Plates 18. and 19 (pp. 99 & 100) and Fig. 2 (p. 90)
Species affected
Representative of many fish families, very common in Cichlidae, Cyprinidae and in
brackish waters in Mugilidae.
Geographic range
Data beyond single species descriptions are available from Egypt (Fahmy et al., 1975),
East Africa (Baker, 1963; Paperna, 1973), Ghana (Paperna, 1968, 1973), the
Cameroons (Fomena et al., 1984/5; Fomena & Bouix, 1987) and Israel (in cichlids and
Clarias lazera Landsberg, 1985, 1986). In some of these reports differentiation to species
is lacking or incomplete (Paperna, 1968, 1973; Fahmy et al., 1975). Infections by
Myxobolus, Thelohanellus and Henneguya occur in both East African and West African
waters. They are apparently host specific and as widespread as their respective hosts.
Ovarian infections of cichlids are thus far known from the Great Lakes, Haplochromis
spp. (Paperna, 1973).
Description taxonomy and diagnosis
Myxosporea cause histozoic (e.g. in tissue) and coelozoic (in internal cavities e.g. in gall
and urinary bladders) infections.
Gross signs of histozoic infection are whitish cysts with a milky substance containing microscopic (5-5 μm longer axis) spores. Large cysts are readily traced, small cysts in tissues, inside viscera and connective tissue and muscles, are detectable when tissue samples are pressed between slides, or in histological material. Spores are readily detected in aqueous methylene blue-stained smears. The so called cyst is a parasite-origin plasmodium which forms a specialised membranous junction of pinocytotic vesicles (canals) with the surrounding host cells (Current & Janovy, 1976).
Spores, hard-walled, with one to six polar capsules are differentiated by sporogenesis within the plasmodium. The polar capsule contains a coiled filament which may be extruded under pressure or other stimulants, including fixation with absolute methanol.
Coelozoic myxosporea of the urinary and bile cavities have a small plasmodium and produce few spores, often only two. Premature plasmodia are attached by pseudopodia to the epithelial lining of the bladder (Paperna et al., 1987). Small diplosporic plasmodia (“pseudoplasmodia”) are characteristic of Myxidium and Sphaerospora which develop from within the kidney tubule into the lumen (Dykova & Lom, 1982) and also in the gut lamina propria (Diamant, 1992).
Genera are identified by spore configuration. Diagnosis to species level is based mainly on measurements of fresh unfixed spores, their polar capsule dimensions as well as the length of the extruded polar filament (Lom & Arthur, 1989). Separation of the genus Myxosoma from Myxobolus by the presence or absence of a iodophilus vacuole does not appear to be taxonomically valid (Walliker, 1968). Ultrastructural studies (scanning) demonstrate interspecific and intergeneric variation in the surface configuration (which consists of 1–6 valves joined by sutures) (Lom & Hoffman, 1971). Location of the cysts/plasmodia in the host may be of diagnostic value in site specific species, while others are not fastidious in their site preferences.
Life cycle and biology
The life histories of myxosporeans in African fish are not known. It has been shown that
transmission of Myxosoma cerebralis of trout and several other myxosporean parasites
of other fish involves tubificid oligochaetes as intermediate hosts (Wolf & Markiw, 1984;
El-Matbouli & Hoffmann, 1989). Goldfish became infected with Myxobolus, Zchokkella
and Thelohanellus after being fed on tubificids infected by actinosporeans (Yokoyama
et al., 1991). It has also been demonstrated that an early migratory proliferative phase
precedes later sporulating plasmodia in tissues or, of Sphaerospora, in the kidneys (Lom
& Dykova, 1986) or the gills (Lom et al., 1983). Such dividing plasmodia occur in blood
(Csaba or C - bodies) and in the swimbladder tissue (Korting, 1982; Csaba et al., 1984;
Molnar & Kovacs-Geyer, 1986) in carp and in the kidney haematopoietic tissue of trout
(PKX cells, Kent & Hedrick, 1985). Myxosporean multinucleate formations preceding
spore formation are generated by endogenous divisions, where cells are formed within
cells. This process occurs in the early proliferative stages and during the process of
sporogenesis. Primary cells from the plasmodia generate, by endogenous division,
secondary inner cells which further divide by mitosis. Their progeny (the sporogenic
cells) produce, by endogenesis, tertiary cells within them. The sequences of divisions
which lead to spore formation also include meiotic division (Lom & Dykova, 1986). The
spore is formed from enveloping (pericytes) and inner cells transforming into shell valves,
polar capsules and to sporoplasm.
