A well designed building should comprise sufficient space for work to be conducted out under adequate hygienic conditions, an area for machinery, equipment and storage, separation of operations that might contaminate food, adequate natural or artificial lighting, ventilation, protection against pests.
There are many technical regulations concerning construction of buildings and processing halls; e.g., outside walls, windows and doors should be constructed such that they are water-, insect- and rodent-proof. The inside walls of the building should be painted white or other light colour and their surface should be smooth, fall-safe, corrosion-proof and easy to clean.
Floors should be resistant to spillage of products, water and disinfectants. They should be slip-proof and maintain their colour. Experience shows that selection and preparation of the floor is one of the most difficult tasks facing the designer. The main problem, however, lies in appropriate general layout and arrangements of rooms which must minimize the risk of contamination of the final product.
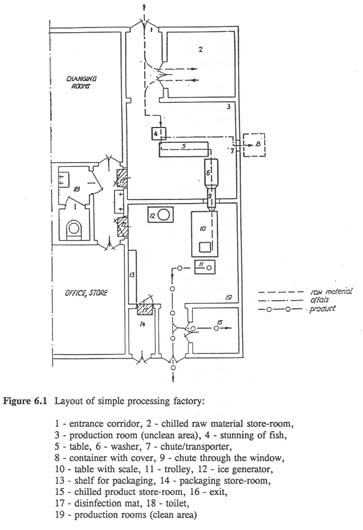
The majority of pathogens and spoilage micro-organisms derive from the raw material. In order to avoid cross-contamination the raw material should be placed in separate cold stores. The best solution seems to be separation by walling-off the unclean area from the clean area. The unclean area is where the raw material is delivered, sorted and possibly processed, e.g., gutted. Clean areas are places of production where any contaminants added to the product could be transmitted to the product, i.e., there is no subsequent processing step that will reduce or destroy the contaminating microbes. Thus separation of these areas has to be complete and there should no movement of people or equipment from unclean to clean area (Figure 6.1).
Proper layout and designs should ensure an uninterrupted and "straight line" process flow, and should meet other requirements listed below (Shapton and Shapton, 1991):
- all functions should avoid zigzagging and backtracking- visitors should move from unclean to clean areas
- conditioned (chilled) air and drainage should flow from clean to unclean areas
- the flow of discarded outer packing material should not cross the flow of either unwrapped ingredients or finished product
- there should be sufficient space for plant operations including processing, cleaning and maintenance; space is also required for movement of materials and pedestrians
- operations are separated as necessary. There are clear advantages in minimizing the number of interior walls since this simplifies the movement of materials and employees, simplified supervision, and reduces the area of wall that needs cleaning and maintenance
The proper design and arrangement of the processing plant greatly influence food production hygiene. Council Directive 89/392/EEC of 14 June 1989 (EEC 1989) on regulations concerning machinery safety and hygiene contains the following most important requirements:
- machinery containing materials intended to come in contact with food must be designed and constructed so that these materials can be cleaned each time they are used- all surfaces and joints must be smooth, with no ridges or crevices that could harbour organic materials
- assembly must be designed so as to minimize projections, edges and recesses; they should be constructed by welding or continuous bonding, with screws, screwheads and rivets used only where technically unavoidable
- contact surfaces must be easy to clean and disinfect, and be built with easily dismantled parts; inside surfaces must be curved so as to allow thorough cleaning
- liquid derived from foods, and cleaning, disinfecting and rinsing fluids should be easy to discharge from machinery
- machinery must be designed and constructed to prevent liquids or living creatures - primarily insects - from entering and accumulating in areas that cannot be cleaned
- machinery must be designed and constructed to avoid ancillary substances, such as lubricants, coming into contact with food
1. Protective clothing, footwear and headgear issued by the company must be worn and must be changed regularly. When considered appropriate by management, a fine hairnet must be worn in addition to the protective headgear provided. Hair clips and grips should not be worn. Visitors and contractors must comply with this regulation.2. Protective clothing must not be worn off the site and must be kept in good condition. If it is in poor condition the supervisor should be informed immediately.
3. Beards must be kept short and trimmed, and a protective cover worn when considered appropriate by management.
4. Nail varnish, false nails and make up must not be worn in production areas.
5. False eyelashes, wrist watches and jewellery (except wedding rings or the national equivalent, and sleeper earrings) must not be worn.
