Related problems to use of insecticides
Compiled by R. L. Semple
Nature of Resistance
Resistance is an increase in tolerance status of a particular pest species to a toxic substance. The onset of resistance as demonstrated by Parkin (1965), assuming that population has the genetic potential, is dependent on
The problem of resistance development is accentuated in bulk grain by the relatively constant and protected microclimate, due to the insulating action of both the storage structure and the grain itself. Insect populations are therefore not subjected to any violent fluctuations in seasonal conditions and breeding is possible throughout the year.
There exists a dynamic variation in tolerance among individuals of any given population, and resistance is therefore initiated by continuous selection of the toxicant of the more resistant individuals in that population. Resistance is due to the selection of hereditary factors and not an adaptation of individual insects to the insecticide. Genes are selected that, alone or in combination, alter the physiology or biochemistry in such a way that progressively higher amounts of insecticide are required to kill them, even up to a point where an insect can tolerate any given dose. Due to economic constraints and potential residue hazards exceeding those maximum residue levels set down internationally, we have for practical purposes a breakdown in control provided alternatives are not available.
The point to remember is resistance will result directly from selection pressure and from this it follows that
Fumigation is recommended in case of control breakdown following use of residual insecticides. Why? Because control breakdown maybe the first indication of tolerant members of that endemic population surviving the recommended treatment. His therefore essential to kill them so as to maintain the use and efficacy of the small number of residual contact insecticides registered for use as grain protectants
Mechanism of Resistance
Insect resistand was first discovered in DDTresistant houseflies, Musca domestica in 1946 (Weismann, 1947) and has since become one of the major constraints/concerns in insect pest control.
The most widely used stored-product insecticide during the 1960's to early 70's was the organophosphate Malathion (S-(1,2dicarbethoxyethyl) 0,0-dimethyl dithiophosphate). Malathion was introduced because of its low toxicity to mammals and birds, its low phytotoxicity and its rapid breakdown rate (Yost, Frederick and Migrdichian, 1955).
In Australia, Malathion was applied at the beginning of the storage period when the risk of infestation was highest with normally only one application is used. Work by Minett and Williams (1971) using 150 9 masses of wheat at 25°C and 70% RH showed that after 10 weeks about 50% of the malathion still remained after an initial application of 12 ppm. Table 1 depicts the half-life of some grain protectants at the reference temperature and relative humidity of 30°C and 50% RH respectively, which are typical of receival conditions for grain in Australia.
Table 1. General rate expressions for 12 protectants plotted to the equation
log t1/2 = log(t1/2)0 - B(T2 - 30) - log RH/50
where t1/2 is the half-life on grain of temperature, T2 in degrees Celsius and percentage relative humidity RH, and (t1/2)0 is a reference half life on grain of 30°C and 50% humidity.
| Pretectant | t1/2 (weeks) | B (degrees-1) |
| Fenitrothion | 14 | .036 |
| Bioresmethrin | 24 | .033 |
| Bioresmethrin* | 38 | .031 |
| d-Fenothrin | 38 | .029 |
| d-Fenothrin* | 40 | .029 |
| Pyrethrum * | 55 | .022 |
| Methacrifos | 8 | .055 |
| Malathion | 12 | .05 |
| Chlorpyrifos-methyl | 19 | .04 |
| Carbaryl | 21 | .031 |
| Pirimiphos-methyl | 70 | small |
| Pyrethrum | 34 | - |
*plus piperonyl butoxide at 20 mg kg-1
Source: Desmarchelier and Bengston "Chemical residues of newer grain protectants pp 108115 in Australian contributions to the Symposium on the protection of grain against insect damage during storage, Moscow, 1978.
Malathion acts as a contact poison which is absorbed through the cuticle. Once inside the body it is oxidized to malaoxon I(CH3O)2P(0)SCH (CO.OC2H5)CH2CO.OC2H5l, which then acts as a powerful cholinesterase inhibitor. This process of conversion of malathion is known as activation, resulting in a disruption of nerve activity due to acetylcholine accumulation at the nerve endings which results in excitability, muscular tremors and finally paralysis (O'Brien, 1967).
