Animal metabolism
Humans. In a volunteer study (Hutson, 1969) a male was given a single oral dose of 12.5 mg of [14C]chlorfenvinphos in olive oil. The radiolabel was rapidly excreted in the urine with 72% of the applied dose excreted in the first 4.5 hours and 94.2% in 26.5 hours. Five metabolites were identified in the urine, two of which were quantified. These were 2-chloro-1-(2,4-dichlorophenyl)vinyl ethyl hydrogen phosphate and 2,4-dichloromandelic acid, which accounted for 23.8 and 23.9 % of the applied dose respectively. The other three metabolites were tentatively identified as [1-(2,4-dichlorophenyl)ethyl-P-D-glucopyranosidyl]uronic acid, 2,4-dichlorophenylethanediol glucuronide and 2,4-dichlorohippuric acid (N-2,4-dichlorobenzoylglycine).
Rats and dogs. In a study on rats and dogs (Hutson and Hathway, 1966) rats were given single oral doses of 2 mg/kg [14C]chlorfenvinphos. Within 96 hours 87% of the applied dose was excreted in the urine, 1.4% in expired air and 11% in the faeces. Most of the radiolabel in the urine was excreted in the first 24 hours.
Dogs were given single oral doses of 0.3 mg/kg [14C]chlorfenvinphos in gelatine capsules. In the first 24 hours 86% of the applied dose was excreted in the urine, and in 96 hours 89.4% was excreted in the urine and 4.5% in the faeces.
The urine was analysed for metabolites: five were identified from the rats and four from the dogs. Their relative proportions are shown in Table 1.
Table 1. Metabolites of chlorfenvinphos in rat and dog urine.
|
Metabolite |
% of 14C in urine |
|
|
Rat |
Dog |
|
|
2,4-dichlorophenylethanediol glucuronide |
3 |
3 |
|
[1 -(2,4-dichlorophenyl)ethyl-P-D-glucopyranosidyl]uronic acid |
47 |
4 |
|
2,4-dichlorohippuric acid |
5 |
absent |
|
2,4-dichloromandelic acid |
8 |
5 |
|
2-chloro-1-(2,4-dichlorophenyl)vinyl ethyl hydrogen phosphate |
37 |
78 |
Cattle. In a briefly reported study (Hutson and Hoadley, 1969; Hunter, 1969), one small (400 kg) Friesian cow was given a single intramuscular injection of 233 mg of [vinyl-l,2-14C]chlorfenvinphos (unspecified radiochemical purity; specific radioactivity 2.8 m Ci/mg) in 'Infonutrol'. The cow had free access to water and hay, was fed 3.6 kg of concentrate per day over the five day duration of the study and was milked twice daily (at 10 am and 4 PHI).
Milk samples were analysed for total radioactive residues by LSC, and were found to contain a maximum initial radioactive residue of 0.076 mg/kg chlorfenvinphos equivalents. Overall, only 0.2% of the administered dose was recovered in the milk (Table 2).
Table 2. Radioactive residues in milk after intramuscular administration of [vinyl-14C]chlorfenvinphos to a cow.