Spores of coelozoic forms are evacuated with the bile and the urine. Cysts in the skin, gills, and the digestive tract release spores by rupture. In grey mullets, entire cysts in the digestive tract wall are discharged into the gut lumen in the form of a white ball. The only other alternative for the release of myxosporean spores is after their host's death.
Pathology
Several myxosporean infections of cultured fish were reported to be pathogenic. Most
notorious is the whirling disease of trout, manifested by skeletal deformities, which is
also claimed to have been introduced with rainbow trout into South Africa (Van Wyk,
1968). In farmed carp, Myxobolus spp. caused locomotory disturbances coupled with
emaciation, and sunken eyes in brain infections (Dykova et al., 1986), anaemia and
haemorrhagic dropsy and mortality in a heavy cardiac infection (Bauer et al., 1991) and
circulatory disfunctions in infections at the bases of the gill lamellae (Kovac-Geyer &
Molnar, 1983). Heavy infection of carp gills with M. koi caused fusion through epithelial
hypertrophy, and congestion; rupture of cysts caused inflammation. Damage to the gills
by dense infestation resulted in respiratory problems, fish were swimming near the
surface with distended operculi (Rukyani, 1990). Severe disaggregation of the
respiratory epithelium is caused by Sphaerospora spp. infections of carp and goldfish
gills (Lom et al., 1983).
Several myxosporeans cause renal pathology in carp and goldfish: Sphaerospora renicola proliferating in the renal tubules damages the renal tubuli epithelium and the released spores congest excretory passages (Dykova & Lom, 1982). Presporulating forms named Hofferellus, with a postulated identity with species of Mitraspora (Molnar et al., 1986), cause Kidney Enlargement Disease by infecting the epithelial cells of the renal tubules (Yokoyama et al., 1990). Proliferative stages of Sphaerospora cause proliferative hypertrophy of the kidney in salmonids (Kent & Hedrick, 1985) and grey mullets (Paperna, 1991). Proliferative, presporulating stages of Sphaerospora renicola were demonstrated to be the aetiological cause of swimbladder inflammation in carp (Korting, 1982; Csaba et al., 1984; Molnar & Kovacs-Geyer, 1986).
Cysts of histozoic myxosporidians provoke inflammation only after their disintegration, and in most instances it is when myxosporan infections become pathogenic to their host and cause clinical conditions (examples: gill infections of American catfish with Henneguya exilis - MacCraren et al., 1975; Channa punctata with Henneguya waltairensis - Kalavati & Narasimhamuri, 1985, and carp with Myxobolus koi - Rukiani, 1990). Rupture of cysts also leads to haemorrhaging, sometimes resulting in considerable loss of blood (Schulman, 1957; Kalavati & Narashimhamurti, 1985).
In some infections, host response may lead to tumour-like proliferations incorporating parasite plasmodia and the capillary net-work (Nigrelli & Smith, 1938). Rupture of dermal and branchial cysts caused intense haemorrhaging and facilitated invasion of secondary opportunistic pathogens (Paperna & Overstreet, 1981).