6. Hands must be washed regularly and kept clean at all times.
7. Personal items must not be taken into production areas unless carried in inside overall pockets (handbags, shopping bags must be left in the locker provided).
8. Food and drink must not be taken into or consumed in areas other than the tea bars and the staff restaurant.
9. Sweets and chewing gum must not be consumed in production areas.
10. Smoking or taking snuff is forbidden in food production, warehouse and distribution areas where 'No Smoking' notices are displayed.
11. Spitting is forbidden in all areas of the site.
12. Superficial injuries (cuts, grazes, boils, sores and skin infections) must be reported to the medical unit or nurse via the supervisor and clearance obtained before entering production areas.
13. Dressings must be waterproof and contain a metal strip as approved by the medical unit.
14. Infectious diseases (including stomach disorders, diarrhoea, skin conditions and discharge from eyes, nose or ears) must be reported to the medical unit or nurse via the supervisor. This also applies to staff returning from travel abroad where there could be a risk of infection.
15. All staff must report to medical unit on return from both certified and uncertified sickness.
Disinfectant residues should be monitored where possible and the bacteriological quality periodically checked. Turbidity, colour, taste and odour are also easily monitored parameters. If there are local problems with chemical constituents (fluoride, iron) or contaminants from industry or agriculture (e.g., nitrate, pesticides, mining wastes) these should (hopefully) be monitored and dealt with by the water suppliers (Huss, 1994).
Very often water must undergo treatment disinfection prior to use. The following chemicals are used as disinfectants: chlorine, chloramine, ozone or UV irradiation. Chlorination is the cheapest form of treatment and monitoring of chlorine is relatively easy. According to WHO (1984) the concentration of chlorine in water should be in the range 0.2-0.5 mg/l. For sanitation purposes it may reach 200 mg/l, but in order to avoid corrosion lower concentrations are advised (50-100 mg/l).
The hygienic standards respected in processing plants depend on kinds of production. For example, in the cannery they will be more strict than in plants where fish is only gutted and stored in ice and its shelf life is rather short.
Regarding all other technological operations and processes, cleaning and disinfection procedures must follow detailed instructions and responsible personnel be assigned.
Various steps should be included in a complete cycle of cleaning and disinfection (Huss, 1994):
1. Remove food products, clear area from bins, containers, etc.As mentioned above, only agents and disinfectants permitted by adequate regulations, can be used for cleaning and disinfection operations. During their use precautionary measures must be observed and this requires proper training of personnel.2. Dismantle equipment to expose surfaces to be cleaned. Remove small equipment, parts and fittings to be cleaned in a specified area. Cover sensitive installations to protect them against water, etc.
3. Clear the area, machines and equipment of food residues by flushing with water (cold or hot) and by using brushes, brooms, etc.
4. Apply the cleaning agent and use mechanical energy (e.g., pressure and brushes) as required.
5. Rinse thoroughly with water to completely remove the cleaning agent after the appropriate contact time (residues may completely inhibit the effect of disinfection).
6. Control of cleaning.
7. Sterilization by chemical disinfection or heat.
8. Rinse off the sterilant with water after the appropriate contact time. This final rinse is not needed for sterilants, e.g., H2O2 based formulations which decompose rapidly.
9. After final rinsing, equipment is reassembled and allowed to dry.
10. Control of cleaning and disinfection.
11. In some cases it will be good practice to re-disinfect (e.g., with hot water or low levels of chlorine) just before production recommences.
In a free market economy the producers are responsible for food quality and they are controlled by the competent authorities according to approved procedures. Certain countries or groups of countries, e.g., European Union, formulate regulations specifying requirements concerning health quality, wholesomeness of raw materials and food/fish products and concerning permissible limits for chemical contaminants (heavy metals, PCBs, etc.) or biological infestants (parasites, microbes, etc.). Other regulations concern quality of water provided for food processing (see 6.3.1. "Water quality in processing and cleaning"). These regulations are of rather general character but there are others which concern health conditions for processing and placing of products on the market. Due to an almost complete lack of detailed standards for individual products, the regulations on labelling (see 5.3. "Requirements for the labelling of freshwater fish products") are of great importance, especially if the "fair trade" principle and consumer interest are to be taken into account. All these groups of obligatory regulations should ensure production of food which is safe for the consumer (Huss, 1994).
