NOTES
1 In the case of grain for export, due regard should be paid to any regulations concerning scheduled pests, and tolerances for such pests, in the importing country. Internal trade may also be affected by such regulations.
2 It should be borne in mind that is is possible for a hidden infestation to mature and produce large numbers of free-living adults shortly after a lot has been reported to be free from infestation or only lightly infested. Rapid changes in insect population density or distribution can result from variations in ambient temperature, cross-infestation or some other reason.
7.2 Inspection of bags, buildings, structures and transport shall be carried out before sampling of the commodity. Information recorded during this inspection may help in the assessment of samples. Any free-living insects found in the samples being taken should be collected and forwarded in a separate sample bag to the laboratory for identification.
8 Sampling of bulk grain
8.1 Extracting samples from moving bulks
At flow rates of 100 t/h, or less, the lot to be sampled shall be not greater than 5 000 kg (5 t) or smaller than 1 000 kg (1 t) and the increments should be equivalent to a minimum of 1 kg per 1 000 kg. Higher rates of flow may require the designation of larger lot sizes, to allow the sampling equipment to cope, but the size of the increments should be proportionately the same. An automatic sampling device or Pelican scoop (see 5.1) shall be used for collecting samples from free-falling grain. If there is no point of free fall, alternative mechanical sampling equipment or hand scoops may be used.
NOTE- It should be pointed out that samples obtained from conveyor belts are less representative than those extracted from a point of free fall.
8.2 Extracting samples from static bulks
In vertical bulk storage bins, sampling for hidden infestation shall first performed at the surface to a depth of 100 mm, or 250 mm if air temperature above the grain is below 15°C. An increment weighing at least 1 kg shall be extracted from each 1 000 kg sample unit in this surface layer, using a hand scoop (see 5.2).
The number of increments to be taken, n, is given by the equation
a) if sampling to a depth of 100 mm
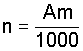
b) if sampling to a depth of 250 mm, by the equation
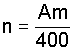
where
A is the suface area, in square metres;
m is the mass of 1 hal of the grain.
Round the value of n to the next highest integer.
If possible, an equivalent number of similar sized increments shall be taken from the bottom of the bulk by running grain out of the outlet spout.
Samples from below the surface shall be obtained using a cylindrical sampler or suction sampler (see 5.3) inserted at selected points on the surface. Increments taken at regular intervals along vertical lines from these surface points shall weigh not less than 1 kg.
Sampling grain for insects in flat bulk stores shall be carried out as described in the preceding paragraph if the surface of the bulk is reasonably level. Pre-sampling information shall be used to select parts of the bulk for sampling.
9 Sampling of grain in bags
9.1 Selection of bags to be sampled
For a stack about to be dismantled, or a lot about to be unloaded from a railway waggon, lorry, ship or lighter, the number of bags to be sampled shall be as specified in the table.
Table · Number of bags to be sampled
| Number of bags in the lot | Number of bags to be sampled |
| Up to 10 | Each bag |
| 10 to 100 | 10, drawn at random |
| More than 100 | Square root (approximately) of total number, taken at random |
In a stack of bags which is to remain in position, it is only possible to sample the top layer. Since most insects are found in the outer bags, including the top layer, no serious disadvantage Is incurred. The scheme for selecting sample units described above may be used, substituting the ex-pression "(in the lot)" by "in the top layer)" The selected sample units shall always include the four corner bags since these are especially prone to infestation. Bags needed to make up the required number to be sampled shall be selected at random.
9.2 Extraction of increments from bags.
A device (see 5.4) capable of taking a representative sample of the contents of a bag shall be used, because of the non-random distribution of insects.
10 Preparation of laboratory samples
10.1 All samples to be submitted for laboratory examination shall be referred to as laboratory samples, whether they are original increments or samples obtained by the reduction of bulk samples.
If information on the distribution of insects within a lot is required, the increments shall not be combined, and each shall be considered as a laboratory sample.
10.2 If necessary, increments shall be combined and thoroughly mixed to form a bulk sample. The bulk sampled shall then be reduced, by the method described in ISO 950 or ISO 951 or any other relevant standard, to a laboratory sample that shall weigh not less than 1 kg.
11 Packaging and labelling of laboratory samples
11.1 Packaging
Laboratory samples shall be packed in sample bags (5.5) which have been cleaned and disinfected.
Sample bags containing laboratory samples shall be closed by knotting the tie ribbons tightly around the bag necks, and shall be secured by attaching metal seals (5.8) to the tie ribbons after closure. Seals shall be attached in such a way as to guarantee the inviolability of the samples.
11.2 Labelling
If paper lablels are used for labelling the samples, they shall be of a suitably high quality for the purpose and, if they are to be attached to the outside of sample bags, the eyelet holes shall be reinforced.
External labels shall be attached by the tie ribbons at the time of closing the sample bags and shall be secured by the metal seals. Alternatively, labels may be placed inside sample bags before they areclosed and sealed, and the bags marked indelibly with simple identification marks. Each label shall bear the information required by terms of the contract.
NOTES
1 It is important to indicate that the samples are intended for the determination of hidden insect infestation and not for the determination of other characteristics of the lot.
2 For examples of the type of information required for the label, see ISO 950 or ISO 951.
12 Despatch of laboratory samples
Laboratory samples shall be despatched as soon as possible, and only in exceptional circumstances more than 48 h after sampling has been completed. Samples shall be packed for transit in such a manner as to protect them from the hazards of the journey.
13 Sampling and inspection reports
A sampling report shall be prepared, giving the usual information and making reference to the condition of the grain sampled, inluding signs of insect infestation visible in the warhouse or silo, or during working the vessel or other carrier The report shall also refer to the technique applies, if this is other than that described in this part of ISO 6639, and to all the circumstances that may have influenced sampling.