In fish in Africa, histozoic myxosporidians occur as skin infections, with cysts formed in the dermis, under the scales, extending to the surface of the head (face, lips) or onto the fins (Myxobolus spp. in juvenile cichlids, Henneguya laterocapsulata in Clarias lazera), and in the gill filaments (Myxobolus spp. in Cyprinidae, siluroids, Characidae, Distichodus rostratus, Heterotis niloticus; Thelohanellus in cyprinids and Henneguya in Lates albertianus as well as in citharinids, cyprinids and siluroids) (Paperna, 1973; Abolarin, 1974; Landsberg, 1986; Fomena et al., 1984/5). Myxobolus cysts occur in the pharyngo-branchial cavity (of Ctenopoma spp.), the interior organs, muscles and viscera. Such infections are best known from cichlids, but also occur in fish from other families (Paperna, 1973). Sphaerospora occur in kidneys of Clarias lazera (Landsberg, 1986) and Grey mullets (Paperna, 1991).
Infections (even heavy infections) often occur in fish otherwise in good condition. Nonetheless, multiple cysts and particularly large cysts in vulnerable organs such as the gills, may compromise fish health. Large Myxobolus cysts caused body deformity or curvature of Barbus lineatus and cichlids (Paperna & Thurston, 1968). Ovarian infection in female cichlids from L. Victoria remained focal in the interstitial tissue, while that (a different species) occurring in cichlids (Haplochromis angustifrons and H. elegans) from L. George enlarged the ovary while displacing the entire ovigerous tissue, evidently causing castration.
The only reported coelozoic infection in freshwater African fish is that of Myxidium clariae from C. lazera gall bladder (Landsberg, 1986).
Rupture of mature cysts leads to host-tissue responses as well as phagocytosis of spores and their capsule residues. Spores accumulate in melanomacrophage centres in the kidney and the spleen (Ogawa et al., 1992). Spores encountered in the spleen are therefore residues of past infections elsewhere (Baker, 1963; Landsberg, 1985).
Epizootiology
Prevalence of infection is variable. In the East African Lakes visceral Myxobolus
infections in Oreochromis were very prevalent (89–100%), while in Haplochromis spp.
they were only exceptionally above 25%. Skin and gill infections in juvenile as well as
larger cichlids are also not too prevalent, although very heavily infected invididuals were
collected. Infection levels seem to change with time (Fryer, 1961; Baker, 1963; Paperna,
1973). Gill infection (Myxobolus, Thelohanellus and Henneguya) of cyprinid fish and
siluroids seems to be very common in Lake Volta, in the Ruaha river, Tanzania, and in
the Cameroons, but quantitative data are limited (Paperna, 1968; Fomena et al., 1984/5),
or non existent (Paperna, 1973). Epizootic infection has been reported in grey mullets
farmed in freshwater ponds in Israel (Sarig, 1971).
Ovarian infections, leading to castration in Haplochromis spp., occurred in less than 4% of female fish, and only in L. George. In L. Victoria only the mild ovo-mesenteric infection occurred.
Neither in Israel, where tilapia are intensively farmed, nor elsewhere in Africa, has the epizootic form of infection ever been observed, except in one farm in Malawi where fish developed large subdermal deforming cysts. Detailed information on this case is, however, lacking.
Of all pathogenic myxosporeans reported in carp or goldfish none have thus far been reported in carp farmed in Africa despite the fact that swimbladder inflammation and kidney infections with Sphaerospora renicola occur in carp reared in Israel (Landsberg, 1989). The carp gill infecting Myxobolus koi has been recently recovered on goldfish stock imported to Israel from China.
Control
To this day no readily effective and easy-to-use drug is available to treat myxosporean
infections. Trials with Fumagillin (also used to control microsporidial infections) produced
encouraging results but at the same time the tolerance of the fish to therapeutic doses
was variable and some workers reported side effects of intoxication following treatment
schedules (Sitja-Bobadilla & Alvares-Pellitero, 1992; Molnar, 1993). Sphaerosporosis of
trout (PKD) and carp, and Dicentrarchus labrax, hoferellosis of goldfish and trout whirling
disease were treated with medicated food containing 0.5–1 g Fumagilin kg-1 fed at 1.5%
body weight (or 0.5–1 g kg-1 fish body mass) for 7–8 weeks (Hedrick et al., 1988;
Yokoyama et al., 1990; El Matbouli & Hoffmann, 1991; Sitja-Bobadilla & Alvares-Pellitero,
1992; Molnar, 1993). Fumagillin when applied to Myxidium giardi infected
elvers arrested development but failed to eliminate the parasite which resumed
development when medication was ended (Szekely, et al., 1988). The cost of Fumagilin,
is however, economically prohibitive for use in food-fish farming (tilapia, catfish and carp).
