomus pumilio, were introduced to Hawaii from Australia to control Psylla uncatoides attacking Acacia spp. D. pumilio failed to establish, but H. conformis established at one of 2 release sites, and controlled the following spring outbreak of the pest (Leeper & Beardsley 1974). D. pumilio was also imported to California in the 1970s, and by 1977 had established in some areas and reduced P. uncatoides populations to low levels (Pinnock et al. 1978).
In the 1960s Anthocoris nemoralis (Hemiptera: Anthocoridae) and 2 other Anthocoris spp. were imported to Canada for control of pear psyllid, Psylla pyricola (Clausen 1978). A. nemoralis established, but it is suggested that the encyrtid Prionomitus mitratus. imported at the same time, may have been present already. In 1977-1981 about 145,000 coccinellids of 5 exotic species were released in Washington State, USA, for control of Psylla pyricola, but without apparent establishment (Fye 1981).
NON TARGET ORGANISMS - None of the predators or parasitoids poses any threat to man, livestock or crops. However, none of the natural enemies described is entirely host specific to leucaena psyllid, so the possible risks of attacks on non-target hosts should be considered.
The two coccinellid species described pose greater risks to non-target organisms than parasitoids in that they are generalist predators, and so may attack many indigenous or introduced species of Homoptera. This is well demonstrated by the fact that they were introduced to Hawaii for control of other pests (Clausen 1978, Funasaki et al 1990), but became predators of leucaena psyllid when it arrived there.
The parasitoids pose less risk in that they have a much narrower host range than the predators, but under particular circumstances their introduction might be undesirable. For example, in Australia and Samoa, a psyllid, Heteropsylla spinulosa, has been introduced as a specific biological control agent of the giant sensitive plant Mimosa invisa (Julien 1992, Muddiman et al 1992). Both P. yaseeni and T. leucaenae attack H. spinulosa, and so biological control of H. cubana might disrupt the biological control of M. invisa. For this reason the parasitoids have not been introduced into Australia. Another psyllid, H. texana, is a possible control agent for Prosopis juliflora (Muddiman et al. 1992), a leguminous shrub which is useful but can become an invasive weed. However, in South Africa seed feeding Coleoptera are being used as biological control agents so H. texana may not be required (PPRI 1994).
Singh & Bhandari (1989) suggested that introduction of the coccinellids to India was undesirable as they might prey on lac insects, Kerria lac, though C. coeruleus was subsequently introduced (Jalali & Singh 1989).
OTHER CONFLICTS OF INTEREST - In some countries Leucaena is regarded as a weed, and the arrival of H. cubana might be viewed as beneficial. In Hawaii L. leucocephala is cultivated in some areas, but in sugar cane growing areas it is the most serious
dicotyledonous weed (Funasaki et al 1990). Such potential conflicts of interest need to be considered before biological control programmes are initiated. In South Africa a compromise solution is being adopted in which seed feeding bruchids are being introduced to reduce the invasive potential of Leucaena without damaging its growth where it is intentionally planted (PPRI 1994).
CONTAMINANTS - When biological control agents are introduced care is required to ensure that they are free of contaminants. One class of contaminant is natural enemies of the agent; coccinellids are attacked by parasitoids, and the parasitoids of leucaena psyllid are attacked by hyperparasitoids.
A second class of contaminant is other pest organisms. During surveys for natural enemies of H. cubana, a potentially serious fungal disease, Captomerus leucaenae, was found attacking L. leucocephala (Baker et al 1993). it is possible that spores of this fungus could survive on plant or even insect material, and so special precautions are required to eliminate that possibility. There are also other insect pests of Leucaena which could be very damaging if inadvertently introduced with natural enemy shipments (Waage 1990).
A third type of potential contaminant is different biotypes of the pest itself, which in this case might be adapted to different species of Leucaena (Waage 1 990). The introduction of these could have serious implications for programmes aimed at breeding resistance to H. cubana.
Contamination can be avoided by following appropriate quarantine procedures. All shipments of natural enemies must pass through quarantine, and if the importing country does not have such facilities, "third country" quarantine must be used. While in quarantine, contaminants such as live H. cubana, other pests and hyperparasitoids, can be killed. To eliminate the risk of fungal spores being transported in a shipment, Baker et al (1993) devised a procedure for treating mummies with sodium hypochlorite and the fungicide benomyl.
