Fig. 7 (p. 175)
Species affected and geographical range
Present in representatives of diverse African fish families (Golvan, 1957, 1965; Khalil,
1971). The geographic range of these parasites is sometimes narrower than that of their
specific hosts, for example, the cichlid parasite Acanthogyrus tilapiae is widespread in
tropical Africa including Madagascar (introduced), but it has not yet been found in the
Near East, the Sudan Nile or South African cichlids (Paperna, 1964; Khalil, 1969; Van
As & Basson, 1983).
Description taxonomy and diagnosis
Acanthocephala are readily recognised by their evaginable proboscis crowned with
several rows of recurved hooks. In the encased larval stage, in tissues, the spiny
proboscis is retracted. The worms are sac-like, containing lemnisci connected to the
proboscis and genital organs opening posteriorly. The sexes are separate and the male
opening is within a membranous bursa. An alimentary canal is absent.
The number and arrangement of the hooks on the proboscis are the main criteria for differentiation of species. A wider range of anatomical details are considered for determination of higher taxa (Kabata, 1985).
Incubation in tap water helps to extract the proboscis prior to fixation in hot or cold alcohol 70%, formol saline 4%, or AFA.
Life history and biology
All acanthocephalans develop via one or more intermediate hosts (heteroxenous). Adult
acanthocephalans are all gut parasites. Eggs are laid into the intestinal lumen and
evacuated with faeces. First intermediate hosts of piscine acanthocephala are
amphipods, isopods, copepods or ostracods. The first larvae, the acanthella (acanthor),
hatch from eggs after being swallowed by a suitable invertebrate host. Some species
will develop to the adult stage when their larvae in the invertebrate host are ingested by
the definitive vertebrate host (George & Nadakal, 1973; Schmidt, 1985). Fish can also
serve as intermediate hosts, harbouring a second larval stage (the acanthor or
cystacanth). Definitive hosts of such acanthocephalans are either predatory fish or
piscivorous birds (Hassan & Qasim, 1960; Hoffman, 1967).
Life histories and intermediate hosts of acanthocephala of African fish are at present unknown.
Pathology
Pathogenic effects of acanthocephalans are due to attachment of the adult parasite in
the digestive tract and also to the encapsulation of larval stages in the tissues. In low to
moderate infections, pathological effects are localised around the attachment of the adult
worm. The extent of damage is proportional to the depth of penetration of the proboscis.
It is negligible when parasites are attached to the epithelial mucosa only (Acanthogyrus
and Acanthocephalus spp.), and becomes extreme, with extensive granuloma and
subsequent fibrosis, when the worm's proboscis is anchored in the muscle layer or
entirely perforates the intestinal wall (Pomphorhynchus spp.) (Paperna & Zwerner, 1976;
McDonough & Gleason, 1981; Kabata, 1985). The depth of penetration of some species,
may vary in different host fish (Taraschewski, 1988). Extensive inflammation, peritonitis
due to perforation of the gut and systemic clinical changes (anurhersia) will occur only
in massive infections, most often in farmed fish (Bullock, 1963; Bauer, 1959). In juvenile
fish (cichlids <60 mm long) a single attached specimen of Acanthogyrus tilapiae
obstructed the digestive tube, apparently with no clinical implications (Douellou,
1992 a,b). Low to moderate infections with larval stages (cystacanths) in visceral organs
(liver, spleen) caused only local changes while heavy infection, in juvenile fish in
particular, led to extensive granuloma, fibrosis and ultimately atrophy through fibrosis of
either a portion of or the entire organ (Paperna & Zwerner, 1976). Information on infection
among fish in Africa is very limited and none of the conditions described above have
ever been reported.
Epizootiology
Host specificity of acanthocephalans is variable and may be evaluated only where
sufficient data are available, which is not the case for most African fish species.