Cereals and pulses Determination of hidden insect infestation Part 3: Reference method
Céréals et légumineuses - Déterminatio de l'infestation cachée par les insectes - Partie 3: Méthode do reference
UDC 633.1: 635.65: 632.7
Descriptors: agricultural products, cereal products, leguminous grains, determination, insects, contamination, analysis methods.
THIS DOCUMENT IS A DRAFT CIRCULATED FOR COMMENT AND APPROVAL. IT IS THEREFORE SUBJECT TO CHANGE AND MAY NOT BE REFERRED TO AS AN INTERNATIONAL STANDARD UNTIL ACCEPTED BY ISO COUNCIL
IN ADDITION TO THEIR EVALUATION AS BEING ACCEPTABLE FOR INDUSTRIAL, TECHNOLOGICAL, COMMERCIAL AND USER PURPOSES, DRAFT INTERNATIONAL STANDARDS MAY ON OCCASION HAVE TO BE CONSIDERED IN THE LIGHT OF THEIR POTENTIAL AS DOCUMENTS TO WHICH REFERENCE MAY BE MADE IN NATIONAL REGULATIONS.
(c) International Organization for Standardization, 1985
Cereals and pulses-Determination of hidden insect infestation Part 3: Reference method
O Introduction
This International Standard deals with methods of determining hidden insect infestation in cereals and pulses.
This part specifies the reference method. The aim of this method is to count all the individuals, at every stage of life, of every insect species that normally feeds and develops within cereals and pulses.
ISO 6639/4 specifies rapid methods of determining hidden insect infestation, while ISO 6639/2 specifies methods of sampling for this purpose.
ISO 6639/1 describes the general principles of the methods.
1 Scope and field of application
This part of ISO 6639 specifies the reference method for determining the nature and number of hidden insects in a sample of cereals or pulses.
It is a slow method because it allows each insect to complete its developmental cycle and to emerge as an adult from the grain before it is removed. It can be used reliably for species that normally feed within within grains, but not for spectes that occasionally feed in holes or cracks in grains. These may be shaken from the grains or be induced to leave them by the disturbance of handling at any stage of the life cycle and some are likely to be killed in the process. The numbers of such species will therefore be underestimated.
2 References
ISO 712, Cereals and cereal products - Determination of
moisture content (routine method).
ISO 5223, Test sieves for cereals.
ISO 6639, Cereals and pulses - Determination of hidden insect
infestation
Part 1: General principles.
Part 2: Sampling.
Part 3: Rapid methods.
3 Definitions
For the purpose of this part of ISO 6639, the following definitions apply.
3.1 initial observed infestation: Those free-living insects that are immediately apparent to the eye when the sample is first examined.
3.2 hidden infestation: Those insects which are present within individual grains either because they are at juvenile stages and have developed from eggs laid inside the grains, or because they have entered the interior of individual grains through cracks or other damage, usually to feed. (Hidden infestation is not normally apparent upon first examination of the sample.)
4 Principle
Maintaining test samples at a controlled temperature and relative humidity such that the greatest possible proportion of the insects present in the sample when collected can develop to the adult stage. Removal of insects that emerge from the grains, identification and counting, at close intervals, to enable the number initially present to be identified.
5 Apparatus
Ordinary laboratory apparatus, and in particular
5.1 Airtight containers, for storage of samples prior to the determination of moisture content (see ISO 712).
5.2 Balance, accurate to about 1 9 and capable of weighing about 300 g.
5.3 Transparent containers, preferable made of glass or plastic, of a size capable of holding up to 300 9 of the sample to be tested at a depth not exceeding 50 mm.
5.4 Closures, to allow exchange of air, but to prevent insects and mites entering or leaving the containers (5.3).
NOTE - Filter papers sealed in place with wax have been found to be suitable.
5.5 Sieves, of suitable aperture sizes to retain the grain but to allow individual insects to pass.
NOTE - For cereals, a sieve of aperture size 2 to 2,5 mm should be suitable, but for pulses a larger aperture size would be necessary to remove some Bruchid beetles. It is desirable for the sieve to have a deep bottom pan to collect the insects removed (see ISO 5223).
5.6 Shallow trays, preferably white enamel, of dimensions about 450 x 300 mm, with a rim about 10 to 20 mm deep, on which large samples can be spread or transparent Petri dishes, of diameter about 200 mm for smaller samples.
5.7 Flexible (entomological) forceps or small brush of soft hair about 10 mm long and not more than 2 mm in diameter, insecticide free.
5.8 Room or incubator, capable of being maintained to within ± 1 °C of a temperature in the range 25 to 30°C and at either 60 to 65 %, or 65 to 70% relative humidity.
NOTE - If is essential that all apparatus and rooms used in connection with this method be kept free of insecticides or other chemicals harmful to insects.
6 Sampling
Use samples obtained as described in ISO 6639/2. The samples shall be protected from extremes of temperature and humidity and from exposure to direct sunlight.
7 Procedure
7.1 Determination of the moisture content of the laboratory sample.
Determine the moisture content of a test portion taken directly from a laboratory sample intended for the determination of insect infestation or a separate sample, according to ISO 712, or failing that, a rapid method.
7.2 Test portion
Weigh the laboratory sample to the nearest 1 9 and divide it into test samples, each weighing not more than 300 9 if the moisture content of the grain is less than 15% (m/m) or not more than 100 9 if the moisture content of the grain is more than 15 % (m/m). Place each test sample in a container (5.3) with a suitable closure (5.4).
7.3 Determination
7.3.1 If Insects are abundant and active, use the test sieve and pan (5.5) to extract them form the sample, taking care not to load the sieve more than three grains deep (if necessary, divide the sample for this purpose).
After sieving, or if the insects are not abundant and active, spread the grain in a single layer on a tray or dish (5.6) and remove all the insects found, using the flexible (entomological) forceps or small brush (5.7).