|
Day |
Time |
l4C |
|
|
% of administered dose |
mg/kg parent equivalents |
||
|
1 |
4 pm |
0.13 |
0.076 |
|
2 |
10 am |
0.04 |
0.011 |
|
2 |
4 pm |
0.01 |
0.006 |
|
3 |
10 am |
0.01 |
0.004 |
|
3 |
4 pm |
0.009 |
0,006 |
|
4 |
10 am |
0.006 |
0.002 |
|
4 |
4 pm |
0.0005 |
0.0003 |
|
5 |
10 am |
0.001 |
0.0005 |
The nature of the residues was investigated in the first milk sample. The second sample was analysed for the parent compound only. The milk was separated into cream, residual whey, and precipitated protein by centrifugation. The cream was extracted with acetone and hexane. The radioactivity was distributed as follows: hexane-soluble fat 52%, acetone-soluble fat 28%, insoluble fat residue 3%, whey 13%, and insoluble protein 4%. A fat sample was prepared by mixing dried cream with sodium sulfate before dissolution in acetone/hexane and concentration by evaporation. The fat content of the milk was estimated as 5%. TLC of the fat solution with reference standards showed mainly chlorfenvinphos (0.049 mg/kg) with the metabolites (found in the range 0.0004 to 0.0023 mg/kg) shown in Table 3. The levels of unchanged chlorfenvinphos in the first and second milk samples represented 75% and 60% of the total radioactive residue (TRR) respectively. The major metabolite found in milk was 2,4-dichloroacetophenone (III), found only at a level of 0.0023 mg/kg (3.6% of the TRR). Of the radioactivity remaining in the whey, 29% was extracted with ether at neutral pH (postulated as parent) and 23% was extracted at pH 2 (considered to be indicative of metabolites VI and IX).
Table 3. Distribution and nature of the radioactive residue in milk fat.
|
Metabolites |
Residue in milk fat expressed as mg/kg in whole milk | |
|
I |
chlorfenvinphos |
0.049 |
|
II |
2,4-dichlorophenacyl chloride |
0.0008 |
|
III |
2,4-dichloroacetophenone |
0.0023 |
|
IV |
1-(2,4-dichlorophenyl)ethanol |
0.0014 |
|
V |
1-(2,4-dichlorophenyl)ethane-1,2-diol |
not detected |
|
VI |
2,4-dichloromandelic acid |
0.0011 |
|
VII |
2,4-dichlorobenzoic acid |
0.0014 |
|
VIII |
2-chloro-1-(2,4-dichlorophenyl)ethanol |
0.0004 |
|
IX |
desethyl-chlorfenvinphos |
0.0007 |
Urine was sampled at an unspecified time and found to contain 29% of the administered dose, of which 90% was extracted with ether/ethanol. Paper chromatography in butanol/ammonia revealed the presence of metabolites IV, V, VI, and IX accounting for 34%, 23%, 12% and 57% of the extracted radioactive residue.
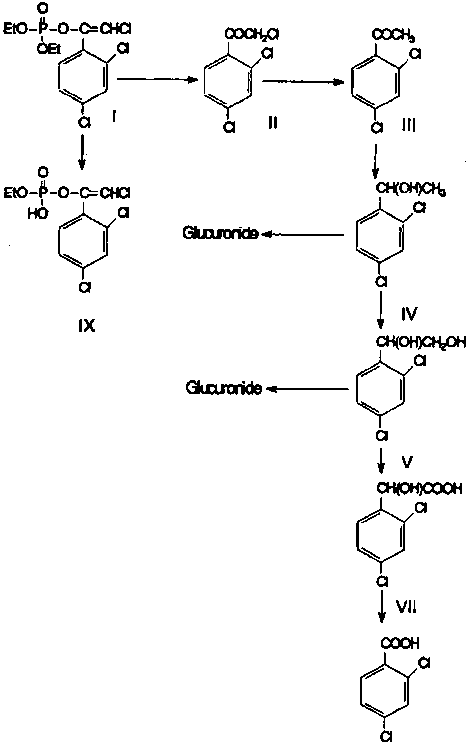
The proposed metabolic pathway for chlorfenvinphos in ruminants is given in Figure 1 below.
A number of investigations with [32P]chlorfenvinphos were briefly reported in a paper published in 1966. In the first of these [32P]chlorfenvinphos (unspecified radiochemical purity) was applied dermally to two calves in two litres of spray (one at 0.25% and the other at 0.05% concentration). Omental fat samples were taken at 3, 7 and 15 days after spraying and were found to contain radioactive residues of 0.675, 0.055 and "0" mg/kg from the 0.25% treatment and 0.06, 0.001 and "0" mg/kg from the 0.05% treatment.