The efficacy of other tested drugs such as furazolidon and toltrazuril remains inconclusive (Yokoyama et al., 1990; Molnar, 1993).
REFERENCES
Abolarin, M.O., 1974. Myxobolus tilapiae sp. nov. (Protozoa: Myxosporidia) from three species of freshwater tilapia in Nigeria. J. West Afr. Sci. Ass., 19: 109–114.
Baker, J.R., 1963. Three new species of Myxosoma (Protozoa: Myxosporidia) from East African freshwater fish. Parasitol., 53: 285–292.
Bauer, O.N., Voronin. V.N. & Yunchis O.N., 1991. Infection of the heart in carp caused by Myxobolus dogieli (Myxosporea, Myxobolidae). Angew. Parasitol., 32: 42–44.
Csaba, G., Kovac-Geyer, E., Bekesi, L., Bucsek, M. & Molnar, K., 1984. Studies into the possible aetiology of swimbladder inflammation in carp fry. J. Fish Dis., 7, 39–56.
Current, W.L. & Janovy, J. Jr., 1976. Ultrastructure of interlamellar Henneguya exilis in the channel catfish. J. Parasitol., 62: 975–981.
Diamant, A., 1992. A new pathogenic histozoic Myxidium (Myxosporea) in cultured gilt-head sea bream Sparus aurata L. Bull. Eur. Ass. Fish Pathol., 12: 64–66.
Dykova, I. & Lom, J., 1982. Sphaerospora renicola n. sp., a myxosporean from carp kidney, and its pathogenicity. Z. Parasitenk., 68: 259–268.
Dykova, I. & Lom, J., 1992. Sphaerospora renicola n. sp., a myxosporean from carp kidney, and its pathogenicity. Z. Parasitenk., 68: 259–268.
Dykova, I., Lom. J. & Cirkovic, M., 1986. Brain myxoboliasis of common carp (Cyprinus carpio) due to Myxobolus encephalicus. Bull. Eur. Ass. Fish Pathol. 6: 10–12.
El Matbuli M. & Hoffmann, R.W., 1989. Experimental transmission of two Myxobolus spp. developing bisporogony via tubificid worms. Parasitol. Res., 75: 461–464.
El Matbouli, M. & Hoffmann, R.W., 1991. Prevention of experimentally induced whirling disease in rainbow trout Oncorhynchus mykis by fumagillin. Dis. Aquat. Org., 10: 109–113.
Fahmy M.A.M., Mandour, A.M. & El Naffar, M.K., 1975. A survey of myxosporidia of freshwater (fish) collected from the river Nile at Assiut province. J. Egypt. Soc. Parasitol., 4/5: 93–102.
Fomena, A., Bouix, G. & Birgi, E., 1984/5. Contribution a l"etude des Myxosporidies des poisson d'eau douce du Cameroun. II: Especes nouvelle du genre Myxobolus Butschli, 1882. Bull. de l'Inst. Fon. Afr. Noir. 46, ser. A: 168–191.
Fomena, A. & Bouix G., 1987. Contribution a l'etude des Myxosporidies des poisson d'eau douce du Cameroun. III. Especes nouvelle des genres Henneguya Thelohan, 1892 et Thelohanellus Kudo, 1933. Rev. Zool. Africaine, 101, 43–53.
Fryer, G., 1961. Observation on the biology of the cichlid fish Tilapia variabilis Boulenger in the northern waters of Lake Victoria (East Africa) Rev. Zool. Bot. Afr., 64: 1–33.