Of the four main candidates for introduction to Africa, three have been released in several other countries. All three species have become established under appropriate conditions, and general observations indicate that all exert some controlling effect on H. cubana . As no detailed impact assessment has been reported on any of the natural enemies, it is difficult to rank their suitability for introduction to Africa based on previous experience. However, the observations suggesting that C. coeruleus is not effective where there is a pronounced dry season, and that it is only effective at reducing high populations rather than preventing them building up, both tend to suggest parasitoids should be considered ahead of the predators.
Data on the impact of natural enemies in the pest's area of origin are scant. The two coccinellids were found rarely, while one or both parasitoid species were found at a number of sites. Of the two parasitoids, T. leucaenae was found more frequently and was usually more abundant.
Biological control of other psyllids has been achieved with both coccinellids and parasitic Hymenoptera. Of particular note are the successful programmes in which Tamarixia spp. have been used (Aubert & Quilici 1983, Chien et al 1989, A. Joomaye, pers. comm.).
From theoretical considerations it is generally agreed that specific parasitoids are preferable as biological control agents to predators, and thus coccinellids tend to be used less frequently than used to be the case. Had C. coeruleus and O. v-nigrum not already been present in Hawaii when H. cubana arrived, it is unlikely that they would have been imported. Given that generalist coccinellids such as Exochomus sp. are already attacking the pest in Africa, and there are unlikely to be any specialised parasitoids doing so, the addition of the latter is more likely to increase overall mortality caused by natural enemies.
There is no evidence to suggest that introducing more than one natural enemy is an unwise strategy, either generally or in the case of H. cubana. From laboratory and field studies in the pest's area of origin, and from consideration of the parasitoids' biology, the simultaneous introduction of P. yaseeni and T. leucaenae would appear to be a promising strategy.
The coccinellids are more likely to attack non-target hosts than the parasitoids, and as Heteropsylla is a New World genus, it is unlikely the parasitoids would attack indigenous species of psyllid in Africa. Potential conflicts of interest with weed biological control need to be considered, though none is apparent.
The following strategy is proposed for the biological control of H. cubana in Africa. All proposed actions would be contingent upon necessary conditions and rulings on the import and release of exotic organisms in individual countries being satisfied.
Choice of agents. The parasitoids P. yaseeni and T. leucaenae should be the first choice for import to Africa. The import of the predators should only be considered if the parasitoids have been shown to be unsuccessful. In the event that coccinellids are imported, C. coeruleus would be preferred to O. v-nigrum.
IMPORT AND QUARANTINE - Sometimes a condition of import and release of natural enemies is that host specificity tests are conducted, to demonstrate that indigenous insects related to the pest are not at risk. In this case such tests are not recommended as previous work indicates that the parasitoids' host ranges are confined to the genus Heteropsylla. As this is a new world species (Muddiman et al 1992), it is very unlikely that there are any indigenous psyllids that the introduced parasitoids would be able to attack.
All agents should be imported via third country quarantine, where appropriate precautions can be made to ensure any contaminants are eliminated. Baker et al. (1993) describe the use of sodium hypochlorite and benomyl for sterilising parasitoid mummies. Under no circumstance should field collected material be sent direct to Africa. Quarantine in Africa is not necessary if third country quarantine has been used.
RELEASE AND EVALUATION - If P. yaseeni and T. leucaenae are imported together, it is proposed that they should be released separately at some sites and together at others, so that detailed studies could be undertaken to determine their performance alone and in concert. It is important that proper evaluation studies are undertaken. The results of such studies would be used to decide whether the import of C. coeruleus was necessary.
If necessary, and providing natural enemies are received through third country quarantine (and appropriate permits are obtained), parasitoid shipments could be released directly into the field. This would avoid the difficulties in culturing the parasitoids that hindered the release of T. leucaenae in South-East Asia (Baker et al 1993).
REGIONAL COLLABORATION - As with the biological control programme for conifer aphids, collaboration between affected countries is recommended, so that relevant information experience and expertise is shared. One possibility for such collaboration would be for one or two countries to rear and supply natural enemies to other countries in the region. This would promote more efficient use of limited resources, and reduce the difficulties that might be encountered if attempts were made to establish rearing programmes in all countries.