Acetylcholinesterase (AChe) is the main site of action for the organophosphorous and carbamate group of insecticides. Messages are transmitted across the nerve synapse by the transmitter acetylcholine, and once the impulse is passed acetylcholinesterase acts on acetylcholine to return it to its active ionic state. The organophosphorous insecticides of the -thionate configuration must be oxidised from the P = S to the P = 0 nucleus before they become active, since the P = 0 nucleus has a strong electronic pull on the hydroxyl group of acetylcholinesterase, thus bonding with it and inactivating it once the insecticide penetrates the synapse.
Resistance can be due to various changes, either singly or due to their interaction such as
In most cases of insecticide resistance, increased detoxification and altered site of action appear to be responsible. This can be brought about by enzymatic changes caused by mutants of single genes. (NB: It is important to realize that insecticides themselves do not perform a mutagenic function, since the genetic variability for resistance already exists in natural populations not previously exposed to insecticides).
Single gene alteration causing a change in the site of action has been demonstrated in spidermites, cattle ticks, leaf hoppers and houseflies where resistance has developed due to an altered cholinesterase displaying a much reduced rate of reaction with the inhibitors (i.e., the insecticide). High degrees of resistance due to single gene alteration resulting in the production of more efficient detoxifying enzymes is known in DDT-dehydrochlovinase and carboxyesterase strains of houseflies degrading DDT and malation respectively. Both resistances are due to the altered properties of the enzyme as well as greater quantities of enzyme produced.
High levels of resistance can also develop through the interaction of more than the resistance gene which may result in decreased penetration as well as detoxification of the insecticide. Resistance to a single insecticide can be due to different causes in various strains of the same species. Malaoxon can be degraded by a carboxyesterase as well as by a microsomal mixed function oxidase in the housefly. They can be present either singly or together in the same strain eliciting different behavioral responses with applied synergists, such as triphenyl phosphate (TPP).
Malathion is unique in that two distinct metabolic resistance mechanisms are possible.
Malathion non-specific resistance (Mal. nonspec R) is a broad resistance to most organophosphates (i.e. cross resistance). This type of resistance is induced by selection or exposure to any organophosphate compound.
Malathion specific resistance (Mal. spec. R) is restricted almost exclusively to malathion. This type of resistance is due to higher levels of a carboxyesterase in the resistant strain. Although first demonstrated in the mosquito, Culex tarsalis, (Matsumura and Brown, 1961), it has since been demonstrated in a number of other insects. The carboxyesterase attacks the CO.OC2H5 group of the malathion molecule and thus prevents activation.
Genetic studies have shown that high carboxyesterase levels and malation specific resistance are inseparable connected.
Increased mixed function oxidase activity is a very important mechanism since it affects insecticides from practically all chemical groups, whereby crossresistance has been conferred in insect growth regulators (Dyte, 1972; Cert and Georghiou, 1972) with the oxidases being amongst the most likely candidates. Through selective pressures from the use of IGR analogues, high levels of resistance may develop (Plapp and Vinson, 1974), but Amos et. al., (1977) detected no crossresistance to either methoprene or hydroprene in malathion susceptible or resistant strains of Tribolium castaneum or T. confusum.
Figure 1. Malathion metabolism.
There is also evidence that the different forms of the detoxifying enzymes and the aberrant mutants of the cholinesterase are produced by alleles of the structural gene. Work by Devonshire and Sawicki (1978) with the peach potato aphid have shown that they have been placed under extreme insecticidal pressure in greenhouses, and through evolutionary change have duplicated some of their genes. The structural gene continues to produce a functional protein while the duplicate is free to evolve without subjecting the population to long periods of selective disadvantage. They found resistant strains contained more carboxylesterase enzyme than non-resistant strains and that enzyme activity increased exponentially with insecticide resistance brought about by gene doublings. Malathion and parathion resistance in different strains of the housefly Musca domestica as well as altered cholinesterase in resistant spider mites Tetranychus urticae is thought to be alleles of structural genes.