Identify all insects found in the test sample and record separately for each species the number of adults, and where possible pupae and larvae. If required, the numbers of living and dead insects shall be recorded separately.
After removing all insects return the test sample to its container (5.3)
Replace the closure (5.4) on the container and place the sample in the room or incubator (5.8).
If the moisture content as determined in accordance with 7.1 was above 15% (m/m) ensure that the relative humidity of the room or incubator in which the sample is placed is between 60 and 65%. If the moisture content is at or below 15% (m/m) maintain the relative humidity between 65 and 70%.
Table - Incubation periods (in days) for the detection of the hidden stages of insects in cereal and pulses samples kept under the suggested conditions
| Species | English common name | Incubation
period (days) |
|
| at 25°C | at 30°C | ||
| Acanthoscelides obtectus (Say.) | Dried bean weevil | 56 | 42 |
| Araecerus fasciculatus Deg. | Coffee bean weevil | 84 | 56 |
| Callosobruchus maculatus(F.) | Cowpea beetle | 49 | 35 |
| Rhyzopertha dominica (F.) | Lesser grain borer | 70 | 49 |
| Sitophilus granarius (L.) | Grain weevil | 56 | 42 |
| Sitophilus oryzae (L.) | Rice weevil | 56 | 42 |
| Sitophilus zeamais Motsch. | Maize weevil | 56 | 42 |
| Sitotroga cerealella (Oliv.) | Angoumois grain moth | 49 | 42 |
| Zabrotes subfasciatus (Boh.) | Mexican bean weevil | 56 | 42 |
7.3.2 Repeat the procedures specified in 7.3 at regular intervals of 3 or 4 days for a period of at least 36 days. The actual length of the incubation period will depend upon the temperature at which the samples are stored, the type of grain involved and the species of insect present.
The recommended lengths of incubation for some insect species are given in the table. If more than one insect species is present in the sample, the incubation period for the species with the longest development shall be adopted.
8 Expression of results
NOTE - An example of a suitable data record sheet is given in the annex.
8.1 Record the numbers of insects found in each test portion at the first examination, by species and by stage (i.e. adults, pupae, larvae and eggs) and whether dead or alive, as required. Calculate the totals for all the test portions and, using the mass of the laboratory sample recorded in 7.2, express the initial observed infestation as number per kilogram for each species and stage.
8.2 Record the numbers of insects found in all the test samples at each subsequent examination by species and stage and calculate the totals for all the test portions.
8.3 At the end of the final examination, calculate the totals for all the examinations and, using themass of the laboratory sample recorded in 7.2, expess the hidden infestation as number per kilogram for each species and stage.
If any adult insects emerge from the test samples during the first 7 days of the examination perio, adults of the same species emerging after the period recommended in the table will be deducted from the total counted before the value for hidden infestation is calculated.
NOTE - It is assumed, in this case, that late emerging insects are progeny of adults emerging after the initial observed infestation has been removed, and, therefore, that they do not belong to the total infestation present at the time of sampling.
9 Interpretation of results
9.1 For each species, the pattern of emergence represents the age distribution at the time the sample was taken. The pattern, when plotted on a graph from right to left, will present a picture of the proportions of life stages from egg to adult in equal time periods.
A high proportion of young stages (late emergents) is a sign that the population in the zone sampled is increasing while a low proportion is a sign that the population is decreasing.
9.2 The significance of the number of insects found depends upon the temperature at which the product is stored. At temperatures below 15°C, none of the species listed in the table can multiply quickly enough for small populations to be dangerous, but at temperatures above 25°C, the presence of even a single individual per kilogram of any of the listed species is a serious hazard.
10 Test report.
The test report shall show the method used and the results obtained. It shall also mention all operating not specified in this part of ISO 6639, or regarded as optional, together with details of any incidents likely to have influenced the results. The test report shall include all the information necessary for the complete identification of the sample.
Céreáles and pulses Determination of hidden insect infestation Part 4: Rapid methods
Céreáles et légumineuses - Détermination de l'infestation cachée par les insectes - Partis 3: Méthodes rapides
UDC 633.1: 635.65: 632.7
Descriptors: agricultural products, cereal products, leguminous grains, determination, insects, contamination, analysis methods.
THIS DOCUMENT IS A DRAFT CIRCULATED FOR COMMENT AND APPROVAL. IT IS THEREFORE SUBJECT TO CHANGE AND MAY NOT BE REFERRED TO AS AN INTERNATIONAL STANDARD UNTIL ACCEPTED BY ISO COUNCIL.
IN ADDITION TO THEIR EVALUATION AS BEING ACCEPTABLE FOR INDUSTRIAL, TECHNOLOGICAL, COMMERCIAL AND USER PURPOSES, DRAFT INTERNATIONAL STANDARDS MAY ON OCCASION HAVE TO BE CONSIDERED IN THE LIGHT OF THEIR POTENTIAL AS DOCUMENTS TO WHICH REFERENCE MAY BE MADE IN NATIONAL REGULATIONS.
(c) International Organization for Standardization, 1985
Cereals and pulses Determination of hidden insect infestation
Part 4: Rapid methods
0 Introduction
This International Standard deals with methods of determining hidden insect infestation in cereals and pulses.
This part specifies rapid methods. ISO 6639/3 specifies the reference method against which the rapid methods can be checked, and ISO 6639/2 specifies methods of sampling for his purpose. ISO 6639/1 describes the general principles of the methods.
1 Scope and field of application
This part of ISO 6639 specifies five rapid methods for estimating the degree of, or detecting the presence of, hidden insect infestation in a sample of a cereal or pulse.
The method described in section one (determination of carbon dioxide production) is primarily intended for testing whole grains. It is not applicable for testing
a) finely ground grain products, as there is a risk that particles of material will be sucked up with air samples; or
b) grain products with moisture contents greater than 15% (m/m), because of the risk of carbon dioxide produced by the products themselves and by micro-organisms interfering with the results.