Aubert, B. and S. Quilici, 1983. Nouvel equilibre biologique observe a la Reunion sur les populations de psyllides apres I'introduction et I'establissement d'hymenopteres chalcidiens. Fruits 38:771-780.
Baker, P.S., N.G. Patil, P.A.C Ooi, S.T. Murphy, and A.E. Cross, 1993. A biological control programme for leucaena psyllid (Heteropsylla cubana), a serious exotic pest of Leucaena leucocephala) in Asia and the Pacific. International Institute of Biological Control, Ascot, UK. 134 pp.
Beardsley, J.W. and G.K. Uchida, 1990. Parasites associated with the leucaena psyllid, Heteropsylla cubana Crawford, in Hawaii. Proceedings of the Hawaiian Entomological Society 30:155-156.
Bellows, T.S., R.G. Van Drieshe, and J.S. Elkinton, 1992. Life-table construction and analysis in the evaluation of natural enemies. Annual Review of Entomology 37: 587-614.
Botto, E.N., M.C. Hernandez, M.E. Boggiatto, and I.S. de Crouzel, 1979. Resultados preliminares de estudios bioecologicos sobre el 'pulgon amarillo de los cereales' Metopolophium dirhodum (Walker), realizados en Castelar, Buenas Aires, durante 1976 a 1979. I Estudios de campo. Revista de la Sociedad Entomologica Argentina 38:37-46.
Boucek, Z., 1988. Tamarixia leucaenae sp. n. (Hymenoptera: Eulophidae) parasitic on the leucaena psyllid, Heteropsylla cubana (Hemiptera), in Trinidad. Bulletin of Entomological Research 38:545-547.
Brewbaker, J.L. 1987. Species in the genus Leucaena. Leucaena Research Reports 7:6- 20.
Catling, H.D., 1 969. The bionomics of the South African citrus psylla Trioza erytreae (Del Guercio) (Homoptera: Psyllidae). 2. The influence of parasites and notes on the species involved. Journal of the Entomological Society of South Africa 32:209- 223.
Chazeau, J., 1987. The Leucaena psyllid in South-East Asia and the Pacific: the problem and the prospects of control (Leucaena leucocephala Lam. de Wit - Heteropsylla cubana Crawford). Quarterly Newsletter, Asia and Pacific Plant Protection Commission, FAO 30:3-4.
Chazeau, J., E. Bouye and L.B. Larbogne, 1991. Cycle de developpement et table de vie d'Olla v-nigrum (Col.:Coccinellidae) ennemi natural d' Heteropsylla cubana (Hom.:Psyllidae) introduit en Nouvelle-Caledonie. Entomophaga 36:275-285.
Chazeau, J., I. Capart and L.B. de Larbogne, 1992. Biological control of the leucaena psyllid in New Caledonia: the introduction of Curinus coeruleus (Coccinellidae). Leucaena Research Reports 13: 59-61.
Chien, C.C., S.C. Chiu and S.C Ku, 1989. Biological control of Diaphorina citrii in Taiwan. Fruits 44: 401-407.
Clausen, C.P., 1978. Introduced parasites and predators of arthropod pests and weeds: a world review. Agriculture Handbook No 480, USDA, Washington DC, USA. 545 pp.
DeBach, P., 1966. The competitive displacement and coexistence principles. Annual Review of Entomology 11:183-212.
Disney, R.H.L and J. Chazeau, 1990. The recognition and biology of Phalacratophora quadrimaculata (Diptera: Phoridae) parasitising Olla v-nigrum (Coleoptera: Coccinellidae) used in attempts to control the leucaena psyllid. Annales de Parasitologie Humaine et Comparee, 65:98-100.
Fullaway, D.T, 1923. Notes on the mealybugs of economic importance in Hawaii. Hawaii Entomological Society Proceedings 5:305-321.
Funasaki, G.Y., P.Y. Lai, and L.M. Nakahara, 1990. Status of natural enemies of Heteropsylla cubana Crawford (Homoptera: Psyllidae) in Hawaii, in Leucaena psyllid: problems and management. Proceedings of an International Workshop held in Bogor, Indonesia, January 1 6-21, 1989. F/FRED, IDRC, NFTA, Bangkok, Thailand, pp 153-158.
