Animal metabolism
The biokinetics and metabolism of teflubenzuron were studied in rats, lactating goats and laying hens.
Rats. The Absorption, distribution, excretion and metabolism of teflubenzuron in the rat were studied by Schlüter (1984, 1985a, 1986a). [14C]teflubenzuron labelled in the aniline ring was administered by oral gavage.
Experiments were performed at dose levels of 25 and 750 mg ai/kg body weight (Schlüter, 1984). The test substance was applied as a suspension in a 1:1 mixture of 1% Tylose C 30 (methylcellulose) and 1% Tween 80 (polyoxyethylene sorbitan mono-oleate) ensuring a high concentration and stable suspension of teflubenzuron. The low dose corresponded approximately to a no-effect level and the high dose was the highest that could be applied without gavage difficulties. The test substance was administered to 6 groups of rats. The general design of the study is shown in Table 1.
Table 1. Metabolism of teflubenzuron in rats, study design (Schlüter, 1984).
|
Group |
Number of rats |
Administration and type of study |
|
A |
1 male, 1 female |
single low dose, analysis of expired air |
|
B |
5 males, 5 females |
single low dose, excretion and metabolism |
|
C |
5 males, 5 females |
repeated low dose, excretion and metabolism |
|
D |
5 males, 5 females |
single high dose, excretion and metabolism |
|
E |
5 males, 5 females |
single low dose, blood level |
|
F |
5 males, 5 females |
single high dose, blood level |
The results revealed that teflubenzuron was excreted completely within a short time with no differences between the sexes and no differences due to the type of exposure. The main route of elimination was in the faeces with more than 85% of the dose being eliminated within 24 h. The main component in the faeces was the unchanged parent compound (90 and 96% of the faecal residue from the low and high dose respectively), indicating low absorption by the gastrointestinal tract. Traces of metabolites (up to at least 15, mostly polar) were also found. One of them was identified as 3,5-dichloro-2,4-difluorophenylurea. The remainder of the total dose (10% and 4% of the low and high doses respectively) was absorbed and subsequently small quantities were excreted in the bile and urine. The amounts in the urine were very low: 0.5-0.9% and 0.1-0.2% of the low and high doses respectively, consisting of several polar products.
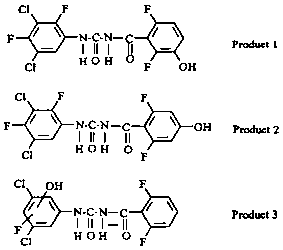
Another study (Schlüter, 1985a) revealed three metabolites in the urine. Two compounds (products 1 and 2) were structural isomers formed by hydroxylation of the benzoyl ring at positions 3 and 4 (Figure 1). The third compound was formed by replacement of one fluorine atom of the aniline ring by hydroxyl.
Figure 1. Metabolites of teflubenzuron in rat urine (Schlüter, 1985a).
The low absorption of teflubenzuron from the gastrointestinal tract of the rat was confirmed by its low levels in the plasma. The administration of 25 mg/kg body weight gave less than 0.5 mg equivalents of parent compound/ml plasma. After treatment with 750 mg/kg body weight the plasma concentrations amounted to 1-3 mg/ml (Schlüter, 1986a).
The examination of organs and tissues showed that even after 7 days administration by gavage the compound is rapidly and completely eliminated. Two days after the last treatment, no residues exceeding 0.05% of the applied radioactivity were found in any organ or tissue except the liver (0.1-0.2%). Five days after treatment the 14C residues in all tissues and organs (including the gastrointestinal tract) were below 0.01 %, except in the liver which contained 0.05%.
No 14C was detected in expired air, indicating that the aniline ring was metabolically stable. Less than 1% of the radioactivity was detected in the carcases of the animals at the end of the study.
The metabolism and biokinetics of teflubenzuron can be characterized as showing poor absorption from the gastrointestinal tract and rapid elimination, mainly in the faeces and largely as the unchanged parent molecule with no accumulation in any organ or tissue, the sum of all metabolites accounting for less than 1% of the total radioactivity (Schlüter, 1984, 1985a, 1986a).
Nendza (1991) considered that phenylurea derivatives, including teflubenzuron, are rapidly metabolized, e.g. by demethylation and hydroxylation, and hence do not accumulate in mammalian tissues.
