FAO plant protection paper 21. "Recommended methods for measurement of pest resistance to pesticide"
J. R. Busvine, 1980.
Statistical Principles of the Detection and Measurement of Resistance Correction for Control Mortality
It should be noted that the mortality observed in bioassay tests may not all be due to the pesticide, since handling and holding operations may also be lethal to some of the arthropods. This can be checked by using batches of controls, which are treated in the same way, except for use of pesticide. If it is assumed that deaths from handling and from the pesticide are quite independent and uncorrelated, then a correction for control mortality can be made by the use of Abbott's formula, as follows:
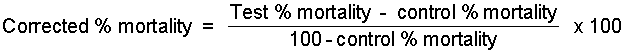
Two comments on this correction may be of interest. Firstly, the change effected by its use is greatest at low mortalities and becomes very small near 99% mortality; secondly, the correction is virtually negligible when control mortalities are below 5%.
Dose-kill Relations
As has been stated, resistance can only be determined by comparison with a reference strain, which provides base-line data. These are obtained by exposing batches of this strain to a series of dosages and recording the mortality produced. The dosage may be the actual dose applied (e.g. in milligrams per arthropod, or per gram weight); or it can be the concentration used, or even the exposure time, since these are normally simple functions of the dose. Since the lethal effects of pesticides (like other poisons) are related to the logarithm of the dose, the dosages used should be in a logarithmic series, or else converted to logarithms for comparison with the kills.
The lethal effects, expressed as percentages killed, are related to the normal distribution of sensitivity in any homogeneous population. Because most susceptibility values are close to the average, there will be bigger changes in percentages killed by a given dose increment in this region than at extremes of high or low susceptibility. Therefore, to obtain a linear relation with log dosage, the percentages must be transformed, which can be done by using a table of "probits". Alternatively, the results may be plotted directly on to logarithmic probability graph paper (as shown in Figure 1). With results plotted on this paper, a straight line may be drawn to the results; and from it, the median lethal dose and other statistics may be estimated graphically with reasonable accuracy. If high accuracy is required, the log doses and probits may be used to compute the position of the line.
Various stages may best be explained by a hypothetical case, illustrated by Figure 1.
Base-line Data. In this hypothetical example, batches of the reference strain have been exposed to doses of 0.25, 0.5, 0.75 and 1.0 dose units, and the resulting percentages killed are plotted as open circles. The kill obtained by 1.0 units was 100%, which cannot be represented on a probability scale; so this point is represented by an arrow. The regression line "A" is drawn to fit these points as closely as possible. From it can be read the best (graphically determined) estimate of the kill to be expected from any dosage. Thus, 0.47 units corresponds to 50% kill, which is known as the median lethal dose, or LD50. If concentration were used for dosage, the expression would be LC50; and LT50 would correspond to a median lethal time of exposure. Similarly, other doses corresponding to 90% (LD90), 99% (LD99), etc., can be estimated.
As has been pointed out, the susceptibility of the reference strain may vary with different intrinsic or extrinsic factors. There-fore, base-line "A" will only be relevant for pests of defined status and conditions. With different status or conditions, the line could be displaced, to "B" for example. It will, however, be assumed that the resistant strain will be directly comparable to base-line "A".
Monitoring for Resistance. For routine monitoring to detect resistance, it is necessary to use a "diagnostic dose". This is chosen with reference to the regression line "A", using it to select a dosage with a high probability of killing all of a sample of normal pests. There is no absolute criterion of the number of individuals to be used in such a sample, nor of the dosage to be employed. The larger the number of pests in the sample, the better the chance of detecting resistance when only a small proportion of the population is affected. Generally a sample of at least 100 should be used, if possible.
With regard to the dosage chosen, it will be clear that one expected to give 99% kill will theoretically allow the survival of one normal individual in 100% that corresponding to 99.9% kill should spare only one in 1000. The lower level will occasionally give false indications of resistance, while the higher one will sometimes miss genuine, but low-level, resistance. Which ever dosage is chosen, the test should be repeated when survivors appear; and if they continue to appear every time, resistance should be suspected.
Detection of Resistance. In the hypothetical example, with pest status and conditions that produced line "a", a diagnostic dose expected to kill 99.9% would be 2.0 units. If conditions or pest status had changed to those which would have given line "f", the dose of 2.0 units would allow about 5% survival. Thus, monitoring with different conditions or status (e.g. lower temperature or insects entering diapause) could give false indications in incipient resistance.