Fye, R.E., 1981. Rearing and release of coccinellids for potential control of pear psylla. United States Department of Agriculture, AAT-W-20, 9 pp.
Graham, M.W.R. de V., 1987. A reclassification of the European Tetrastichinae (Hymenoptera: Eulophidae), with a revision of certain genera. Bulletin of the British Museum of Natural History (Entomology) 55:1-392.
Hardi, T.W.T., 1989. Penyeberan predator kutu loncat, Curinus coeruleus Mulsant di KPH Banyumas Timus, Jawa Tengah. Buletin Penelitian Hutan 518:23-31.
Hardi, T.W.T., S.E. Intari and A.H. Bahagiawati, 1988. Siklus hidup dan potensi daya memangsa Curinus coeruleus terhadap kutu loncat lamtoro. Buletin Penelitian Hutan 504: 51-57.
Heydon, D. and M. Affonso, 1989. Economic review of psyllid damage on leucaena in South East Asia and Australia. Report prepared for the Australian International Development Assistance Bureau, CAB International Development Service, Wallingford, U.K. 129 pp.
Jalali, S.K. and S.P. Singh, 1989. Release and recovery of an exotic predator, Curinus coeruleus Mulsant on subabul psyllid Heteropsylla cubana Crawford in India. Journal of Insect Science 2:1 58-1 59.
Jalali, S.K. and S.P. Singh, 1992. Biology and feeding potential of Curinus coeruleus Mulsant and Chrysoperla carnea Stephens on subabul psyllid Heteropsylla cubana Crawford. Journal of Insect Science 5:89-90.
Julien, M.H., 1992. Biological control of weeds: A world catalogue of agents and their target weeds. 3rd Edition, CAB International, Wallingford, UK. 186 pp.
Leeper, J.R. and J.W. Beardsley, 1974. The biological control of Psylla uncatoides (Ferris & Klyver) (Homoptera: Psyllidae) on Hawaii. Proceedings of the Hawaiian Entomological Society 22: 307-321.
Keller, M.A. 1984. Reassessing evidence for competitive exclusion of introduced natural enemies. Environmental Entomology 13: 192-195.
Luck, R.F., M. Shepard, and P. Kenmore, 1988. Experimental methods for evaluating arthropod natural enemies. Annual Review of Entomology 33, 367-391.
Mangoendihardjo, S. and F.X. Wagiman, 1990. Some experiences in mass breeding, release and evaluating the establishment of Curinus coeruleus Mulsant in Yogyakarta and Central Java, in Leucaena psyllid: Problems and Management, Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand: 166-170.
Mangoendihardjo, S., F.X. Wagiman, Y.A. Trisyono and M. Sujono, M., 1990. Seasonal abundance of leucaena psyllid populations in Yogyakarta, Indonesia, in Leucaena psyllid: Problems and Management, Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Thailand: 111- 113.
McClay, A.S., 1990. Distribution of leucaena psyllid and its natural enemies in Mexico: implications for biological control, in Leucaena psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989. F/FRED, IDRC, NFTA, Bangkok Thailand: 139-143
Muddiman, S.B., I.D. Hodkinson and D. Hollis, 1992. Legume-feeding psyllids of the genus Heteropsylla (Homoptera: Psylloidea). Bulletin of Entomological Research, 82: 73-117.
Murai, K.T., 1986. Host range study of the psyllid parasitoid Psyllaephagus sp. nr. rotundiformis (Howard). Report of the Hawaii Department of Agriculture, Honolulu, Hawaii, USA, 6pp.
Napompeth, B., 1990a. Leucaena psyllid problems in Asia and the Pacific, in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand: 1-7.
Napompeth, B., 1990b. Leucaena psyllid in Thailand - a country report. in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand. pp 45-53.
Napompeth, B. and K.G. MacDicken, eds, 1990. Leucaena Psyllid: Problems and management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand.