Hedrick, R.P., Groff, J.M., Foley, P. & McDowell, T., 1988. Oral administration of fumagillin DCH protects chinook salmon Onchorhynchus tschawytscha from experimentally-induced proliferative kidney disease. Dis. Aquat. Org., 4: 165–168.
Kalavati, C. & Narasimhamurti, C.C., 1985. Histopathological changes in the gills of Channa punctatus BL. infected with Henneguya waltairensis. Arch. Protistenk., 129: 199–202.
Kent, M.L. & Hedrick, R.P., 1985. PKX, the causative agent of proliferative kidney disease (PKD) in pacific salmonid fishes and its affinity with the Myxozoa. J. Protozool., 32: 254–260.
Korting, W., 1982. Protozoan parasites associated with swimbladder inflammation (SBI) in young carp. Bull. Eur. Ass. Fish Pathol., 2: 25–28.
Kovac-Geyer, E. & Molnar, K., 1983. Studies on the biology and pathology of the common carp parasite Myxobolus basilamellaris Lom & Molnar, 1983 (Myxozoa: Myxosporea) Acta Vet. Hungar., 31: 91–102.
Landsberg, J.H., 1985. Myxosporean infections in cultured tilapia in Israel. J. Protozool., 32: 194–201.
Landsberg, J.H., 1986. Myxosporean parasites of the catfish, Clarias lazera (Valenciennes). System. Parasitol., 9: 73–81.
Landsberg, J.H., 1989. Parasites and associated diseases of fish in warm water culture with special emphasis on intensification. In: Shilo, M. & Sarig, S. (ed.) Fish Culture in Warm Water Systems: Problems and Trends. CRC Press Inc. Boca Raton, Flo. pp. 195–252.
Lom, J. & Arthur, R., 1989. A guideline for the preparation of species descriptions in Myxosporea. J. Fish Dis., 12: 151–156.
Lom, J. & Dykova, I., 1986. Comments on myxosporean life cycles. Symposia Biol. Hung., 33: 309–318.
Lom, J., Dykova, I., Pavlaskova, M. & Grupcheva, G., 1983. Sphaerospora molnari sp. nov. (Myxozoa: Myxosporea), an agent of gills, skin and blood sphaerosporosis of common carp in Europe. Parasitol., 86: 529–535.
Lom, J. & Hoffman, G.L., 1971. Morphology of the spores of Myxosoma cerebralis (Hofer, 1903) and Myxosoma cartilaginis (Hoffman, Putz and Dunbar, 1965). J. Parasitol., 57: 1302–1308.
McCraren, J.P., Landolt, M.L., Hoffman, G.L. & Meyer, F.P., 1975. Variation in response of channel catfish to Hennegua sp. infections (Protozoa: Myxosporidea). J. Wildl. Dis., 11: 2–7.
Molnar, K., 1993. Recent achievements in the chemotherapy of myxosporean infections of fish. Acta Veterinaria Hungarica, 41: 51–58.
Molnar, K., Csaba, Gy. & Kovacs-Geyer, E., 1986. Study on the postulated identity of Hoferellus cyprini (Doflein, 1898) and Mitraspora cyprini Fujita, 1912. Acta Vet. Hungar., 34: 175–181.
Molnar, K. & Kovacs-Geyer E., 1986, Experimental induction of Sphaerospora renicola (Myxosporea) infection in common carp (Cyprinus carpio) by transmission of SB-protozoans. Applied Ichthyol., 2: 86–94.
Nigrelli, R. F. & Smith, G.M., 1938. Tissue response of Cyprinodon variegatus to the myxosporidian parasite, Myxobolus lintoni Gurley. Zoologica, 23: 195–202 + 7 plates.
Ogawa, K., Delgahapitiya, K.P., Furyta, T. & Wakabayashi, H., 1992. Histological studies on the host response to Myxobolus artus Akhmerov, 1960 (Myxozoa, Myxobolidae) infection in the skeletal muscles of carp, Cyprinus carpio L. J. Fish Biol., 41: 363–371.