Let it be assumed, however, that conditions and status are constant and that about 20% survivors have been repeatedly found at the 2.0 unit dose. This constitutes a warning signal, indicating a strong possibility of resistance. Additional tests are now done over a wider range of doses, the results of which are indicated by crosses in Figure 1. It will be seen that, above the diagnostic dose, there is little increase in kill over a large increase in dosage. The regression line of the suspected population ("c") forms a plateau, corresponding to about 80% kill, up to the point where resistant forms begin to be killed. This is consistent with the existence of 20% of resistant pests in the population.
It may be noted that, if changed conditions or status had been responsible for survivors at the diagnostic dose, there would have been no plateau, because increased doses would have soon cause a complete kill.
Measurement of Resistance. It is clearly impossible to draw a straight line to the results of tests with the suspected population, nor can one logically determine its LD50, as can be done with a homogeneous population (though some workers have tried to assess resistance levels of field populations by doing so). It should be noted that the dose to kill 50% of the suspect population has not changed much.
To measure the resistance level, it is necessary to obtain a homogeneous resistant strain, by appropriate breeding and selection in the laboratory. In the hypothetical example, a very high resistance has been postulated, as indicated by the results of tests which gave line "d" 1. Because of technical limitations in the test method, it was not possible to get kills higher than about 50%. If the resistance had been at a lower level, corresponding to line "d2", tests with the suspected field population would have begun to kill some of the resistant individuals in the population at the higher doses, diving line "c2".
Cross-resistance Tests. When the existence of resistance has been confirmed, it is clearly desirable to investigate its crossresistance characteristics by tests with other pesticides. This work, which would involve laboratory studies, should help to select an alternative pesticide.
EVALUATION OF INSECTICIDES FOR GRAIN TREATMENT, THE PRACTICAL DETAILS FOR DETERMINING EFFICACY
During the last 10 years work on the development of insecticides for grain treatment in Australia has concentrated on the control of malathion resistant strains. Samples of live insects were collected from storages throughout Australia and typical resistant strains are now maintained under continuous selection with insecticides in laboratory culture.
LABORATORY TRIALS
initial testing of candidate compounds in the laboratory involves modifications of the standard FAO resistance tests with impregnated papers. This provides a rapid assessment and is valid for comparison of compounds within one chemical group. It is not valid for use with highly volatile compounds.
Promising compounds are then bioassayed in treated grain. With wheat, desired quantity of insecticide is pipetted into bottles of grain at 11 % moisture content in sufficient volume of water to increase the moisture content to 12%. Grain is mixed by tumbling on a mechanical tumbler for 10 minutes. In general each bioassay involves six doses of an insecticide plus and untreated control. At each dose reacts there are 3 replicates of 50 parent insects in 83 9 of wheat for Sitophilus oryzae or Rhyzopertha dominica or in 167 9 for Tribolium castaneum. For Ephstia cantella 100 eggs are added to 167 9 of grain. The test insects are added 16 hours after treatment.
Initial assessments of mortality are made on parent insects after 3 days using as a criterion of response the inability to stand and walk.
All insects are returned to the treated grain and mortality again assessed at 26 days when all parent insects are removed. Most tests are conducted at 25°C and 55% RH but at this time those involving Rhyzopertha and Tribolium are transferred to 30°C and 55 RH for progeny development. The numbers of F1 progeny developing at 10 weeks are recorded and also the number of arbitrary F2 progeny at 16 weeks.
The efficacy of residues of highly promising compounds is determined after storage at 25°C for 0,112,3,4 - 1/2,6,9 and 12 months after treatment.
Data are analysed using probit analysis and compounds are compared with standards using relative potency analyses.
The rate of decay of residues is determined by chemical analysis of samples at the same time.
FIELD TRIALS
Silo scale experiments are carried out in commercial silos using standard industry equipment. The candidate insecticides are applied by spraying into the grainstream, 11 per tonne, during unloading of grain into storage. Silos larger than 500 tonnes are preferred since in smaller grain masses temperatures approximate ambient conditions which are often at lower temperatures than grain in large silos.
Grain samples are taken at 12 points each 2 m below the grain surface using a vacuum sampler. Samples are bulked, subsampled and sealed into sample tins before leaving the treated bin. Significant losses of residue have occurred from newly treated grain during subsampling using more conventional techniques.
Bioassays and chemical assays are carried out on samples of treated grain in the laboratory as already described.
Necessary records for silo scale experiments include grain quality, grain moisture and grain temperature. The presence of any naturally occuring infestations are determined by sieving samples collected either with the vacuum sampling probe from the grain surface or directly from the grain stream during turning. The presence of such infestations indicates a failure but the absence does not necessarily indicate success. The species could simply be absent.