Acanthogyrus tilapiae is specific to Cichlidae, while other species have been found in
Cyprinidae, Paragorgorhynchus albertianum is indiscrminate in its choice of hosts
(Khalil, 1971). Epizootiological data are limited to natural infections: In the Sudan White
and Blue Nile, 5–27 Tenuisentis niloticus occurred in 93% of Heterotis niloticus, 6–43
Neoechinorhynchus sp. in 26% of Citharinus citharus and 2–5 unidentified
acanthocephala in 60% of Synodontis batensoda (Khalil, 1969). Similarly abundant
infections were found in the same fish in L. Chad (Troncy, 1974, 1977; Troncy &
Vassilides, 1973). Acanthogyrus tilapiae is a fairly common parasite of cichlid fish in
tropical Africa, in Lake Kariba, 63% of the Tilapia rendalli, and all four Oreochromis
mortimeri examined harboured worms, of which, one specimen had over 100 worms
(Douellou, 1992a,b).
Control
To control infections in coldwater fish farms, medicated feed with Bithionol (2.2-thio bis
(4,6-dichlorophenol), is recommended, at a dose of 0.2 g/kg fish (Hoffman, 1983). Feeds
medicated with Di-N-butyl tin oxide are also potentially effective (see 14.1).
REFERENCES
Bauer, O.N., 1959. The ecology of freshwater fish. Inves. Gosud. Nauch. -Issled. Inst. Ozer. Rech. Ryb. Khoz. 49: 5–206 (In Russian, English transl. Israel Prog. Sci. Trans. cat. No. 622, 1962, 3–215).
Bullock, W.L., 1963. Intestinal histology of some salmonid fishes with particular reference to the histopathology of acanthocephalan infections. J. Morphol., 112: 23–44.
Douellou, L., 1992a. A survey of fish parasites in Lake Kariba, Zimbabwe (1989–1992), Final Report. ULKRS [University of Zimbabwe Lake Kariba Research Station] Bulletin, 1/92. 71 p.
Douellou, L., 1992b. Parasites of Oreochromis (Oreochromis) mortimeri (Trewavas, 1966) and Tilapia rendali rendali (Boulanger, 1836) in Lake Kariba, Zimbabwe. University of Zimbabwe Lake Kariba Research Station Bull., 2 (Proc. of seminar series): 14–31.
George, P.V. & Nadakal, A.M., 1973. Studies on the life cycle of Pallisentis nagpurensis Bhalerao, 1931 (Pallisentidae; Acanthocephala) parasitic in the fish Ophiocephalus striatus (Bloch). Hydrobiol., 42: 31–43.
Golvan, Y.J., 1957. Acanthocephala des poissons. Expl. Hydrobiol. Lac Kivu, Eduard et Albert, 1952–54. Bruxelles, 3: 55–64.
Golvan, Y.J., 1965. Acanthocephales de Madagascar recoltes par E.R. Bgygoo. Premiere note). Ann. Parasitol. Hum. Comp., 40: 303–316.
Hassan, R. & Qasim, S.Z., 1960. The occurrence of Pallisentis basiri Farooqi (Acanthocephala) in the liver of Trichogaster chuna (Ham.). Zeit. Parasitenk. 20: 152–156.
Hoffman, G.L., 1967. Parasites of North American Freshwater Fishes. University of California Press, Berkeley & Los Angeles.
Hoffman, G.L., 1983. Prevention and control of parasitic diseases of fishes. First Internat. Symp. Ichthyoparasitol., Ceske Budejovice, Czechoslovakia, pp. 38–39 (abstract).
Kabata, Z., 1985. Parasites and Diseases of Fish Cultured in the Tropics. Taylor & Francis, London & Philadelphia.
Khalil, L.F., 1969. Studies on the helminth parasites of freshwater fishes of the Sudan. J. Zool. London, 158: 143–170.
Khalil, L.F., 1971. Checklist of the helminth parasites of African freshwater fishes. Tech. Comm. 42, Comm. Inst. Helm., C.A.B. England, 80pp.
