Laboratory studies.
Degradation. The degradation of unlabelled teflubenzuron in two different types of soil was studied by Heupt (1984). Analytical grade teflubenzuron was added at a starting concentration of 100 mg per 100 g soil (1 mg/kg).
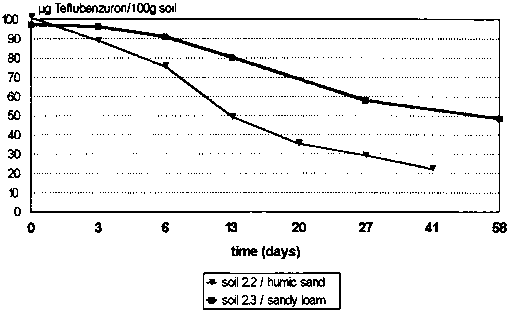
The results showed a big difference in the rates of degradation in the different soil types. In humic soil (humic sand) degradation was more rapid than in sandy loam soil which showed less microbiological activity. The results emphasize that the microbiological factor is of primary importance. The course of the degradation curves (Figure 3) confirm this microbial metabolism. Both curves after an initial linear course show a distinct break after 3 weeks in humic soil and 4 weeks in loam soil. Thereafter they are approximately linear again with a reduced gradient. This effect is probably determined by a change of microbial activity, owing to a partial 'intoxication' of some micro-organisms involved in the degradation by the metabolites formed. The half-life of teflubenzuron in humic sand soil was 2 weeks and in sandy loam soil 6 weeks.
The aerobic and anaerobic degradation of teflubenzuron in a sandy loam soil (5 mg/kg) was studied by Schlüter (1985c). The compound was uniformly labelled with 14C in the aniline ring. The results show that teflubenzuron is degraded in a sandy loam soil under anaerobic conditions about six times as rapidly as under aerobic. This is confirmed by the fact that the sample taken about 1 h after treatment in the anaerobic test already showed considerable degradation (about 14%) of the parent compound whereas the aerobic test showed about 3% degradation at day 0. Under practical conditions in the field, degradation is likely to be considerably higher than under aerobic conditions in the laboratory because anaerobic degradation is involved.
Figure 3. Degradation of teflubenzuron in soil (Heupt, 1984).
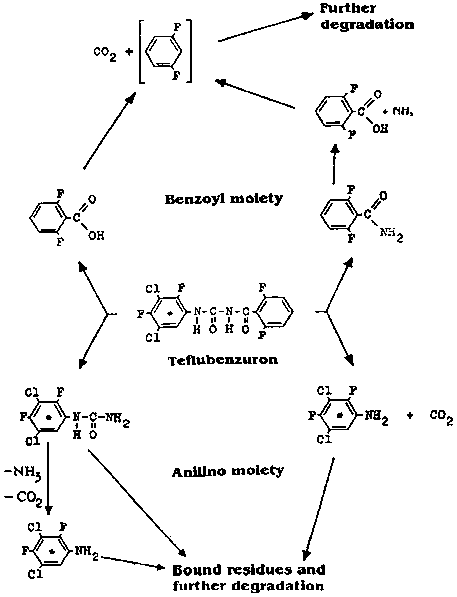
There is no fundamental difference in the initial degradation pathway under anaerobic and aerobic conditions (cleavage of the parent compound and formation of unextractable residues). In both cases 3,5-dichloro-2,4-difluorophenylurea and 3,5-dichloro-2,4-difluoroaniline were found, indicating that the initial steps in the degradation route were not affected by the difference in conditions. This is consistent with the previous results which demonstrated that 3,5-dichloro-2,4-difluorophenylurea was the major product under both aerobic and anaerobic conditions.
A proposed pathway for the degradation of teflubenzuron in soil under aerobic and anaerobic conditions is shown in Figure 4.
In an additional 150-day study the aerobic and anaerobic degradation of teflubenzuron in silty clay loam (0.5 mg/kg) was investigated by Croucher and Edwards (1990), using the benzoyl-14C compound. Soil samples were incubated under aerobic conditions for 30 days, when conditions in some of the samples were made anaerobic by flooding with water and purging with nitrogen. Soils incubated in both aerobic and anaerobic conditions were analysed 60 days and 90 days after treatment.
The degradation of teflubenzuron in aerobic soil occurred at a moderately rapid rate with a half-life (DT-50) of 29 days and a DT-90 of 108 days. After the change to anaerobic conditions the rate of 14CO2 evolution slowed considerably but the overall rate of degradation did not change, indicating that the initial steps in the degradation route were not affected by the change in conditions.
Figure 4. Proposed pathway of teflubenzuron degradation in soil under aerobic and anaerobic conditions (Schlüter, 1985c).
The proportions of the radioactive products recovered after 90 days from the anaerobic and aerobic soils were different. Only 3% of the applied radioactivity was evolved as 14CO2 in the anaerobic phase of the experiment compared with 24% from the aerobic soils over the same time period. The bound residue was higher in the anaerobic than the aerobic soil.