In a second investigation, two calves were similarly treated dermally with 2 litres of a spray emulsion, one at 0.25% and the other at 0.5% concentration. Both animals were killed 7 days after treatment and samples of renal and omental fat, heart, kidney, and muscle were taken for radiometric analysis. The results are shown in Table 4.
Table 4. Radioactive residues in fat and tissues of calves 7 days after treatment with a [32P]chlorfenvinphos spray.
|
Sample |
32P as chlorfenvinphos, mg/kg |
|
|
0.25% spray |
0.5% spray |
|
|
Renal fat |
0.042 |
0.204 |
|
Omental fat |
0.036 (0.361) |
0.223 |
|
Heart |
0.002 |
0.015 |
|
Kidney |
0.001 |
0.008 |
|
Muscle |
0.001 |
0.008 |
1 Additional sample taken by omentectomy 24 hours after treatment
Figure 1. Proposed metabolic pathways of chlorfenvinphos in ruminants.
In a third investigation, three Hereford calves were treated "to saturation" with a 0.25% spray emulsion of chlorfenvinphos. The calves were killed 7 (calf A), 16 (calf B) and 28 (calf C) days after treatment. Samples of omental and renal fat, muscle, heart, kidney, liver, brain, and spleen were analysed for the parent compound by GLC (Table 5).
Table 5. Residues of chlorfenvinphos in fat and tissues of cattle sprayed 'to saturation' with a 0.25% spray of chlorfenvinphos.
|
Sample |
Chlorfenvinphos, mg/kg, at intervals, days, after spraying |
||
|
7 |
16 |
28 |
|
|
Omental fat |
0.085 |
0.006 |
<0.005 |
|
Renal fat |
0.021 |
<0.005 |
<0.005 |
|
Muscle |
<0.004 |
<0.004 |
<0.004 |
|
Heart |
<0.004 |
<0.004 |
<0.004 |
|
Kidney |
<0.004 |
<0.004 |
<0.004 |
|
Liver |
<0.004 |
<0.004 |
<0.004 |
|
Brain |
<0.004 |
<0.004 |
<0.004 |
|
Spleen |
<0.004 |
<0.004 |
<0.004 |
In a fourth, more comprehensive, investigation (Ivey et al., 1966) six Hereford cattle (group A) were sprayed 12 times at weekly intervals with a 1% emulsion of chlorfenvinphos. Another group (B) of six cattle was sprayed six times at two-week intervals with the same concentration of spray. Control animals were sprayed with "formulation blank". Fat samples were taken by omentectomy from three animals from group A, one week after the 1st, 2nd, 4th, 6th, 8th, 10th and 12th spray treatments, and from three animals from group B two weeks after each treatment. The samples were analysed for chlorfenvinphos and the metabolite 2,4-dichlorophenacyl chloride by GLC. 2,4-dichlorophenacyl chloride was not detected in any of the samples. The residues of chlorfenvinphos in the omental fat of the cattle in groups A and B are shown in Tables 6 and 7 respectively. All results were corrected for blanks and a recovery of 80%.
Table 6. Residues of chlorfenvinphos in omental fat from cattle sprayed weekly with a 0.1% emulsion.
|
Animal |
Residues, mg/kg, in omental fat 7 days after indicated spray |
||||||
|
1st |
2nd |
4th |
6th |
8th |
10th |
12th |
|
|
A.1 |
0.012 |
|
|
|
0.161 |
|
0.010 |
|
A.2 |
0.009 |
|
0.065 |
|
0.121 |
|
0.010 |
|
A.3 |
0.056 |
|
0.142 |
|
0.245 |
|
0.020 |
|
A.4 |
|
0.047 |
|
0.051 |
|
0.020 |
|
|
A.5 |
|
0.070 |
|
0.065 |
|
0.019 |
|
|
A.6 |
|
0.020 |
|
0.035 |
|
0.009 |
|
Table 7. Residues of chlorfenvinphos in omental fat from cattle sprayed biweekly with a 0.1% emulsion.