McDonough, J.M. & Gleason, L.M., 1981. Histopathology in the rainbow darter, Etheostoma caeruleum, resulting from infections with the acanthocephalans Pomphorhynchus bulbocolli and Acanthocephalus dirus. J. Parasitol., 67: 403–409.
Paperna, I., 1964. The metazoan parasite fauna of Israel inland water fishes. Bamidgeh (Bull. Fish Cult. Israel), 16: 3–66.
Paperna, I. & Zwerner, D.E., 1976. Parasites and diseases of striped bass, Morone saxatilis (Walbaum) from the lower Chesapeake bay. J. Fish Biol. 9: 267–287.
Schmidt, G.D., 1985. Development and life cycles. In: Crompton, D.W.T. & Nickol, B.B. (ed.) Biology of Acanthocephala, Univ. Press, Cambridge, pp. 273–286.
Taraschewski, H., 1988. Host-parasite interface of fish acanthocephalans. 1 Acanthocephalus anguillae (Palaeacanthocephala) in naturally infected fishes: LM and TEM investigations. Dis. aquat. Org., 4: 109–119.
Troncy, P.M., 1974. Acanthocephales parasites de poisson d'Afrique, 2'eme note. Bull. Inst. Fond. Afr. Noire (A Sci.Nat.), 36: 902–910.
Troncy, P.M., 1977. Recherches sur les cestodes, nematodes et acanthocephales, parasites des poisson d'eau douce du bassin Tchadien. Ph.D. thesis, Universite des Science et Technique du Languedoc.
Troncy, P.M. & Vassilides, G., 1973. Acanthocephales parasites de poisson d'Afrique. Bull. Inst. Fond.Afr. Noire (A Sci. Nat.), 35, 522–539.
Van As, J.G. & Basson, L., 1984. Checklist of freshwater fish parasites from southern Africa. S.Afr. J. Wildl., 14: 49–61.
ILLUSTRATIONS - Fig. 7 page 175 with legend
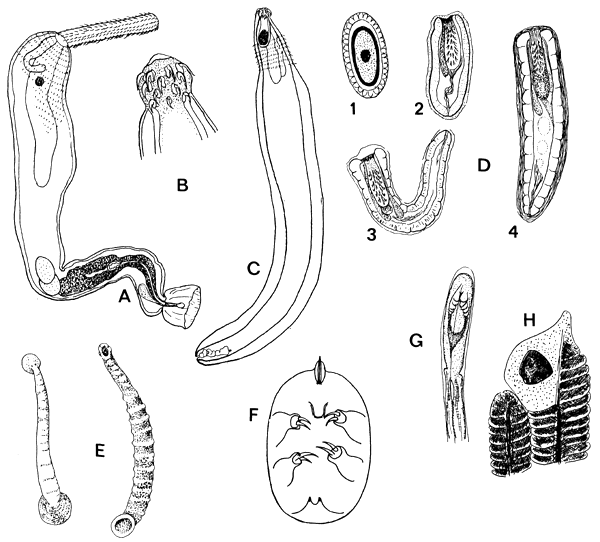
Fig.7. Acanthocephala and various other parasites of fish. A. Paragorgorhynchus chariensis, male, 10–11 mm long. B,C. Pallisentis tetraodontis, female, 4.5 mm long, proboscis and whole view (A–C, after Troncy, 1977). D. Larval stages of acanthocephalans: 1. egg (of Neoechinorhynchus, 60×25 μm); 2. Acanthella from Gammarus amphipod, 1–4 mm long; 3. Acanthella from ostracods (2–4 mm long). 4. Cystacanthus (Acanthor) from fish (3–6 mm long). E. Piscicolid leeches (Hirudinea) (80–100 mm). F. Pentastomid larva. G. Parasitic larva of mutelid bivalve (after Fryer, 1970). H. Unionid glochidium embedded in the gill tissue.