In addition, the method is not applicable to the rapid testing of grain products on to which carbon dioxide has already been adsorbed in large quantities, for example grain stored in a confined atmosphere or when there are clear external indications of heavy infestation.
The method can be used for coarsely milled or kibbled grain products, provided that they have been sieved before testing to remove fine particles and loose insects.
The method does not permit the presence of dead adults, pupae, larvae or eggs to be detected.
The method described in section two (ninhy-drin method) is applicable to any dry grain prone to internal insect infestation, particularly wheat, rice and similar sized grains.
Large grains, such as maize, have to be partially broken (kibbled) before they can be tested. This treatment of large grains can cause some insects to be lost or fragmented, thus rendering the interpretation of results unreliable. Numbers of eggs and early instar larvae may be underestimated, but, in this respect, the method is no less efficient than any other.
The method described in section three (whole grain flotation method) is suitable for detecting hidden infestation in most cereals and pulses but only on a qualitative basis.
The method described in section four (acoustic method) is suitable for detecting living insect adults and larvae feeding inside grains. It does not permit dead adults and larvae or living eggs and pupae (nonfeeding stages) to be detected.
The method described in section five (X-ray method) is suitable for detecting living and dead larvae and adults within grains. Insects which have been recently killed (for example by fumigation) may be difficult to distinguish from those still living.
2 References
ISO 520, Cereals and pulses-Determination of the mass of 1 000
grains.
ISO 565, Test sieves-Woven metal wire cloth, perforated plate and
electroformed sheet-Nominal sizes of openings.
ISO 712, Cereals and cereal products-Determination of moisture
content (Routine method).
ISO 950, Cereals-Sampling (as grain).
ISO 591, Pulses in bags-Sampling.
ISO 6639, Cereals and pulses-Determination of hidden insect
infestation
Part 1: General principles.
Part 2: Sampling.
Part 3: Reference method.
Section one: Method by determination of carbon dioxide production
3 Principle
Incubation of a test portion of the material at a standard temperature and estimation, by a gasometric method or an infra-red method, of the amount of carbon dioxide generated during a standard period as a measure of the total metabolism of the material.
NOTE - This method is based on work in which it was shown that respiration could be detect insects in produce and that the volume of airspace is approximately constant in bulk grain packed tight. The metabolic rate of dry grain, or a grain product is very low. That of insects is so much higher that the generation of carbon dioxide in dry grain or grain product can be regarded as a sigh of infestation, provided care has been taken to avoid contamination with this gas and to ensure that none is adsorbed on the grain.
4 Apparatus
4.1 Sieve, of suitable aperture size such that fine particles and insects can pass, but the material under test is retained (see ISO 565).
4.2 Balance, accurate to 0,1 g
4.3 Apparatus for gasometric analysis (see figure 1).
4.3.1 Airtight sample containers, of capacity not exceeding 750 ml. Each container shall be closed with a rubber septem.
4.3.2 Syringes and needles, for withdrawing samples of interstitial air. The syringes shall be completely airtight and shall be of sufficient capacity for the analysis. All-glass syringes of capacity 20 ml are suitable.
4.3.3 Incubator or climatic chamber, capable of being maintained at 25 ± 1 °C (see 4.4.1).
4.3.4 Gas analysis apparatus, suitable for measuring carbon dioxide concentrations to within 0,2 % (V/V)
4.4 Apparatus for infra-red gas analysis (see figure 2).
4.4.1 Controlled climate room.
The analytical apparatus should be housed in a controlled climate room, preferably maintained at 25 ± 1 °C and a relative humidity of 70 ± 5%.
4.4.2 Infra-red gas analyser, with two interchangeable measurement ranges for carbon dioxide (0 to 50 ul/l and 0 to 500 uI/I), capable of operating with dry air as the carrier gas supplied by a compressed air cylinder, and air pressure line or a leakproof diaphragm pump at a flow rate of 2 000 ml/mint
4.4.3 Airtight sample containers, of capacity not exceeding 750 ml. These containers comprise a cylinder made of gasproof material, approximately 100 mm in diameter, sealed at the bottom and accommodating a removable lid with an airtight closure at the top (see 4.3.1), having two orifices with nozzles permitting air to be introduced into the lower part of the cylinder after connection to the purified air line (see figure 2) and to be expelled at the top.
4.4.4 Supply of compressed dry air (air pressure line, compressed air cylinder or diaphragm pump) with a pressure reducing valve. A flow regulating valve and a flowmeter are necessary in the circuit.
4.4.5 Three-way valves, manually or electrically controlled.
4.4.6 Air washing and drying tubes, installed in the circuit before the sample container. The washer comprises a flask to allow the air to be bubbled through 10 % (m/m) sodium hydroxide solution. The desiccator contains desiccant, for example anhydrous calcium chloride.
4.4.7 Moisture indicator, placed between the sample container and the analyser (silica gel with saturation indicator).
5 Sampling
Use samples obtained as described in ISO 663912.
6 Procedure
6.1 Preparation of test sample
Use the sieve(4.1) to remove any fine particles and insects from the sample. If required, the insects may be identified and the number of adults, pupae and larvae recorded separately for each species.
In order to bring the sample to a suitable condition for testing, keep it for 24 h in the incubator (4.3.3), controlled at 25°C, or in the controlled climate room (4.4.1) in a close-woven cloth bag, or a widemouthed jar, tray or open tin, suitably covered to prevent the entry or escape of free living insects, while allowing exchange of air (see ISO 6639/3, subclause 5.4).
Before preparing the airtight sample container (6.2), resieve the sample to remove any insects which may have emerged during the preparatory period.
Spread the sample thinly on a tray or other suitably flat surface, and leave to air for 15 to 30 min (to permit adsorbed carbon dioxide to escape). Airing is less important for infra-red analysis, but, if this is not done, the test report (clause 9) shall mention the fact.