Paperna, I., 1968. Ectoparasitic infections of fish of Volta lake, Ghana. Bull. Wildl. Dis. Ass., 4: 135–137.
Paperna, I., 1973. Occurrence of Cnidospora infections in freshwater fishes in Africa. Bull Inst. Fond. Afr. Noir, 35 (A-3): 509–521.
Paperna, I., 1991. Diseases caused by parasites in the aquaculture of warm water fish. Annual Rev. Fish Dis. 1: 155–194.
Paperna, I. Hartley, A.H. & Cross, R.H.M., 1987. Ultrastructural studies on the plasmodium of Myxidium giardi (Myxosporea) and its attachment to the epithelium of the urinary bladder. Int. J. Parasitol. 17: 813–819.
Paperna, I. & Overstreet, R.M., 1981. Parasites and diseases of Mullets (Mugilidae). In: Oren, O.H. (ed.) Aquaculture of Grey Mullets. IBP 26, Cambridge University Press, U.K.
Paperna, I. & Thurston, J.P., 1968. Report on ectoparasitic infections of freshwater fish in Africa. Bull. Off. int. Epizoot., 69: 1192–1206.
Rukyani, A., 1990. Histopathological changes in the gills of common carp (Cyprinus carpio) infected with the myxosporean parasite Myxobolus koi, Kudo, 1920. Asian Fish. Sci. 3: 337–341.
Schulman, S.S., 1966. Myxosporea of the fauna of the USSR (in Russian). nauka, Moscow, 504 pp.
Sitja-Bobadilla, A. & Alvares-Pelleteiro, P., 1992. Effect of Fumagillin treatments on sea bass Dicentrarchus labrax parasitized by Sphaerospora testicularis (Myxosporea: Bivalvulida). Dis. Aquat. Org., 14: 171–178.
Szekely, Cs, Molnar, K. & Baska, F., 1988. Efficacy of fumagillin against Myxidium giardi Cepede, 1906 infection of the European eel (Anguilla anguilla): New observations of myxidiosis of imported glass eels. Acta Vet. Hungar., 36: 239–246.
Van Wyk, G.F., 1968. Annual report No. 24, 1967. Jokershoek Hatchery, Division of Inland Fisheries, Department of Nature Conservation, Province of Good Hope, Republic of South Africa.
Yokoyama, H., Ogawa, K. & Wakabayashi, H., 1990. Chemotherapy with fumagillin and toltrazuril against kidney enlargement disease of goldfish caused by the myxosporean Hofferellus carassii. Fish Pathol., 25: 157–163.
Yokoyama, H., Ogawa, K. & Wakabayashi, H., 1991. A new collection method of Actinosporeans - a probable infective stage of Myxosporeans to fishes - from tubificids and experimental infection of goldfish with the Actinosporean, Raabeia sp. Gyobyo Kenkyu, 26: 133–138.
Walliker, D., 1968. The nature of the iodophilous vacuole of myxosporidian spores, and a proposal to synonymize the genus Myxosoma Thelohan, 1892 with the genus Myxobolus Butschli, 1882. J. Protozool., 15: 571–575.
Wolf, K. & Markiw, E., 1984. Biology contravenes taxonomy in Myxozoa: new discoveries show alternation of invertebrate and vertebrate hosts. Science, 225: 1449–1452.