Additionally the monitoring of raw materials is a complementary part of activities carried out according to requirements contained in regulations. It provides the competent authorities, responsible for supervision of production, with information about potential hazard.
As mentioned earlier, producers are responsible for food quality. Besides the competent authorities such as the Ministry of Health, Ministry of Agriculture and Veterinary Services and consumer organizations or associations, producers also participate in creating new food laws. Such cooperation enables rules corresponding to industrial reality to be created which at the same time ensure consumer safety. Moreover, guides such as: Good Manufacturing Practice, prepared by producer associations, or Codes of Good Manufacturing Practice elaborated by FAO/WHO, are complementary tools widely used in assuring product quality (Codex Alimentarius, 1969). They lay down detailed technological procedures and recommendations for production. Provided they are respected by the producer the expected product quality is reached and consumer safety ensured.
Familiarization with principles contained in these guides and codes is important especially in the case of small food processing plants which unfortunately are often directed by people without adequate professional qualifications and training.
Rules and regulations of US FDA codes and standards of FAO/WHO, Council Directives of EEC (EU) set out an approach to the issue of health quality assurance. According to the above regulations, the main principle is that fish and fish products constitute a source of potential health hazard and danger for consumer safety. Many requirements, regulations, supervision, controls, inspections and governmental interventions stem from that principle.
Listed below are some basic regulations on requirements for fish and fish products from aquaculture, which are either compulsory or have been introduced in European Union countries. These documents concern also Third Countries which export fish and fish products to the EU market. Requirements in this context are covered by the following documents:
1. Council Directive (91/67/EEC) of 28 January 1991 concerning the health conditions of animals destined for marketing and originating from aquacultureThis first directive states that animals must:2. Council Directive (91/493/EEC) of 22 July 1991 laying down the health conditions for the production and the placing of fishery products on the market
3. Council Regulation (EEC No 3759/92) of 17 December 1992 on the common organization of the market in fishery and aquaculture products
- be free of clinical signs of disease on the day of loading;- not be directed for processing in order to liquidate such diseases as:
- Infectious haematopoietic necrosis (IHN),
- Viral haemorrhagic septicaemia (VHS),
- Infectious pancreatic necrosis (IPN),
- Bacterial kidney disease (BKD),
- Spring viremia of carp (SVC),
- Enteric red mouth disease (ERM)
- Gyrodactylosis (Gyrodactylus salaris)
- Myxobolosis (Myxosomiasis - whirling disease);
- not come from a farm which is closed due to diseases, and must not be in contact with fish from such a farm;
- be subject to the same requirements if directed for farming;
- be delivered, in the case of aquaculture fish, in the shortest possible time to the destination, and the change of water must only be done in specified places. Such places must be known to European Union countries;
- the Commission checks if regions are free of diseases, approves them and can, within reason, also revoke approval of the decision; and finally makes a list of approved fish farms;
- permission may be granted for placing on the market fish from aquaculture and from regions not approved but under special conditions. Such instances require documentation confirming the wholesomeness of fish from this region, and this must be issued by official inspectors, for example, by the competent veterinary authorities.The region can be approved as free of diseases if it meets at least two requirements:
- the diseases listed above did not occur for at least four years,
- all fish farms located in this region are under continuous veterinary supervision and are inspected at least twice a year.Veterinary inspection should cover the visual assessment of aquaculture fish wholesomeness, taking of fish samples and immediately sending them to the competent laboratory. Each farm must record all necessary data pertaining to the wholesomeness of fish including the official certificate of laboratory analysis. It is pointed out that only certificates issued by official control authorities are valid and placing of fish in the fish farm or for sale has to be formally documented.
The above rules concern all the fish being sold domestically and not only the fish directed for European markets.
Fish processing plants which, apart from farming carry out processing must obtain the approval of veterinary authorities to export their products. Acquiring a registration number is a formal approval to export fish. This registration number enables identification of the fish product, and this number must be shown on the label of each package and on the relevant documents.