Immediately before filling the airtight sample container, determine the moisture content of the sample by the method described in ISO 712, using test portions obtained in accordance with ISO 950 or ISO 951.
6.2 Preparation of the airtight container for test and test portion
Weigh the airtight sample container (4.3.1 or 4.4.3) to the nearest 0,1 9, having first ensured, by leaving it open, that it contains no trace of carbon dioxide.
Pour approximately 300 9 of the test sample into the airtight container. Tap the container to shake the sample down, and add more of the test sample until container is completely full.
Weigh the container containing the test portion to the nearest 0,1 9 and deduct the mass of the test portion.
NOTE - Constancy of filling and packing of the airtight sample container is not essential if the infra-red method is used.
Seal the container hermetically by means of its airtight device (see 4.3.1 and 4.4.3).
Return the prepared sample container to the incubator or climatic chamber (4.3.3) and leave for 24 h if the carbon dioxide is to be measured by the gasometric method. If the infra-red method is to be used, the prepared sample container may be connected to the gas analyser immediately.
6.3 Determination by the gasometric method
Expel all air from the syringe (4.3.2), insert the needle through the rubber septum on the sample container and move the piston of the syringe backwards and forwards several times so as to mix the air in the needle thoroughly with the atmosphere in the container. Draw about 10 ml of the atmosphere in the container into the syringe and withdraw the needle from the septum.
Promptly transfer a suitable quantity of the gas sample from the syringe to the gas analysis apparatus (4.3.4). (If the gas sample cannot be transferred promptly, insert the needle into a rubber bung). Determine the concentration of carbon dioxide in the gas sample, expressing it as a percentage by volume. Repeat the analysis on the same test portion.
6.4 Determination by the infra-red method
Position the valves (4.4.5) so as to isolate the circuit near the container containing the test portion. After 5 min of scanning with purified air at a rate of 1 I/min, set the analyser to zero and to the most sensitive scale (measuring range 0 to 50 = l/l).
Connect the sample container nozzles to the air inlet pipe and to the analyser (see figure 2).
Direct the flow of air throuth the sample by operation the three-way valves, with the analyser now set on the least sensitive scale (measuring range 0 to 500 = I/I). Circulate the purified air at a rate of 1 I/min through the sample for 15 min. Then switch the analyser to the most sensitive scale (measuring range 0 to 50 = I/I). Take the reading, in microlitres per litre per minute, of the emission of carbon dioxide in the sample directly from the analyser screen or form the recorder.
NOTE - The automatic operation of the valves and sensitivity scales may be performed by and electronic programmer and electric control valves. The measurement may also be carried out cyclically, but an integration system is required to measure the area of the successive peaks and for accurately determining the production of carbon dioxide in the sample.
Figure 1-Apparatus for gasometric analysis
Figure 2-Diagram of apparatus for infra-red gas analysis with operating accessories
With analysers with a non-linear scale, the value obtained should be converted into microlitres per litre using the analyser calibration curve.
6.5 Number of determinations
Carry out two determinations on the same test portion.
7 Expression of results
7.1 Gasometric method
7.1.1 Calculiation and formula
The concentration, expressed as a percentage by volume, of carbon dioxide in the intergranular air of 1 kg of grain after 24 h incubation at 25°C is given by the formula
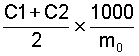
where
C1 and C2 and the results of the two determinations of the
carbon dioxide concentration, as percentages by volume, measured
on each test portion;
mo is the mass, in grams, of the test portion.
Take as the result the arithmetic mean of the values obtained in the two determinations, if the repeatability conditions are met.
7.1.2 Repeatability
The difference between the results of two determinations carried out one after the other by the same analyst should not exceed 0,2 % (V/V).
7.2 Infra-red method
7.2.1 Calculation and formula
The concentration, expressed in microlitres per litre, of carbon dioxide produced in 1 min in the intergranular air in 1 kg of grain is give by the formula
C × 1 000 / m0
where
C is the concentration, in microlitres per litre, of carbon
dioxide produced in 1 min in the intergranular air of the test
portion;
m0 is the mass, in grams, of the test portion.
Take as the result the arithmetic mean of the Values obtained in the two determinations, if the repeatability conditions are met.
7.2.2 Repeatability
The difference between the results of two determinations, carried out one after the other by the same analyst, should not exceed 2 = I/I min.
8 Test report
The test report shall show the method used, the number of determinations carried out, and the results obtained. It shall also mention any operating details not specified in this part of ISO 6639, or regarded as optional, together with details of any incidents likely to have influenced the results.
The test report shall include all the information necessary for the complete identification of the sample.
9 Interpretation of results
9.1 Gasometric method
For wheat, peas, split, haricot beans, butter beans, polished rice, small yellow maize, and similar small huskless hard grains, tested by the gasometric method, the interpretation given in table 1 applies.
NOTE - For other grains, it is necessary to make a correction for the characteristic volume of interstitial air and the observed carbon dioxide concentration should be multiplied by the correction factor. Some correction factors are:
Table 1 · Interpratation of results obtained by the gasometric method
| Production of Carbon dioxide, % CO2(V/V) for 1 kg after 24 h incubation |
Interpretation |
| Probably no infestation | |
| < 0,2 | present, Repeat test on |
| another sample to confirm. | |
| Possible light infestation. | |
| 0,2 | Repeat test on another |
| sample to confirm. | |
| Light to moderate infestation. | |
| Grain unsuitable for storage | |
| 0,3 to 0,5 | longer than 2 months without |
| treatment. | |
| Moderate to heavy infestation. | |
| 0,6 to 0,9 | Grain should be fumigated |
| immediately. | |
| Heavy infestation. Grain in | |
| 1,0 and higher | dangerous condition and |
| Highly unsuitable for storage. |
9.2 Infra-red method
The interpretation given in table 2 applies.