Truly sublethal exposures to applied insecticides do not induce resistance in known susceptible strains of M. domestica or increase the levels of DDTdehydrochlorinase (DDT-ase) as demonstrated by Moorfield (1958) and Brown (1964). Rather, the cumulative effect of daily sublethal amounts of DDT, gamma BHC, dieldrin or diazinon renders the housefly more susceptible to exposures at the discriminating dose. Bond and Upitis (1972) with sub-lethal treatments of phosphine, found cumulative effects which differed with species when repeatedly applied to insects. For example Tribolium consusum adults recovered from a sublethal treatment in 10 days while Sitophilus granaries adults required 40 days although the tolerance level regained was less than in untreated individuals. Tenebroides mauritanicus larvae showed a varying response after an initial dose that achieved no mortality in the first two weeks; some individuals died slowly, some remained permanently as larvae, some developed into pupae but subsequently died, while some adults that emerged were physically deformed and some appeared morpholigically normal.
These observations on increased susceptibility of insects exposed to sub-lethal doses of phosphine maybe important in terms of economic control if for example, survivors from a treatment where incomplete control is obtained, are more responsive to subsequent treatments applied soon after the first. The normal criterion for effectiveness of a treatment is usually mortality, but various sterilizing effects that inhibit further development of insect populations becomes important in considering combined control strategies.
The mechanism of resistance is by no means clearcut, but certain established criteria enable pest control operators to implement counter measures where suspect resistance occurs.
When resistance has been observed, the pesticide in use should be replaced by one of an unrelated chemical groups and attention given to its efficient utilization. With residual pesticides the first indication of resistance is a reduction in the time that residual deposits remain effective; thus a grain protectant that inintially gave 6 months protection may subsequently not control insect populations although residue analyses may indicate no change in the normal decay pattern of the pesticide on the commodity. Early warning indications such as this often go unheeded through mismanagement and it is not until obvious signs of resistance such as large scale buildup of pest populations in or on treated commodities that the problem is recognized. Regular monitoring of the tolerance status is a valuable means of early detection of resistance and can provide unequivocal evidence of the contribution of resistance to control failures.
Breakdown in control measures other than those initiated by insecticide resistance are as follows:
1. Use of pesticides that have deteriorated in storage.
2. Incomplete coverage or application due to faulty equipment or treatment:
3. Use of unstable preparations: rapid breakdown or disregard of comparability warning on labels.
4. Variation of pest susceptibility with temperature. Dosage levels maybe effective at normal temperature but inadequate in extremely hot or cold weather. Also affects stability, reaction rates of insects and rates of diffusion with chemicals.
5. Also, under conditions of extremely hot weather and light, pesticides maybe decomposed or otherwise lost at abnormally high rates.
Resistance in stored products has advanced from 3 species in 1960 to approximately 17 species of Coleoptera and Lepidoptera in 1979. Resistance to fumigants detected in the field is of major concern because of the global dependence on fumigation both as routine disinfestation and for combating insecticide resistance strains. From the 1972-73 FAO global survey of tolerance status of the major pests of grain, methyl bromide resistance was detected in 23 countries and phosphine resistance in 35 countries, where resistance factors were generally low and in most instances would not have resulted in breakdown of pest control. The species of particular concern were Rhyzopertha dominica Fabricus and Tribolium confusum Jag. du Val.
The emergence of strains of different species may not be a practical problem as yet in the field, but the widespread occurrence of strains surviving discriminating doses which would normally prove fatal to susceptible strains and the case at which this level of tolerance can be increased under laboratory selection, poses a serious threat to international trade.
Most cereal products moving in international trade is almost exclusively in bulk ships and the infestation either originates from the exporting country or, from cross-infestation from residues in previous cargoes or other infested cargoes on board.
Malathion resistance was first detected in Tribolium castaneum in the early 1960's and now can almost be considered to be a normal attribute of a T. castaneum population. The frequency of resistance in other species is still comparatively low and although a much later detected resistance in these species (i.e., Sitophilus oryzae, 1969; R. dominica, 1971) indicates genotypes which were not as conducive to development of resistance.