|
Animal |
Residues, mg/kg, in omental fat 14 days after indicated spray |
|||||
|
1st |
2nd |
3th |
4th |
5th |
6th |
|
|
B.1 |
<0.005 |
|
<0.005 |
|
0.247 |
|
|
B.2 |
0.006 |
|
0.006 |
|
0.170 |
|
|
B.3 |
<0.005 |
|
<0.005 |
|
0.080 |
|
|
B.4 |
|
0.009 |
|
<0.005 |
|
0.180 |
|
B.5 |
|
0.008 |
|
0.007 |
|
0.110 |
|
B.6 |
|
<0.005 |
|
<0.005 |
|
|
No residues of chlorfenvinphos were detected in omental or renal fat taken from animals of group A or B slaughtered 14 and 28 days after the last spray respectively.
In a very briefly reported study (Roberts et al., 1961) two dairy cows were sprayed with 32P-labelled chlorfenvinphos (unspecified radiochemical purity; specific activity 3.4 mCi/g). One cow (Holstein) was treated with 400 ml of a water-based spray formulated from a simple EC containing 5 g of the radiolabelled compound. This was done by spraying 200 ml on each side of the cow, avoiding the udder, and working into the hair with a comb. The second cow (Jersey) was similarly treated with 5 g of 32P-labelled chlorfenvinphos (unspecified radiochemical purity; specific activity 1.7 mCi/g), using a different EC formulation based on xylene and lanolin in a total spray volume of 60 ml; this was not worked into the hair, and resulted in a loss of about 5%. Duplicate milk samples (200 ml) were taken from the morning milk just before treatment and up to 12 days after treatment. The organosoluble radioactivity was extracted and determined with a Geiger tube. The maximum residues were found in the milk sampled 5 hours after treatment, 0.06 mg/kg in the Holstein and 0.03 mg/kg in the Jersey. One day after treatment the residues had decreased to 0.011 mg/kg and 0.005 mg/kg in the Holstein and Jersey milk, and residues were finally eliminated in 12 and 10 days after treatment respectively.
Chamberlain and Hopkins (1962) applied [32P]chlorfenvinphos (radiochemical purity in the range 76 to 87%) at 55, 25 and 8 mg/kg body weight to the back and sides of three steers, A, B and C respectively, in a volume of 300 ml as an EC spray using a chromatography spray bottle held 1.2 cm from the surface of the skin, with subsequent combing into the skin. Blood samples and excreta were taken at regular intervals for 1 week after treatment and radioassayed with a gas-flow proportional counter. The results are shown in Table 8. It was stated that 18 to 42% of the chloroform-soluble radioactivity in the blood co-chromatographed with unchanged chlorfenvinphos. Twenty five to 35% of the applied radioactivity was excreted in the urine, but only 2% was recovered from the faeces.
It was reported, although full details were not given, that 9 or 10 radioactive compounds were excreted in the urine, one of which (representing 2 to 14% of the TRR) co-chromatographed with dimethyl hydrogen phosphate. Another metabolite (in the range 0.4 to 7%) was tentatively identified as diethyl l-methyl-2-chlorovinyl hydrogen phosphate. The predominant component, which represented "49% of all the radioactive material in early hourly samples", remained unidentified. It was stated to decrease in concentration with time. A further unidentified component was reported in the range 6 to 44% of the TRR.