Table 2 ·Interpretatlon of results obtained by the infra-red method
| Rate of carbon dioxide production, -I/l min. for 1 kg of grain |
Interpretation |
| Probably no infestation | |
| present, Persistent small | |
| < 10 | peaks could indicate a very |
| light infestation. Repeat test | |
| on another sample to confirm. | |
| Possible light infestation. | |
| 1,0 | Repeat test on another |
| sample to confirm. | |
| Light to moderate infestation. | |
| Grain unsuitable for storage | |
| 2,0 to 3,0 | longer than 2 months without |
| treatment. | |
| Moderate to heavy infestation. | |
| 4,0 to 6,0 | Grain should be fumigated |
| immediately. | |
| Heavy infestation. Grain in | |
| 6,0 and higher | dangerous condition and |
| highly unsuitable for storage. |
Section two: Ninhydrin method
10 Principle
Crushing a test portion, from which any visible living insects have been removed, against white paper impregnated with ninhydrin.
When an infested dry grain is crushed, the amino acids in the body fluid of insects with the ninhydrin in the paper to give a purple spot, but the amino acids of the grain are not released and do not react.
Counting of the purple spots on the paper. The number of spots is taken to indicate the level of hidden infestation in the sample.
11 Apparatus
11.1 Sieve (see 4.1).
11.2 Kibbling device, if required, to partially break large grains.
11.3 Grain sample divider (see ISO 950).
11.4 Infestation detector, manually or electrically operated, which consists essentially of two rough surfaced steel rolls 0,75 mm apart between which passes a continuous strip of ninhydrin treated paper (see figure 3).
NOTE - The Ashman Simon apparatus is suitable.
11.5 Ninhydrin treated paper
Use a roll of white paper 57 mm wide and 50 m long, already impregnated with ninhydrin, or prepare as follows.
Pass the untreated paper through a 10 g/l solution of ninhydrin in industrial denaturated alcohol. Roll the paper up and leave it to dry at 20 to 25°C and 40 to 60% relative humidity, in a dark place for at least 3 days. Wrap the dry treated roll in metal foil and store away from light, if possible at 20 to 25°C and 40 to 60% relative humidity. Under these conditions, the ninhydrin treated paper will remain stable for 2 to 3 years.
11.6 Balance, accurate to 0,1 g.
12 Sampling
Use samples obtained as described in ISO 6639/2.
13 Procedure
13.1 Preparation of test sample and test portion
Use the sieve (11.1) to remove all foreign matter and free insects from the sample. If required, the free insects may be identified and counted according to species and stage.
Weigh the sifted sample and divide it, using the grain sample divider (11.3), to obtain the test portions required (see 13.3 and clause 15). Each test portion should contain at least 1 000 grains (see ISO 520). Test portions of large grains should be kibbled and resifted before testing.
Weigh a test portion and/or count the number of grains in it. Prepare the infestation detector (11.4) and pass the test portion through it in accordance with the manufacturer's instructions.
13.2 Determination
Remove the paper strip corresponding to the test from the detector, taking care only to handle the ends of the strip as amino acids on the skin of the fingers also react with ninhydrin to give purple stains (this may be obviated by wearing of surgical gloves or using tweezers), and allow time for purple spots to develop. At 20°C and at higher ambient temperatures, purple spots develop within 1 h, although they can take up to 24 h to reach maximum intensity. At lower temperatures, or if more rapid development is required, the paper can be heated in an oven maintained at 50°C, or it can be passed cautiously (to prevent burning) over a spirit lamp flame or electric light bulb.
Figure 3 - Apparatus for ninhydrin detection of hidden insect infestation
When purple spots have developed, mark the boundary of each with a pencil line, taking care to distinguish spots which may be so close as almost to merge.
Ignore any spots on the paper which are not purple in colour.
Count the number of marked spots.
13.3 Number of determinations
Carry out two determinations on the same test sample. (See also clause 15.)
14 Expression of results
Express the infestation as the number of hidden insects per kilogram or per 100 grains, and take as the result the arithmetic mean of the two determinations.
15 Interpretation of results
If no insects are detected in the first pair of test portions, the test should be repeated with up to a total of 10 test portions before it can be reasonably concluded that the grain is free from infestation. Even then it should be remembered that eggs and small larvae can escape detection by the method. Therefore, if it is desired and practical, apparently infestation-free grain should be tested again after 2 to 4 weeks.
The efficiency of method also varies according to the species of insect and size and type of grain under test. It is doubtful whether a correction coefficient valid for different grain types and defferent insect species can, or should, be recommended or whether it is necessary in commercial practice.
In general, a positive result should be taken to indicate that the grain is potentially unsafe for storage. Relatively few purple spots occurring irregularly on the paper indicate a light to moderate infestation, and that the grain cannot be stored for more than 2 months without treatment. Many purple spots indicate a heavy infestation requiring immediate fumigation. However, before taking any action, it should be determined whether the grain has already been effectively treated, and how recently. This is because dead insects continue to give positive results by the method until their body fluids have dried up. A large dead insect can take several weeks to dry out.
16 Test report
The test report shall show the method used, the number of determinations carried out, and the results obtained. It shall also mention any operating details not specified in this part of ISO 6639, or regarded as optional, together with details of any incidents likely to have influenced the results.
The test report shall include all the information necessary for the complete identification of the sample.
Section three: Whole grain flotation method
17 Principle
Hidden insect infestation reduces the mass of grain. When a mixture of sound and infested grains is immersed in a test solution in which sound grains just sink, infested grains float to the surface. The separation is usually imperfect because grains containing early instar larvae tend to sink, and uninfested grains with air pockets under the testa or some other defect may float. Floating grains are dissected, to confirm the presence or absence of insects.