The movement of resistant strains in trade is providing a mechanism allowing rapid dissemination throughout the world, as well as distributing new resistances as they develop, which is probably happening at present with any new strain acquiring fumigant resistance. Pest control methods now take into account the problems associated with excessive pesticide usage and dependence to obtain control at an economic level.
There exist three very good reasons for reduction in pesticide use:
1. Development of resistance.
2. Cost of pesticides includes development, toxicology and registration
3. Environmental effects
4. Misuse and abuse particularly in home garderners
The application of pesticides since the turn of the century can be classified under 2 broad categories:
1. Utilization of marginally effective inorganic pesticides combined with labour intensive cultural control methods (ca 1900-1945).
This included some extremely deleterious compound such as the mercury based insecticides, arsenicals such as parts green, lead and calcium arsenate and the flouride insecticides. More recently, compounds of botanical origin such as nicotene, pyrethrum and rotenone, have 'had widespread application for field crop, orchard and domestic pest control.
2. Nearly sole reliance upon synthetic organic compounds applied on a calendar or preventative schedule (1945-present).
DDT was the first of these insecticides, first synthesized by Zeidler in 1874, and recognized for its potenial in insect control in 1939 by Mueller against clothes moths. From the synthesis of DDT came a succession of related compounds (methoxychlor, dicofol) followed by the cyclodienes which are chlorinated cyclic hydrocarbons developed in 1945. Next came the organophosphates, carbonates, the synthetic pyrethroids to compounds attempting to mimic naturally occurring insect hormones applied at such a stage as to disrupt their normal life processes.
Both these categories in pesticide development have had associated and related problems such as:
Attempts have been made recently to resolve the contradictory demands of society that on the one hand asks for a constant supply o high quality food free of pest damage, but on the other hand is very much concerned with the potential negative side effects of pesticide usage. This approach to pest control has been referred to as integrated pest management (IPM).
The objectives of the system are to utilize an array of suitable control techniques rather than relying on a single disruptive tactic.
It is important to realize that field applications of IPM allow sub-economic pest densities rather than demanding total eradication as the criterion for successful control; for the utilization of biological control agents as a viable tactic depends on residual pest levels to insure the survival of natural enemies.
This is a practical approach which has proved to be an effective and economical control method in growing crop situations with minimum use of pesticides. But the situation is far more dramatic in storage where even the presence of beneficial parasites and predators are not tolerated. The growing crop can also absorb insect damage to a limited extent without any undue exonomic loss, but once a kernel of grain has been attacked, its nutritional value or potential for regeneration is lost forever. The further limitations imposed by some importing countries on levels of acceptable pesticide residues has precipitated the need for higher degrees of management of stocks to meet these constraints.
SUMMARY
Development of resistance is an incremental process which is related to the number of generations under selection pressure as well as the multiplication within each generation (the Net Reproduction Rate, Ro). These factors are largely dependent on the temperature and moisture of the grain in which the selected population is breeding (Heather, 1981) thus modifying the intrinsic rate of natural increase per week or rm.
Temperature and moisture also affect the rate of decay of pesticides during storage, where some are more effective under high temperature regimes (i.e., pirimiphos-methyl, azamethiphos) while the pyrethroids (i.e., permethrin) are more effective at lower temperatures, both groups benefiting from low moistures. Target rates of application of pesticides which are lethal to all insects in the population is desirable but if infestations do develop, a treatment must be introduced that does not posses any crossresistance correlation of which include fumigation with either methyl bromide or phosphine.
Pest management systems which aim to delay the rapid development of resistance must therefore; aim for complete mortality of target species, since only the survivors can develop resistance. Application techniques, maintenance of spray equipment and correct concentrations and rates of application assume importance.
Low initial populations mean a smaller genetic "pool" from which resistance development can occure, and consequently there will be fewer survivors of any applied pesticide treatment which are much easier and less costly to deal with.