Table 8. Total radioactive residues in blood, urine and faeces of dermally treated steers.
|
Time |
32P as chlorfenvinphos, mg/kg |
||||||||
|
Steer A |
Steer B |
Steer C |
|||||||
|
Blood1 |
Urine |
Faeces |
Blood1 |
Urine |
Faeces |
Blood1 |
Urine |
Faeces |
|
|
1 h |
7.1 (1.4) |
741 |
|
|
|
|
0.7 |
2.8 |
|
|
2 h |
7.8 (1.2) |
2504 |
|
3.9 (0.3) |
|
|
0.9 |
16 |
|
|
3 h |
6.7 (0.8) |
2966 |
7.2 |
3.9 (0.7) |
1148 |
1.1 |
0.8 (0.04) |
27 |
0.5 |
|
6 h |
3.8 (0.8) |
2589 |
13 |
2.2 (0.3) |
1117 |
2.9 |
0.6 |
84 |
2.2 |
|
9 h |
3.2 (0.4) |
1556 |
113 |
|
|
|
0.4 |
74 |
|
|
12 h |
3.3 |
1445 |
|
|
|
|
0.3 |
57 |
|
|
18 h |
2.9 |
918 |
428 |
0.8 (0.2) |
408 |
56 |
0.2 |
56 |
|
|
1 day |
2.1 |
684 |
441 |
0.7 |
193 |
52 |
0.2 |
38 |
7.6 |
|
2 days |
1.5 |
196 |
108 |
|
121 |
32 |
0.2 |
26 |
4.9 |
|
4 days |
1.1 |
46 |
26 |
0.6 |
57 |
42 |
0.2 |
17 |
5.0 |
|
7 days |
0.9 |
18 |
21 |
0.4 |
18 |
7 |
0.3 |
6.6 |
3.8 |
1 Chloroform-soluble residues are shown in parentheses
A further study on the toxicology and metabolism of chlorfenvinphos (Herbst and Herbst, 1995) was submitted but was not evaluated because it was written in German.
Plant metabolism
In a 1965 study, later described in two papers and summarized in a further review (Beynon and Wright 1965, 1967; Beynon et al. 1973; Anon, undated) [14C]vinyl-labelled (E)-chlorfenvinphos (radiochemical purity not specified) was applied to soil around cabbage plants at a rate of 4 mg per plant (growth stage not specified) to soil eight weeks after it had been sown with carrots at an application rate of 3.4 kg ai/ha, and to soil ten weeks after it had been sown with onions at a rate of 4.5 kg ai/ha. Cabbages were harvested 12-14 weeks, and carrots and onions 18 weeks, after treatment. All three crops were grown in the laboratory.
The samples were extracted with acetone and analysed by TLC (only brief details supplied). Quantification of the unextractable residues was by combustion analysis.
The results are summarized in Tables 9-11 below. In cabbages no radiolabel (<0.01 mg/kg as chlorfenvinphos) was detected in the heart but 0.11 mg/kg was found in the outer leaves, of which 0.05 mg/kg was extractable but not characterized. An acetone extract of the stump/root was found to contain a residue of 0.26 mg/kg, of which 95% was chlorfenvinphos and 5% 2,4-dichloroacetophenone. A total residue of 0.15 mg/kg was found in the roots of carrots, of which 0.12 mg/kg was chlorfenvinphos, and a total residue of 0.08 mg/kg in onion bulbs, of which 0.07 mg/kg was chlorfenvinphos.
Table 9. Residues of (E)-[vinyl-14C]chlorfenvinphos and its breakdown products in cabbages grown indoors following application to the soil around the roots at transplanting.
|
Sample |
14C as chlorfenvinphos1 |
|
|
Acetone-extractable |
Acetone-unextractable |
|
|
Heart |
0.005 |
0.005 |
|
Outer leaf |
0.05 |
0.06 |
|
Dead leaf (on soil) |
0.15 |
0.04 |
|
Stump and root |
0.262 |
0.26 |
1 Controls <0.005 mg/kg
2 95% chlorfenvinphos, 5% 2,4-dichloroacetophenone
Table 10. Residues of (E)-[vinyl-14C]chlorfenvinphos and its breakdown products in carrots grown indoors.