18 Apparatus
18.1 Hydrometer floats, to measure relative densities in the range 1,100 to 1,300.
18.2 Measuring cylinder, of capacity 500 ml.
18.3 Sieve (see 4.1).
18.4 Balance, accurate to 0,01 9.
18.5 Grain sample divider (see ISO 950).
18.6 Beaker, of capacity 1 000 ml.
18.7 Skimmer, for removing floating grains.
19 Sampling
Use samples obtained as described in ISO 6639/2.
20 Test solution
A suitable test solution can be prepared by dissolving sodium silicate, ammonium nitrate or glycerol in water. The quantity of solute required to make 1 000 ml of test solution of approximately the correct relative density can be calculated by reference to figure 4. Check the relative density of the solution by using a suitable hydrometer float (18.1) and the measuring cylinder (18.2). If necessary, add small amounts of solute or water until the relative density is within ± 0,005 of that required.
NOTE - As grain densities vary according to type, variety and other factors, the required densities of test solutions also vary. Where preatical, the required relative density should be determined by experiment. The following values for relative densities of test solutions are intended only as a guide:
Figure 4 - Guide to the preparation of test solutions of given relative densities
21 Procedure
21.1 Preparation of test sample and test portion
Use the sieve (18.3) to remove all foreign matter from the sample. Weigh the sifted sample and divide it, using the grain sample divider (18.5), into test portions, each containing about 500 grains. Count the grains in a test portion.
21.2 Determination
Place the test portion in the beaker (18.6) containing the test solution. Mix thoroughly and allow to stand for 10 min. stirring briefly at 1 min intervals to release air bubbles by the grains. When the grains have settled for the last time, use the skimmer (18.7) to remove all floating grains. Sort out and count all grains bearing visible evidence of insect infestation ("windows" in the testa or tunnels visible through it). Cut open the remaining grains with a suitable instrument, and count those found to contain insect larvae, pupae or adults.
21.3 Number of determinations
Carry out two determinations on the same test sample.
22 Expression of results
22.1 Calculation
Express the infestation as a percentage of grains which are infested and take as the result the arithmetics mean of the two determinations.
22.2 Repeatability
The difference between the result of either determination and the mean shall not exceed the limit indicated in figure 5. If this limit of repeatability is exceeded, repeat the determination on other test portions until the requirement is satisfied.
23 Interpretation of results
In view of the limitations referred to inclause 17, the method is most likely to produce an underestimate of the level of infestation present. Therefore, results are of qualitative, rather than quantitative, value. One of the more accurate methods should be used if quantitative results are i m portent.
24 Test report
The test report shall sholw the method used, the number of determinations carried out, and the results obtained. It shall also mention any operating details not specified in this part of any incidents likely to have influenced the results.
The test report shall include all the information necessary for the commplete identification of the sample.
Section four: Acoustic method
25 Principle
Placing a test portion in a sample container inside a well soundproofed box. An acoustic vibration sensor, fitted inside the sample container and connected to an amplification system, transmits noise from the feeding activity of hidden insects for direct listening or for recording. Estimation of the approximate degree of hidden infestation from the noise level transmitted or recorded.
26 Apparatus
26.1 Acoustic detection equipment, comprising the following elements (see figure 6).
26.1.1 Box, sound-proofed by an internal lining of high performance sound-insulating material (for example rock wool or high density polyester foam with lead), the opening of which has a seal impervious to external noise, and within which is placed a removable sample containe in the form of a thick plastic cylinder (for example high density PVC) fitted with a hermetic closure. A vibration sensor is placed in the centre of the sample container and connected by a flexible or expanding cable to a socket outlet placed on the outer face of the box.
The box is mounted on an elastic suspension system (for example rubber pads).
NOTE - The arrangement of the various components of the soundproofed box may vary while achieving the same insulation result for airborne and mechanical vibrations.
26.1.2 Electronic amplification system, composed of a pre-amplifier giving an amplification of 50 to 100 dB, according to the equipment, compatible with the characteristics of the vibration sensor, with a pass band of 600 to 4 000 Hz and a signal to back ground noise ratio, on average, of-120 dB/V.
NOTE-In order to restrict the background noise, a fillter may be added to reduce the width of the pass band (the central tuning frequency for the rice weevil is approximately 2 kHz). A filter is recommended when using a recording system.
26.1.3 Headphones or recording system:
a) headphones or loudspeaker connected to the amplifier for direct listening to the noise produced by the hidden insects;
b) voltage threshold detector and recording system for electrical impulses exceeding the adjustable threshold.
26.1.4 Mat of high density insulating material, placed between the box and the horizontal support to limit the transmission of mechanical vibrations. (This precaution is optional with wellinsulated boxes.)
26.2 Sieve (see 4.1)
26.3 Grain sample divider (see ISO 950).
27 Sampling
Use samples obtained as described in ISO 6639/2.
28 Procedure
NOTES
1 It is essential to carry out the test on samples having a temperature greater than or equal to 20°C.
2 Some equipment incorporates a system for heating the sample in order to increase insect activity; this preliminary heating requires about 20 min before each determination.
28.1 Preparation of the test sample and test portion
Use the sieve (26.2) to remove all free insects. If required, the insects may be identified and counted according to species and stage. Divide the sample, using the grain sample divider (26.3), into the required number of test portions (see 28.3). Each test portion shall be equal to or slightly in excess of the quantity required to fill the cylinder of the box (26.1.1).
28.2 Preparation of the apparatus
Place the box on the insulating mat (26.1.4) if necessary. Close the empty sample container, place it in the box and close the box. Connect the amplifier and listening or recording system and adjust the gain until a low continuous background noise is obtained. Switch off the now pre-set apparatus.
NOTE - With a recording system, the threshold setting should be calibrated periodically (for example with a test recording on magnetic tape).
28.3 Determination
Fill the sample container with the test portion. Settle the grain down gently by vibration, seal the container, place it in the box and seal the box. Wait for 5 min for the grain to stabilize, then switch on the detection system. Listen for the characteristic noises of insect activity in five listening periods of 1 min or by recording for a period of 5 min.