Napompeth, B. and T. Maneeratana, 1990. Biological and partial ecological life tables of Heteropsylla cubana Crawford and its predator, Curinus coeruleus Mulsant, in Thailand, in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand: 130-138
Napompeth, B., 1994. Leucaena psyllid in the Asia-Pacific Region: Implications for its management in Africa. FAO, Regional Office for Asia and the Pacific (RAPA) Publication 1994/13, 27 pp.
Noyes, J.S., 1990. A new encyrtid (Hymenoptera) parasitoid of the leucaena psyllid (Homoptera: Psyllidae) from Mexico, Central America and the Caribbean. Bulletin of Entomological Research 80:37-41.
Oka, I.N., 1990. Progress and future activities of the leucaena psyllid research program in Indonesia, in Leucaena Psyllid: Problems and Management, Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand: 25-27.
Pan, F.J., 1990. Leucaena psyllid in the Republic of China, in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Thailand: 12-13.
Patil, N.G., P.S. Baker, and G.V. Pollard. 1993. Life histories of Psyllaephagus yaseeni (Hym., Eíncyrtidae) and Tamarixia leucaenae (Hym., Eulophidae), parasitoids of the leucaena psyllid Heteropsylla cubana. Entomophaga 38:565-577.
Pinnock, D.E., K.S. Hagen, D.V. Cassidy, R.J. Brand, J.E. Milstead and R.L. Tasson, 1978. Integrated pest management in highway landscapes. California Agriculture 32:33- 34.
PPRI, 1994. Plant Protection News 36. Bulletin of the Plant Protection Research Institute, Pretoria, South Africa.
Rauf, A., S. Rasyid, and A. Nurmansyahulur, 1990. Laboratory life-table of Curinus coeruleus Mulsant (Coleoptera:Coccinellidae), an introduced predator for controlling Heteropsylla cubana Crawford (Homoptera: Psyllidae), in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Thailand: 119-121.
Sanchez, F.F. 1990. Leucaena psyllid in the Philippines - a country report, in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21,1 989, F/FRED, IDRC, NFTA, Thailand.pp 40-42
Singh, P. and R.S. Bhandari, 1989. Further spread of the Leucaena psyllid, Heteropsylla cubana in India. Indian Forester 115:303-309.
Tedders, W.L., 1978. Important biological and morphological characteristics of the foliar feeding aphids of pecan. USDA Technical Bulletin No. 1579. 29 pp.
USAID, 1991. Environmental assessment of the classical biological control of the leucaena psyllid in Indonesia, Laos, Malaysia, Nepal, the Philippines and Thailand. Office of Forestry & Natural Resources, Bureau for Science and Technology, United States Agency for International Development, Washington D.C., 94 pp. plus appendices.
Van Den Beldt, R.J.V. and B. Napompeth, 1992. Leucaena psyllid comes to Africa. Agroforestry Today 4:11-12.
Villacarlos, L.T., R.V. Paglinawan and R.P. Robin, 1990. Factors affecting leucaena psyllid populations in Leyte, Philippines, in Leucaena Psyllid: Problems and Management. Proceedings of an International Workshop held in Bogor, Indonesia, January 1 6-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand:1 22-1 29.
Villacarlos, L.T. and R.P. Robin, 1992. Biology and potential of Curinus coeruleus Mulsant, an introduced predator of Heteropsylla cubana Crawford. Philippine Entomologist 8:1247-1258.
Waage, J., 1990. Exploration for biological control agents of leucaena psyllid in tropical America, in Leucaena Psyllid: Problems and Management, Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand:144-1 52.
Wagiman, F.X., S. Mangoendihardjo and E. Mahrub, 1990. Performance of Curinus coeruleus Mulsant as a predator against leucaena psyllid, in Leucaena Psyllid: Problems and Management, Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989,F/FRED, IRDC, NFTA, Bangkok, Thailand: 163-165.
Watve, C.M. and D.F. Glower, 1976. Natural enemies of the bandedwing whitefly in Louisiana. Environmental Entomology 5:1075-1078.
Yao, A.L., Y.S. Hwang and W.Y. Wang, W.Y., 1990. Natural enemies and biological control of Heteropsylla cubana on Leucaena leucocephala in Taiwan, in Leucaena Psyllid: Problems and Management, Proceedings of an International Workshop held in Bogor, Indonesia, January 16-21, 1989, F/FRED, IDRC, NFTA, Bangkok, Thailand: 162.