|
Sample |
Acetone extractability |
Component |
14C as chlorfenvinphos1 |
|
edible root |
Extractable |
Chlorfenvinphos |
0.12 |
|
|
|
2,4-dichloroacetophenone |
0.01 |
|
|
Unextractable |
Unidentified |
0.024 |
|
leaf |
Extractable |
Chlorfenvinphos |
0.33 |
|
|
Unextractable |
Unidentified |
0.02 |
1 Recovery of [14C]chlorfenvinphos at approximately 1 mg/kg was 82%
Table 11. Residues of (E)-[vinyl-14C]chlorfenvinphos and its breakdown products in onions grown indoors.
|
Sample |
Acetone extractability |
Component |
14C as chlorfenvinphos1 |
|
Bulb |
Extractable |
Chlorfenvinphos |
0.07 |
|
|
Unextractable |
Unidentified |
0.01 |
|
Leaf |
Extractable |
Unidentified |
0.05 |
|
|
Unextractable |
Unidentified |
0.01 |
1 Recovery of [14C]chlorfenvinphos at approximately 0.7 mg/kg was 90-95%. Control plants showed 14C corresponding to <0.01 mg/kg
In reviews of the metabolism and degradation of vinyl phosphate insecticides (Beynon et al., 1973; Beynon and Wright, 1968) it was reported that [14C]vinyl-labelled (E)-chlorfenvinphos of unspecified radiochemical purity was foliar-applied (precise method and rate not specified) to potatoes, cabbage and maize growing in a greenhouse. Analyses of crop samples taken 28-112 days after treatment gave the results shown in Table 12. The methods used to extract and analyse the samples were not described.
In potatoes, 39% of the applied 14C was found in the foliage after 28 days and less than 0.5% in the tubers after 80 days. Evidence for identification was not given, but the authors indicated that 21% of the applied radiolabel represented chlorfenvinphos, 11% a conjugate of 1-(2,4-dichlorophenyl)ethanol and 7.2% could not be extracted with acetone. They suggested that plant metabolism studies with tetrachlorvinphos indicated that the unextracted residues were mainly further quantities of conjugates of 1-(2,4-dichlorophenyl)ethanol.
Twenty per cent of the radiolabel applied to cabbages was found in the foliage 24 days after treatment: 6.7% of the dose as chlorfenvinphos and 6.7% as the 1-(2,4-dichlorophenyl)ethanol conjugate; 6.7% could not be extracted with acetone and again appeared to consist mainly of conjugates of 1-(2,4-dichlorophenyl)ethanol.
In maize, 54% of the applied radiolabel was found in the foliage after 24 days and less than 0.5% in the grain after 112 days. In the foliage 26% of the dose was chlorfenvinphos, 12% the 1-(2,4-dichlorophenyl)ethanol conjugate and 16%, unextractable with acetone, apparently also conjugates of 1-(2,4-dichlorophenyl)ethanol.
Table 12. Metabolites found after foliar treatment of glasshouse crops with [14C]chlorfenvinphos.
|
Crop |
Sample |
Days from treatment to sampling |
% of applied 14C |
|||
|
Chlorfenvinphos |
Conjugate of 1-(2,4-dichlorophenyl)ethanol1 |
Unextracted by acetone2 |
Total |
|||
|
Potato |
Whole plant above ground |
28 |
21 |
11 |
7.2 |
39 |
|
Tubers |
80 |
- |
- |
- |
<0.5 |
|
|
Cabbage |
Whole plant above ground |
24 |
6.7 |
6.7 |
6.7 |
20 |
|
Maize |
Whole plant above ground |
24 |
26 |
12 |
16 |
54 |
|
Grain |
112 |
- |
- |
- |
<0.5 |
|
1 Approximately 1% of the activity ascribed to the conjugate could be from desethyl-chlorfenvinphos
2 Probably also mainly conjugates of 1-(2,4-dichlorophenyl)ethanol
The metabolic pathway proposed on the basis of foliar application is shown in Figure 2.
Figure 2. Metabolism of chlorfenvinphos in plants following foliar treatment.