FURTHER READING
BOND, E. J. and E. Upitis (1972). Response of three insects to sublethal doses of phosphine. J. Stored Prod. Res., 1973. Vol. 8, pp. 307-313.
BUSUINE, J. R. (1980). Recommended methods for measurement of pest resistance to pesticides. FAO Plant Production and Protection Paper 21.
CHAMP, B. R. and M. J. CAMPBELL-BROWN (1969). Insecticide resistance in Australian Tribolium castaneum (Herbst) - 1. A test method for detecting insecticide resistance. J. Stored Prod. Res., 1970. Vol. 6, pp. 53-70.
CHAMP, B. R. and C. E. DYTE (1976). Report of the FAO Global Survey of Pesticide Susceptibility of Stored Grain Pests. Rome, FAO Plant Production and Proteciton Series, No. 5.
DYTE, C. E. and D. G. ROWLANDS (1968). Metabolism and synergism of malathion in resistant and susceptible strains of Tribolium castaneum (Herbst). J. Stored Prod. Res. 4:157173.
FINNEY, D. J. Probit Analysis: A statistical treatment of the Sigmoid Response Curve. Second Edition. 1952. Cambridge at the University Press. 316 pp.
HARTLEY, G. S. and 1. J. GRAHAM-BRYCE. Physical principles of pesticide behaviour: "The dynamics of applied pesticides in the local environment in relation to biological response" Vol. 2: Academic Press.
McEWEN, F. L. and G. R. STEPHENSON (1979). The use and significance of pesticides in the environment. University of Guelph, Guelph, Ontario, Canada. pp. 155-213.
O'BRIEN, R. D. (1967). Insecticides - Action and metabolism. Academic Press.
OPPENOORTH, F. J. Development of resistance to insecticides extract from "Future of insecticides needs and progress". Edited by R. L. Metcalf and J. L. McKelvey Jr. pp. 41-57.
The Pesticide Manual: A world compendium. The British Crop Protection Council. Edited by Charles R. Worthing. Glasshouse Crops Res. Ins. 6th Edition, 1979.
METHODS OF TESTING CHEMICALS
Several techniques for testing insecticides and other chemicals are discussed extensively by Busvine (1971). This lecture will discuss specifically methods used in the laboratory screening and evaluation of insecticides on stored product insects. The common method used for determining the toxicity of insecticides to stored product insects are: topical application, exposure to residual films, exposure to insecticideimpregnated grains, and; direct spraying of test insects. The compounds found promising in any of the tests are evaluated in a laboratory-field simulated conditions by sack treatment, admixture with grain and residual treatments.
A. Topical Application
The most commonly employed method of applying contact insecticides to individual insects is by topical application. This offers the precise means of measuring toxicity of known doses of insecticides. Several equipment are used: wire loop, capillary pipettes and microsyringe. The microsyringe with adaptors for regular delivery of small volumes is the one recommended. It can be manually or electrically operated. The syringe size is either 1 ul or 0.25. The small syringe is more sensitive. The syringe should be calibrated individually using mercury. For application work, a 27 gauge hypodermic needle (0.4 mm) is suitable with the tip blunted on a fine abrasive stone. For convenience, the needle may be bent at right angles with the fine wire supplied for cleaning left inside.
This method is commonly used on larger larva or adults of stored product Lepidoptera.
There are three factors which are associated with the delivery of the chemical and thus the toxicity: solvent system, droplet size and site of application.
A-1. Choice of Solvent. - Solvents which tend to destroy or disrupt the waxy epicuticular layer accelerate penetration of the solution to the aqueous region of the exo-and endocuticle. The solvents most commonly used to apply them have been acetone or various oils (mineral or vegetable) or a mixture of acetone with other solvents. A preliminary test to determine the toxicity of the solvent system to the insect before using it as carrier of the insecticide should be conducted. The solvent may or may not be toxic depending upon the method of application and insect species.
A-2. Droplet Size and Insecticide Concentration. - In insects, generally, the smaller amount of droplet size is more toxic than bigger ones. The size of the droplet will vary with the solvent carrier and insecticide. Most often they apply 1 ul of the insecticide of a known dose.