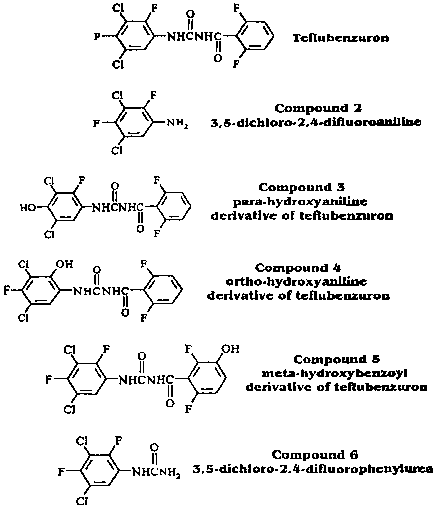
The biliary excretion and metabolism of aniline ring-labelled [14C]teflubenzuron was studied in two groups of 6 rats (3 males and 3 females) with bile-duct canulae by Hawkins and Mayo (1988a). After the oral administration of [14C]teflubenzuron at 25 mg/kg, about 16% and 1% of the dose was excreted in 48 h in the bile and urine respectively. After dosing with 750 mg/kg the corresponding figures were about 2% and 0.4%. Summation of the radioactivity in the urine, bile and liver indicated a total absorption of about 18% and 2% of the dose after administration at the 25 and 750 mg/kg levels respectively. It is evident that absorption is dose-dependent. Virtually all the absorbed teflubenzuron has been shown to be transformed. A radioactive component which yielded material which co-chromatographed with compound 5 (Figure 2) after hydrolysis indicated that one biotransformation pathway was meta-hydroxylation of the benzoyl ring and conjugation. Only very small amounts of radioactivity were co-eluted with the hydroxylated aniline derivatives of teflubenzuron (compounds 3 and 4). A radioactive component associated with 3,5-dichloro-2,4-difluorophenylurea (compound 6) confirmed that scission of the benzoyl-urea bond was an additional degradation pathway. Hydrolytic treatments of bile indicated that this phenylurea may also be present as a sulfate conjugate.
The biotransformation products in bile and urine included much unidentified polar material. The proportion of this material decreased only slightly after various enzyme treatments, to produce the m-hydroxybenzoyl derivative of teflubenzuron and the dichlorodifluorophenylurea. Acid and alkaline hydrolysis decreased the proportion of this polar material further to produce some unidentified products. There was no appreciable difference between the high-level and low-level doses in the proportions of radioactive components produced in bile or the effects of various hydrolytic treatments.
Figure 2. Reference compounds used in the teflubenzuron bile-duct cannulation study (Hawkins and Mayo, 1988a).
Lactating goats. Cameron et al. (1987a) carried out a study to determine the rates and routes of excretion of orally administered [14C]teflubenzuron uniformly labelled in the aniline ring in two lactating goats and to quantify and identify the radioactive metabolites in the milk, plasma, urine, faeces, bile, organs and tissues. The nature of the radioactivity in the faeces and bile was also investigated. The goats were dosed orally twice daily for 7.5 days at a level of 7 mg/kg body weight/day.
The main route of elimination of radioactivity was in the faeces, accounting for 99% of the total administered dose (including intestinal contents at post-mortem). The major radioactive component in goat faeces had identical HPLC and TLC retention characteristics to teflubenzuron and accounted for 76.9% of the radioactivity. A minor radioactive component with similar retention characteristics to compound 5 in Figure 2 accounted for 3.6% of the radioactivity, and a second unknown minor component for 5.9%.
The levels of total radioactivity in the plasma following the first dose remained at or close to the limit of detection. During the dosing period they increased to a maximum of 8-10 ng teflubenzuron equivalent/ml by day 4.
The levels of total radioactivity in the milk were similar to those in the plasma at the same times. The highest levels were found in the day 5 evening milk (10-15 ng equivalent/ml) and represented 0.002-0.005% of the cumulative administered dose up to that time. The radioactivity in the milk accounted for 0.03% of the total administered dose. Cameron et al. (1989) showed that the radioactive residues had the chromatographic characteristics of teflubenzuron.
The radioactive residues in all organs, tissues and body fluids examined post mortem were low in relation to the total dose. The highest mean levels in organs were in the liver and lung with 486 ng equivalent/g and 136 ng equivalent/g respectively, which corresponded to 0.14% and 0.02% of the total administered dose in the whole organs. Relatively high levels were also detected in bile (mean level 1306 ng equivalent/ml, 0.002% of the total administered dose). The levels of radioactivity in the liver and bile indicate biliary excretion as being important in the elimination of the absorbed fraction of an orally administered dose. The absence of similar levels in the plasma suggests that much of the absorbed radioactivity is removed by 'first-pass metabolism' in the liver.