NOTE - When recording, the direct listening device can be used for checking any defects in recording and adjusting the setting of the detection threshold.
Once the apparatus is switched off, remove the test portion from the cylinder and weigh it to the nearest 0,1 9.
28.4 Number of determinations
Carry out two determinations on the same test sample.
Figure 6 · Acoustic detection equipment
29 Expression of results
29.1 Direct listening
The result of each 1 min listening period shall be noted separately, with an indication of the existence or absence of activity of hidden insects. The relative intensity of the insect may be assessed in order to classify the extent of infestation.
29.2 Recording
The number of impulses recorded over 5 min shall be converted to the mean number per minute.
30 Interpretation of the results
With less than one period of insect activity activity recorded per minute, the sample shall be free from infestation.
With one period of activity per minute, the sample is probably infested but the presence of hidden insects has to be confirmed.
31 Sample
Use a sample as described in ISO 6639/2. 32 Procedure
35.1 Sieving
Remove all free living insects from the sample using the sieve (33.2)
35.2 Test portion
35.2.1 Standard test portion (recommended in cases of dispute)
Take, and weigh to the nearest 0,1 9, a test portion that is sufficiently large to cover completely a minimum film area of 750 cm² when placed in a layer one grain thick.
NOTE - This quantity corresponds to approximately 10 000 grains of wheat or 3 000 grains of maize.
35.2.2 Reduced test portion
It may be possible to detect infestation to an acceptable degree of accuracy by using a smaller test portion (for example 1 000 to 1 200 grains of wheat). This reduced test portion, which is particularly applicable for rapid checking, may be substituted for that specified in 35.2.1 on agreement between the interested parties.
35.3 Spreading the test portion
Place the wire grid on the envelope containing the film. Spread the test portion in a layer one grain thick. In this way, it is ensured that all the grains will lie on one side or other of the grid lines when the radiograph is examined.
35.4 Identification of the film
At the side of the grains, prains, place figures or letters made of X-ray opaque material which apear on the fillm after exposure and will allow the film to be identified.
35.5 Exposure
During exposure, the film remains inside its lighttight envelope. Position it in accordance with the instructions for the apparatus being used.
Ensure that all safety conditions have been fulfilled.
Choose a duration of exposure to suit the nature of the sample and the fillm being used, so as to reach a satisfactory film density (see 38.1).
If an apparatus for measuring the density is available, a density of 1,0 should be sought.
35.6 Development
After exposure, develop the film in accordance with the menu facturer's instructions (see 38.1).
35.7 Examination and interpretation of the radiograph (see 38.1).
Examine the radiograph using the negatoscope or viewing screen (33.5) and count the infested grains.
In general, cereals or pulaes appear white or grey on the negative. Any cavity within a grain is represented by a dark region and an insect within the cavity appears light in colour.
35.8 Number of determinations
Carry out three determinations on the same test sample.
33 Expression of results
36.1 Count the number of infested grains found in the three determinations and calculate the number of infested grains per kilogram.
36.2 The result may also be expressed as the number percentage of infested grains, provided that the number of grains in the test portions has been counted.
34 Test report
The test report shall show the method used, the number of determinations carried out and the results obtained, indicating clearly the method of calculation used. As far as possible, the stages of development of the insects present should be recorded. If shall also mention any operating conditions not specified in this part of ISO 6639, or regarded as optional, together with details of any incidents likely to have influenced the results.
The test report shall include all the information necessary for the complete identification of the sample.
35 Notes on exposure and development of the film and interpretation of radiographs
38.1 Exposure and development
The exposure and voltage required vary according to the product being examined and the degree of penetration and contrast required. Low voltages give less penetration of grain than high voltages. For small grains, it may be preferable to use a low voltage in order to achieve the image resolution required to detect eggs, for example.
The moisture content of the grain is also important: a grain with a high moisture content will require a high voltage for satisfactory penetration by the X-rays.
It is essential to develop the film in accordance with the manufacturer's instructions, for example for the the developer concentration and the temperature. Time of film development will be variable and, until experience has been gained, the middle of the manufacturer's range should be chosen.
The most satisfactory exposure time may be determined in the following way:
a) Expose the entire area of the film covered by the grains for 15 at 20 kV and 5 mA, for example;
b) Cover one-third of the area of the film by placing a sheet of tin plate, steel or copper (of thickness 1,25 mm) over the grains and expose for a further period of 5 s;
c) Cover a further third of the surface and expose again for 5 s;
d) the film now has areas which have been exposed for 15, 20 and 25 s.
If, after determination of the most satisfactory exposure time, the penetration of X-rays at 20 kV seems too great or too little, repeat the procedure
as described above, adjusting the voltage in steps of 5 kV between 15 and 30 kV in order to find the most satisfactory voltage.
Ater development and fixing of the film or films under the above conditions, the most satisfactory voltage and period of exposure may be selected, and used on future occasions for similar grains.
38.2 Interpretation of the radiograph
Eggs and small larvae can occasionally be recognized in a general test exposure. However, the proportion found will depend on the orientation of the grain at the time of exposure, the voltage of the apparatus, the insect species, the grain type and the operational conditions. The X-ray technique cannot be relied on for the detection of every egg or early larval instar. If this point is important, the test portion should be kept at 25°C after the test and re-examined at appropriate intervals.
Living larvae may sometimes be distinguished from recently dead larvae by a blurring of the image which is caused by the movement of live individuals during long exposure of the film. This requires considerable skill to detect, and furthermore, a living individual may not move for some minutes.
The X-ray technique gives an accurate assessment of late larval instars pupae and adults.
If it is desired to check the infested grains, they may be cut open and examined for the presence of larvae.
36 Disposal of test portion
When the test portion used in the test is being disposed of, it should be borne in mind that materials sold for food after irradiation may be required to comply with national legislative requirements.