A-3. Size of application. - There is some evidence that effectiveness of application declines with distance from the presumed site of action (the head or CNS). Applications are less effective as they move distally or toward the legs or abdomen.
B. Residual Exposure Methods
Investigations using insecticide films are generally of two types: (1) experiments concerned with the performance in the field, thereby demanding some approximations to practical conditions, or (2) experiments using artificial media, either for simplicity (screening tests) or precision (resistance tests or bioassay). For the first type, residues are sometimes produced by dipping, spraying or painting the substrate. Residues for the second type are often prepared by application by pipette of solutions in volatile solvents.
B-1. Dipping and Spraying. - Insecticide solutions are applied on sacks, concrete or wood to determine the toxicity of stored product insects to the residual films at different storage intervals. This is applied generally by spraying. Sacks could be treated simple by dipping in an insecticidal solution of known concentration, in a formulation to be used in practice.
B-2. Painting. - This method is used to investigate the possibility of incorporating insecticides in ordinary wall treatments such as paints or whitewash. To determine the residual activity, insects are exposed to it at different time intervals.
B-3. Application in Volatile Solvents. - The insecticides are applied in dry or oil solution residues, some workers have urea a very simple method of applying residual insecticides by dissolving them in a volatile solvent (usually acetone), and spreading a measured quantity, as evenly as possible, over a test surface. Among the glass vessels that could be treated are petri dishes, wide conical flasks, wide cylinders and ball jars. The solution is usually spread inside the vessel by tilting or rotating it and evaporation may be accelerated by a stream of compressed air.
Volatile solvents may also be applied in treating paper by applying a small volume from a pipette. However, dry residues from volatile solvents are not so uniform, therefore some workers have used a mixture of volatile and non-volatile (improves the dispersion) solvents to deposit the insecticide. This is the one recommence for treating filter papers for toxicity and resistance tests. The commonly used oils are "Risella" oil and olive oil. The mixture is spread especially over the paper which is mounted on a bed of pins. The concentration is expressed as mg per sq. cm. For resistance studies, Whatman Filter Paper No. 1, 7 cm. diameter is recommended and enclosed in a glass mug or inverted funnel. For insects which crawl up, the glass rings may be smeared with grease or Fluon.
The pick-up and spread of the carrier oil is temperature dependent but if temperature is controlled, uptake of insecticide is a linear funciton of concentration and exposure time. Exposure of insects to impregnated paper can provide a precise and convenient method of treatment. Insects are not handled individually, anaesthesia is not necessary and the mumber of insects and of samples that can be handled in a convenient time is large.
B-4. Exposure to Treated Grains. - The use of this method is to preliminary screen insecticides that could be used for protectant treatment and to measure the residual toxicity of the insecticides on the grains at different time intervals. This method is not recommended for resistance tests, since the grain particle size and adsorption of insecticide by the grain and grain dust, and in case of OPs, instability associated with enzymatic breakdown (Rowlands, 1967) may all influence the availability of insecticide for toxic action.
D. Direct Spraying of Test Insects
Spray towers, which have been evolved as a precision instrument for laboratory research on toxicological problems, are used for direct spraying of test insects. Two types of spray are available. The direct spray type makes the droplets fall directly from the atomizing nozzle to the target. In the other form, the indirect sprayer, a cloud of particles is blown into a settling chember to form aerosol, and subsequently allowed to settle on the target. The amount applied is measured. Specialized apparatus is required for this method. There are many spray towers used and for detailed information see Busvine (1971).
E. Evaluation Methods
The promising compounds found in the screening tests are further evaluated in field experiments. Sack treatment either by dipping or spraying, direct treatment of grain or combination of these methods are used for evaluation. The experimental samples are kept in laboratory-field simulated conditions for a certain period, i.e. 6-12 months. Damage, number and kind of insects, dead and alive are noted before treatment and at different time intervals after treatment, e.g. 1, 3, 6, 9 and 12 months after treatment.