Aquaculture fish and fish products exported to the EU must fulfil the conditions specified in Council Directive 91/493/EEC. The level of requirements in this Directive indicates that many fish processing plants will face great difficulty in obtaining an export licence. Thus each establishment should draw up its own production programme covering for example:
- kind of production (for example fresh fish, frozen fish, canned products, etc.),
- volume of production (daily, annual),The competent veterinary authorities evaluate the production capacity and possibilities of fulfilling the production programme, taking into account the technical abilities and insurance of adequate sanitary conditions.- production rooms and store rooms,
- technical equipment,
- sanitary facilities for staff,
- written schedule of quality assurance system and adherence to it
- temperature control of fish during transportation (temperature record)The temperature of the fish hold in the transport system is usually registered automatically or periodically by a driver. This temperature record is part of the documentation on fish shipment. Measurements of real temperature of fish tissue and control of icing are made on random samples. Apart from these elements the cleanliness of the means of transport and the containers, and the labelling, are checked. The number of samples/packages with fish to be further assessed depends on lot size, and it should be specified clearly in compulsory procedures or codes of good manufacturing practice, perhaps in the standards or contract specifications. The temperature of purchased fish should be close to ice melting temperature and not higher than 4° C. The samples of fish taken for temperature measurement are at the same time the samples examined for quality control of raw material. Usually in the case of medium size batches eight packages are taken and in each package three temperature checks are made.- temperature control of fish and control of icing
- quality control of purchased fish
Detailed quality assessment is made according to requirements laid down in procedures, codes or standards if the latter exist. Such an assessment is carried out on an average sample from a set of randomly selected packages.
The sensory analysis of raw material is a main part of control, and it allows full characteristics of the fish investigated to be obtained. This analysis includes appearance of skin, eyes, gills and fish as a whole, colour of fish tissue; damage to fish, springiness of meat tissue, flavour of individual organs; flavour, taste and texture of meat tissue after cooking. Occurrence of inadmissible features like for example sour smell of gills, strange/unfamiliar smell of meat or fish as a whole causes that raw material is disqualified and excluded as a material intended for processing. In the case of live fish their appearance and movement in the water in a container are assessed.
The kind and the degree of infestation with parasites determines further procedure. If the presence of parasites which are harmful for humans is detected, fish cannot be sold as fresh. As mentioned above, this matter should be considered by the receiver when the contract is prepared. The final result of quality control of raw material is decisive with respect to further procedure during fish processing. Generally when fish is qualified as conforming with requirements and cooled properly it is placed in cold stores or transported direct to the processing line. Ice is added to fish cooled insufficiently and this is placed in cold store. The temperature inside the cold store should be close to 0° C and should be continuously recorded. If temperature cannot be registered automatically, measurements should be taken not less frequently than every two hours.
Technological supervision is responsible for use of adequate processing parameters. Quality control personnel are responsible for monitoring these parameters and in the case of deviation they should undertake proper corrective action.
The final step in production control is the quality control of the final product according to technical requirements and specifications included in the contract or standards if the latter are compulsory. Such assessment is carried out according to approved procedures with special regard to health quality requirements pertaining in a given country. This type of control will disappear in the future because an introduction of quality assurance systems, as a continuous control throughout the entire processing procedure, will eliminate this traditional form of control (Bonell, 1994; Jakobsen and Lillie, 1992; Huss, 1994).
Quality control personnel are also responsible for supervision of assurance of cleanliness and disinfection of production lines and processing rooms. Maintenance of cleanliness and disinfection should be carried out in accordance with a programme approved by the local veterinary service. The quality control staff assures adherence to this programme which especially concerns:
- types of detergents/disinfectants and concentrations used;In summary, the quality control staff is responsible for carrying out this programme and for the sanitary-hygienic conditions of the processing plant and for maintaining the documentation relating to these activities.- compliance with procedures of cleaning/washing and disinfection;
- arrangement of periodic microbiological measurements on the surface of equipment and processing machines;
- control of personal hygiene of staff including working clothes and sanitary fittings in the plant.
- proper packaging materials and labelling (according to official requirements);- duration and temperature of storage;
- proper conditions of storage, for example adequate ice, temperature etc;
- choice of means of transportation and hygienic conditions (cleanliness, temperature record, etc.);
- proper loading (e.g., arrangement of load in vehicles).