Environmental fate in soil and water/sediment systems
In the study of plant metabolism following soil application described above (Beynon and Wright, 1965), further work was carried out to identify degradation products in the soil. In addition, a second phase of the study involved the treatment of different soil types with higher rates of [vinyl-14C]chlorfenvinphos (15 mg/kg) in closed containers. Acetone extracts of soil samples taken from below the onion crop were reported to contain chlorfenvinphos at 2.4 mg/kg, desethyl-chlorfenvinphos (near 0.02 mg/kg) and 2,4-dichlorophenacyl chloride. Further treatment of the soil with acid extracted 0.35 mg/kg chlorfenvinphos equivalents, which consisted of chlorfenvinphos (0.28 mg/kg), desethyl-chlorfenvinphos (0.07 mg/kg) and a trace of 2,4-dichlorophenacyl chloride. The authors stated that the desethyl-chlorfenvinphos in the acid extract may have been present as such in the soil but was more likely to have been in the form of a salt or conjugate which was hydrolysed to desethyl-chlorfenvinphos by the acid. Few further details were given, and no results of the second phase were presented.
In a summarized study of the degradation of chlorfenvinphos in soil under laboratory conditions (Anon., undated; Beynon et al., 1973) [vinyl-14C]chlorfenvinphos was applied to 4 different soils at an initial concentration of 15 mg/kg. The pH and water contents of the soils are given in Table 13. The soils were incubated in the dark at 22°C and samples were taken for analysis at intervals for 4 months.
Table 13. Characteristics of experimental soils.
|
Soil type |
pH |
Water content (% w/w) |
|
Clay |
8.0 |
21.1 |
|
Loam |
8.0 |
15.1 |
|
Sand |
7.9 |
13.9 |
|
Peat |
6.4 |
88.6 |
Extracts of the soils were examined for products of degradation by TLC with radio-analysis, with the results shown in Table 14. Radioactivity designated as unextractable was obtained by oxidation of the treated soil by "Van Slyke oxidation".
Table 14. Residues of [14C]chlorfenvinphos and its degradation products in soils four months after treatment.
|
Compound or fraction |
Residue, mg/kg moist soil |
|||
|
Clay |
Loam |
Sand |
Peat |
|
|
desethyl-chlorfenvinphos |
0.2 |
0.1 |
0.2 |
0.1 |
|
(2,4-dichlorophenyl)ethan-1,2-diol |
£ 0.02 |
£ 0.02 |
£ 0.03 |
£ 0.02 |
|
unknown |
0,07 |
0.06 |
0.04 |
0.1 |
|
1 -(2,4-dichlorophenyl)ethanol |
1.0 |
0.1 |
0.06 |
0.2 |
|
chlorfenvinphos |
2.0 |
4.2 |
1.0 |
4.7 |
|
2,4-dichloroacetophenone |
0.5 |
0.2 |
0.1 |
0.2 |
|
2,4-dichlorophenacyl chloride |
£ 0.005 |
£ 0.005 |
£ 0.005 |
£ 0.005 |
|
2,4-dichlorophenyloxirane |
£ 0.005 |
£ 0.005 |
£ 0.005 |
£ 0.005 |
|
salts or conjugates of desethyl-chlorfenvinphos |
0.1 |
0.5 |
0.6 |
<0.05 |
|
unextractable radioactivity |
2.0 |
1.8 |
- |
- |
The pathways for the degradation of chlorfenvinphos proposed by the authors are shown in Figure 3. Structures enclosed in brackets were described as "transient intermediates", although no derivative of the phenethyl alcohol "intermediate" is suggested.
Figure 3. Proposed degradation pathways of chlorfenvinphos in soil.
The high application rate was employed to identify products which might not be identified at lower rates. Radioactivity which was not recovered from the soils represented 60-80% of the applied dose; it included 14CO2 and residues which could not be extracted with common organic solvents. The predominant products were 1-(2,4-dichlorophenyl)ethanol, 2,4-dichloroacetophenone and the sodium salt of desethyl-chlorfenvinphos.