TOXICOLOGICAL STATISTICS
A. Importance of Ouantal Response Assessment
A great deal of research on insecticides involves either comparison of the potencies of different compounds or comparison of the susceptibility of different species or strains of insects. In either case, the most useful method of comparison is on the basis of equitoxic doses. There are three general ways of assaying poisons 6Finney, 1952) to find these critical doses: (1) to direct assay, or by indirect assay (2) quantitative response or (3) quantal response.
In insects, the indirect assay based on quantal response is most feasible, precise and practical assay. In tests based on quantal response, the data required are the proportions of each batch reacting in a particular way. The object of the method is to estimate the magnitude of the dose which is sufficient to produce death or knockdown within a given proportion of population of insects. Comparisons may then be made on the basis of this critical dose.
For statistical reasons, it is easiest to estimate the median (50%) response level of a population rather than the most susceptible as tolerant. The median lethal dose is commonly expressed as LD50 for the 50% lethal dose; and correspondingly LD90 or LD95 for other equitoxic levels. Equivalent expressions for other dosage parameters are used. LC50 for lethal concentrations, LT50 for lethal exposure time, KD50 for knock-down dosage, and ED50 for effective dose.
The median lethal dose and similar data are quantitative expressions of the tolerance of a particular species (or strain) of insects under certain conditions. The same data as expressed as the tolerance of an insect, provides a measure of the toxicity of the insecticide used. The higher the LD50 the lower will be the toxicity.
In all the above evaluation, the criteria of toxic action is death or knockdown at a certain time (i.e. 24 or 48 hrs). This measures the acute toxicity of a compound. For those with side effects, the onset of paralysis or knockdown is often used as a criterion of toxic action. In most cases, knockdown and death are just lumped together in obtaining data.
B. Obtaining Date for Quantal Response
It is necessary to expose batches of insects to a range of doses of a toxicant. Both test insects and environmental conditions need to be standardized. The number required for each batch is governed largely be practical considerations. With stored product beetle which can be reared without difficulty in large numbers, more insects should be used per replicate per dose. The larger the number per batch, the greater the accuracy; but there is generally little advantage in exceeding 30 or 50 per batch per replicate.
In selecting doses or concentrations for testing, it is desirable to space them evenly over the mortality range. since toxic effect is more conveniently realted to the logarithm of the dose than to the dose itself, the doses chosen should be a geometric series.
In choosing for the time of assessment of mortality, it is desirable to make preliminary observations, for any insect/poison, on the reactions displayed at different periods of dosing. If an insecticide with rapid action, which allows some individuals to recover from paralysis, is being compared with a slow-acting irreversible poison, the conclusions reached will vary greatly according to the time chosen to assess their effects. Changes are more pronounced at high doses. the most common practrice is to assess at 24 and 48 furs.
C Statistical Procedures Correction for Control Mortality
The data collected should be corrected for the control mortality, which if appreciable, may affect the precision of the results. This is corrected by Abbott's formula:
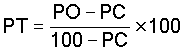
where PT = corrected mortality; PO = observed mortality in treated and PC = control mortality (all%). Rejection of expriments is recommended with control mortalities of 10 to 20%.
The Use of the Probit/Log Dosage Transformation The most usual way of interpreting quantal response is from the regression line (either plotted graphically or computed) relating the log dosage to a transformed percentage response. This will correct for the sigmoid form of curve when the percentages per se are plotted against the dose in ordinary graph paper.
The use of probits and log doses to obtain estimates of critical dosage levels and their limits of accuracy can be done in three ways with different degrees of precision. these are (1) simple arithmetical and graphical methods (2) standard method of computation using desk calculator and (3) using a computer programme.
In graphical method ( Appendix A), the critical doses or susceptibility can be estimated with sufficient accuracy from a probit/log-concentration graph. The two transformed variables are also plotted on plain paper of the original data (% kill and dose) can be plotted on logarithmic/probability paper. A straight line is fitted by eye (a celluloid ruler is useful) and the critical doses determined by inspection.
For the calculations of the regression line relating probits and dose consult Finney (1952a & b).