The major extractable radioactive component at all sampling times under both aerobic and anaerobic conditions was teflubenzuron, but at least 6 other components were observed in extracts of 60-day anaerobic soil accounting for a total of 7% of the applied radioactivity. The radioactive product expected from the initial cleavage of the [benzoyl-14C]teflubenzuron was 2,6-difluorobenzoic acid. It was not observed under aerobic conditions, which indicated that its rate of depletion exceeded its rate of formation, but trace amounts were identified in the anaerobic soil where the rate of mineralization of the benzoyl ring was slower. Another minor degradation product identified under anaerobic conditions was formed by replacement of fluorine by hydroxyl in the 4-position of the aniline ring. This may be an aerobic transformation which was detected because of the slower rate of subsequent catabolism under anaerobic conditions. Alternatively reductive defluorination is a possible first step, followed by replacement of hydrogen by hydroxyl. Decarboxylation of 2,6-difluorobenzoic acid would also be expected. This provides good evidence for the mineralization of the bound residues under aerobic conditions.
Hawkins et al. (1987) studied the photodegradation of [14C]teflubenzuron, uniformly labelled in the aniline ring, applied to thin layers of soil on glass plates at a rate of 30 mg/m2 and irradiated with artificial sunlight from a xenon arc lamp for periods up to a maximum of 15 days. The compound was also applied to control plates which were maintained in the dark under similar conditions of temperature and ventilation.
On exposed plates, teflubenzuron was degraded with a half-life of about 104 days. The major degradation product was volatile and accounted for about 7.2% of the applied radioactivity after 15 days exposure; it was probably 14CO2. Most of the radioactivity remaining in the soil was extractable into acetone and chromatographed with teflubenzuron, although after 15 days exposure 7.5-11.9% of the applied radioactivity remained bound to the soil. 3,5-Dichloro-2,4-difluorophenylurea was a minor degradation product (about 2% of the applied radioactivity) after 15 days exposure. No volatile labelled products were formed on the control plates. Apparently photodegradation has relatively little significance in the degradation of teflubenzuron in soil.
Leaching. The fate and behaviour of compounds in soil depends on the extent to which they are leached. The leaching of unlabelled teflubenzuron was studied in three types of soil: (i) sand with a low humus content, (ii) loam sand with a high humus content, and (iii) sandy loam with low humus (Celamerck, 1980). The application rate was 0.6 kg ai/ha (0.12 mg/column) and the columns were leached with a simulated rainfall of about 200 mm during two days. The leachates were extracted with benzene and analysed by HPLC. The LOD was 5 mg/l. Teflubenzuron was not detectable in the drainage water of any of the three soils.
In a more recent study (Schlüter, 1986b) a sandy loam soil was treated with [14C]teflubenzuron uniformly labelled in the aniline ring at 0.5 mg/kg and aged for 30 days under aerobic conditions. After ageing 90.7% of the applied radioactivity could be extracted with solvents of increasing polarity. The radiolabelled material consisted of teflubenzuron (75.5% of the applied activity), 3,5-dichloro-2,4-difluoroaniline (2.1%), 3,5-dichloro-2,4-difluorophenylurea (5.6%), and traces of various unidentified products (7.45% in total).
Aged soil samples each equivalent to 100 g of air-dried soil were placed on top of a 30 cm segmented leaching column of untreated soil to which 11 ml distilled water was added each day for 45 days. After this simulated irrigation most of the applied radioactivity was found in the originally treated soil (80.5% of the applied activity) and the first 5 cm column segment (13.3%). In these segments teflubenzuron was still the main residue and was accompanied by the two compounds identified in the aged soil before the start of the irrigation period.
From the first 5 cm segment only 0.03% of the applied radioactivity could be found in the aqueous effluent obtained during the 45 days period. Neither teflubenzuron nor its identified degradation products could be found in the effluent, and all three compounds show almost no tendency to migrate into deeper soil layers. The contamination of ground water by these compounds is extremely improbable in agricultural practice.
The lack of leaching is related to high adsorption of teflubenzuron by soil as is evident from the next study.
Adsorption/desorption in soil. The adsorption/desorption of teflubenzuron in soil was studied by Schlüter (1986c) in sand, sandy loam, silt loam and clay loam using a solution of the compound in 0.01 M CaCl2, at a level of about one-half saturation (9.46 mg/l). After 6 hours the concentration in the supernatant solutions in contact with sand, sandy loam, silt loam and clay loam had decreased to 0.24, 0.09, 0.07, and 0.05 mg/l respectively. The concentration of the solution without soil was still 7.94 mg/l.
From these results it was calculated that the proportions of teflubenzuron adsorbed after the 6-h treatment period were 96.9% from sand, 98.8% from sandy loam, 99.1% from silt loam and 99.4% from clay loam, showing very strong adsorption of the compound to all the soils tested.
Desorption tests with the treated soil samples revealed that 6.1%, 3.7%, 1.2%, and 1.3% of the adsorbed radioactivity could be desorbed again from sand, sandy loam, silt loam and clay loam soils respectively during two desorption periods of 24 h each. It is very improbable that these trace amounts still consist only of the unchanged parent compound, since the results of the degradation studies under aerobic and anaerobic conditions indicate appreciable degradation after a corresponding period of time.
It was concluded that teflubenzuron itself shows practically no tendency to migrate once it is applied to soil. This is attributable to the very low solubility of the compound in water, and strong adsorption with very little leaching in all types of soil tested.