In additional summarized experiments (Beynon et al., 1973) onions and carrots grown in boxes containing John Innes No 2 compost under glasshouse conditions were treated with [14C]chlorfenvinphos at the commercial rate of 3.4-4.5 kg/ha. Eight weeks after application of the insecticide the 14C in the compost, expressed as mg chlorfenvinphos equivalents/kg moist soil, was accounted for by 2.7 mg/kg of chlorfenvinphos, 0.09 mg/kg of desethyl-chlorfenvinphos and 0.03 mg/kg of 2,4-dichloroacetophenone or 2,4-dichlorophenacyl chloride.
A summarized study (Anon., undated), presented as a poor copy which was illegible in places, described three further experiments on degradation in field soils. In all of these it was unclear whether the application rate referred to product/ha or active ingredient/ha. In the first experiment, chlorfenvinphos was applied at 4.5 or 9 kg/ha to crops in the field at 4 sites in the UK in spring or summer. Soil samples were taken for analysis at intervals up to 6 months. The soils were a brick-earth, a sandy loam, a loam and a peat. Half-lives of chlorfenvinphos were in the range of about 14-84 days in the mineral soils and more than 150 days in the peat soil. 2,4-dichlorophenacyl chloride was found in peat samples taken 4 weeks or more after treatment at concentrations up to 0.1 mg/kg of soil (105 day sample) after application of chlorfenvinphos at 9 kg/ha. The properties of the soils were not given.
In the second experiment, chlorfenvinphos was applied to field soils at rates of 4.5, 6.7, 9 or 22 kg/ha. Samples of soil were taken for analysis at intervals up to 6 months after application in spring or summer and examined for the degradation products 1-(2,4-dichlorophenyl)ethanol, 2,4-dichloroacetophenone and 2,4-dichlorophenacyl chloride. There was no evidence of isomerisation of the (Z)- isomer in soil. 2,4-Dichlorophenacyl chloride was not detected in the soils within 6 months of application at 4.5 or 6.7 kg/ha but was found at a concentration of 0.1 mg/kg 105 days after application at 9 kg/ha. The highest residue of 2,4-dichloroacetophenone was 0.2 mg/kg, found 30 days after application at 9 kg/ha. 1-(2,4-dichlorophenyl)ethanol was not detected within 6 months of treatment at 4.5-9 kg/ha with a limit of detection of 0.2 mg/kg, but was found at 0.6 mg/kg 28 days after application of the unrealistically high rate of 22 kg/ha.
In the third experiment, carried out in 1966-7, labelled chlorfenvinphos was applied as a GR to a brick loam soil and as an EC to clay loam soil in the UK at 4 kg ai/ha. The residues remaining in soil samples taken at intervals are given in Table 15.
Table 15. Decay of chlorfenvinphos residues in soils.
|
Interval |
Chlorfenvinphos equivalents, mg/kg |
|
|
Faversham brick loam |
Woodstock clay loam |
|
|
0 days |
- |
3.2 |
|
2 days |
4.6 |
- |
|
1 week |
4.4 |
- |
|
2 weeks |
2.6 |
- |
|
4 weeks |
4.4 |
3.3 |
|
10 weeks |
1.1 |
1.9 |
|
20 weeks |
- |
1.1 |
|
52 weeks |
0.11 |
0.4 |
|
82/86 weeks |
0.05 |
0.3 |
|
99 weeks |
Illegible |
- |
|
107 weeks |
|
0.04 |
A further paper was submitted which provided an overview of the occurrence and fate of residues in soil, mainly of the work described above (Anon., 1985). Laboratory data on the degradation of chlorfenvinphos in water/sediment systems (Wable, 1993) and in fresh water aquatic systems (Edwards and Gibb, 1981) were also submitted but not reviewed.