The radioactivity in the bile was mainly in b-glucuronide (or possibly sulfate) conjugates. When these were hydrolysed the main product had similar chromatographic characteristics to 1-(3,5-dichloro-2,4-difluorophenyl)-3-(2,6-difluoro-3-hydroxybenzoyl)urea (compound 5 in Figure 2). No unchanged teflubenzuron was found in bile either before or after enzymic hydrolysis.
The levels of radioactivity in all other organs, tissues and body fluids were generally less than 100 ng equivalent/g. Teflubenzuron was, therefore, shown to be poorly absorbed after oral administration: the absorbed fraction appears to be metabolized in the liver and conjugated before elimination, mainly in the bile.
Cameron et al. (1989) examined the nature of the radioactivity in extracts of the liver. The major component was a polar compound which was not identical to any of the reference compounds. Traces of material co-chromatographing with 1-(3,5-dichloro-2,4-difluorphenyl)-3-(2,6-difluoro-3-hydroxybenzoyl) urea (compound 5 in Figure 2) were also detected. The distribution of the radioactivity was unaffected by treatment with deconjugating enzymes, indicating that the polar material was not a glucuronide or sulfate conjugate.
None of the extracts contained any radioactive components with similar characteristics to either 3,5-dichloro-2,4-difluoroaniline or 3,5-dichloro-2,4-difluorophenylurea (compounds 2 and 6 in Figure 2).
Laving hens. Cameron et al. (1987b) investigated the disposition of teflubenzuron in laying hens and the levels and identity of the radioactive compounds in the plasma, bile, organs, tissues and eggs. [14C]teflubenzuron uniformly labelled in the aniline ring was administered orally to 3 groups of 6 laying hens twice daily for 7.5 days at a level of 1.25 mg/kg/day.
The administered radioactivity was almost quantitatively recovered from the excreta (mean recovery 95.6%). The low levels of radioactivity found in plasma, eggs and post-mortem tissue samples suggest that teflubenzuron is only poorly absorbed after oral administration to hens. The absorbed radioactivity was readily eliminated from the eggs and plasma when dosing stopped (half-life of elimination 1.5-2 days).
The main radioactive component in extracts of the excreta had identical HPLC retention characteristics to teflubenzuron.
The levels of radioactivity detected in the liver and bile indicate biliary excretion as being important in the elimination of the absorbed fraction of orally administered doses. The radioactivity in the bile was again mainly in b-glucuronide (or possibly sulfate) conjugates which yielded a product with the chromatographic characteristics of 1-(3,5-dichloro-2,4-difluorophenyl)-3-(2,6-difluoro-3-hydroxybenzoyl)urea (compound 5 in Figure 2) on hydrolysis. No unchanged teflubenzuron was found in bile either before or after enzymic deconjugation.
A radioactive component with identical retention characteristics to teflubenzuron was found in the plasma, fat, liver and egg yolk. One or possibly two very polar radioactive components were also observed in the plasma and liver which had similar retention characteristics to the main constituent of untreated bile.
In summary teflubenzuron was found to be poorly absorbed after oral administration with the absorbed fraction passing into body tissues, especially fatty tissues and egg yolk. It was readily eliminated when dosing was stopped. The absorbed fraction is apparently metabolized in the liver and conjugated before elimination, mainly in the bile. The main route of metabolism of teflubenzuron in the liver seems to be by hydroxylation of the difluorobenzoyl ring followed by conjugation of the hydroxyl group to form a b-glucuronide.
An additional study with similarly labelled teflubenzuron was carried out to identify the radioactive components in hen excreta, liver, kidney, egg yolk, and fat by HPLC and TLC (Cameron et al., 1988).
Liver extracts were shown to contain two components, one with characteristics identical to teflubenzuron and one very polar compound which could not be identified. No significant differences were noted following treatment with deconjugating enzymes, indicating that this polar species was not a conjugate. No compounds were found with the chromatographic characteristics of 3,5-dichloro-2,4-difluoroaniline or 3,5-dichloro-2,4-difluorphenylurea (compounds 2 and 6 in Figure 2).
Kidney extracts were shown to contain both species detected in liver extracts and also traces of material which co-chromatographed with 3,5-dichloro-2,4-difluorphenylurea.
Most of the radioactivity in yolk extracts and all of it in fat extracts co-chromatographed with teflubenzuron.