ILLUSTRATIONS
Plate 18. Myxosporea: a. Myxobolus cysts beneath scales of Oreochromis variabilis from northern Lake Victoria (× 4.0). b,c. Tilapia spp. from a fish pond in Malawi with one (arrow) or two large transverse myxosporean (reported as Myxobolus) cysts. d. Myxosporan cysts in viscera of Haplochromis sp. from L. Edward. e. Cyst of Myxobolus unicapsulatus on gills of Labeo cubie from northern L. Victoria (× 2.8). f. Ovary of H. angustifrons from L. George, hypertrophic due to Myxobolus infection (× 2.8). g. Myxobolus spores from visceral cysts of Haplochromis sp. (× 1400) (fig. d). h. Myxobolus cysts from H. angustifrons ovary (× 700) (fig. f). i. Myxobolus cyst on gills of Heterotis niloticus from Landja, Central African Republic (Coll. by J.C. Micna) (× 2.8). j. Myxobolus sp. spores from large dermal cysts from young Oreochromis niloticus from Kisumu bay, Lake Victoria. k,l. Henneguya sp. spores from cysts on gills of Lates niloticus from northern Lake Victoria.
Plate 19. Myxosporea continued and Microspora: a. Subdermal node due to Myxobolus in Barbus (Tor) canis from L. Kinneret, Israel. b. Spores of Thelohanellus sp. from cysts on gills of Labeo senegalensis, Volta lake, Ghana (× 1500). c. Sphaerospora sp. from kidneys of Mugil cephalus, Kowie Lagoon, southeastern Cape, South Africa. d. Gill sphaerosporosis in farmed goldfish, Israel. e. Henneguya sp. from a cyst on gills of Distichodus rostratus, Volta lake, Ghana (× 1500). f. Microsporidian infection of glomeruli in Oreochromis aureus kidneys, L. Kinneret, Israel. g. fibroblastic nodules containing microsporidian spores in kidneys of farmed goldfish, Israel. h–j. Plistophora infected swimbladder of Haplochromis angustifrons from L. George: histological view of infected cells of the bladder's fibrous wall (h,i) and view of the hypertrophic bladder.
Fig. 2. Haemosporida, Myxosporea and Microspora. (pp. 89–90 with legend)
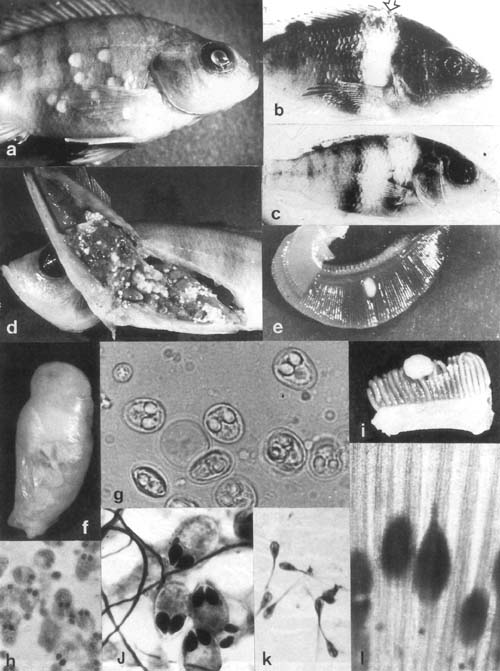
Plate 18. Myxosporea (legend p. 98).
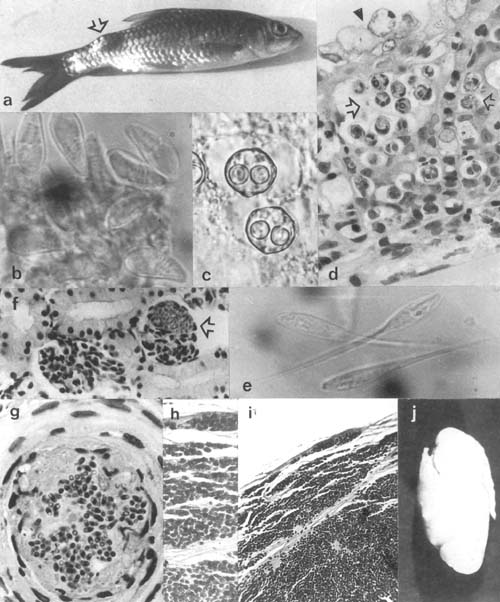
Plate 19. Myxosporea continued and Microspora (legend p. 98).