Supplementary observations are made on the stability of insecticide formulations, the effect of insecticide on grain conveyor belts, the concentration of insecticide in grain dust and the reaction of workmen to the treatment.
Residues are determined in appropriate pro-. ducts manufactured from treated grain e.g., bran, germ, wholemeal flour, white flour, wholemeal bread and white bread. Additional laboratory studies are made of the rate of breakdown of residues on treated grain of different moisture contents at different temperatures. These data then enable the prediction of breakdown of residues under any given temperature or moisture conditions.
The final stage of testing involves Pilot Usage Trials at a minimum of 20 sites throughout the major grain producing areas of Australia. During this stage scientific staff are involved only with residue analyses and bioassays. All of the treatment operations are carried out by industry personnel.
The participation of industry personnel both at the administrative and operational levels is considered an essential aspect of the field testing programme.
Recommended Methods for the Detection and measurement of Resistance of Agricultural Pests to Pesticides
Tentative method for adults of some major pest species of stored cereals, with methyl bromide and phosphine · FAO Method No. 16
Synopsis
A test method is described for detecting and measuring resistance to the fumigants methyl bromide and phosphine in Sitophilus oryzae, S. zeamais, S. granarius, Rhyzopertha dominica, Tribolium castaneum, T. confusum, Oryzaephilus surinamensis and O. mercator.
The method is based on exposure of adult insects to discrete atmospheres containing fumigant. Exposure periods are 5 hours for methyl bromide and 20 hours for phosphine. Responses are determined 14 days following termination of the exposure.
Base-line data are established with reference strains of known susceptibility from which it is possible to select discriminating doses that may be used to monitor samples of beetles for resistance. Survival in such tests is indicative of resistance, following which extensive testing should be carried out to determine the degree of resistance present.
Equipment and materials
Insectary. Facilities should be available for rearing insects at both 25 and 30°C at approximately 70 percent relative humidity. A large incubator can be used, in which case the humidity can be adjusted by introducing an open tray filled with a saturated solution of sodium nitrate or strontium chloride.
Breeding materials. A summary of the breeding media and appropriate temperatures for the different species is given in Appendix 1. The media should be disinfested by heating in a sealed container at 60°C for one hour after this temperature is reached throughout the medium. For breeding containers, small jars or wide-mouthed bottles sealed with filter paper (Whatman No. 29, black) and paraffin wax (to restrict movement of mites) are suitable.
Containers held in the incubator should be placed on trays containing paraffin oil as a further precaution to prevent contamination by mites.
All grain pests are subject to disease in laboratory culture. Tribolium species are particularly susceptible to the protozoan parasite Farinocystis tribolii. All equipment used for handling insects and media should be sterilized in a hot-air oven at 110 to 120°C between each use and when not in use should be stored in this oven. At least one-half to one hour exposure at this temperature is necessary to ensure the destruction of the protozoan spores. all plastic ware used should be polypropylene, nylon or polycarbonate which is not affected by this temperature.
A supply of Fluon GP1 (an aqueous dispersion of polytetrafluoroethylene, obtainable from Imperial Chemical Industries Ltd.) must be available to assist in restraining all species other than Tribolium spp. and R. dominica. A film of this material is applied to the top 2 cm of the inside wall of breeding containers to prevent insects congregating on the filter paper seals and boring out. All handling containers must be treated similarly.
Test area. A room with normal illumination maintained at 25°C is required. Should this room have a controlled humidity of 70 percent it will also serve to condition the atmosphere of fumigation chambers before commencing tests; however, preconditioning of fumigant chamber atmospheres may be carried out in a breeding room.
Funigants. Pure methyl bromide (excluding chloropicrin) is recommended. Suitable sources include commercially available ampoules and cans. Aluminium phosphide formulations available commercially provide a suitable source of phosphine. Glassware, etc.
1. Fumigation chambers. Desiccators, 20 cm (8 in.) in diameter, fitted with a plate of stainless steel or other chemically inert material, and with lids containing ground sockets of a standard glass joint size make suitable fumigation chambers. Cone/screw thread adapters (e.g. Quickfit) should be fitted to the sockets of desiccator lids. A pure gum-rubber septum (or other rubber with good resealing properties when pierced with a syringe needle) is placed under the screw cap. Septa for this purpose are conveniently obtained from sheet rubber 3.2 mm (0.12 in.) thick with a wad punch.
Ground surfaces of desiccator flanges and cone/screw thread adapters should be lightly coated with silicone grease. (The use of cone/screw thread adapters is not essential. Any method providing in the lid of desiccators a septum that ensures both a gastight seal and chemically inert surfaces will be satisfactory.)
2 Fumigation cages Glass rigs 30 mm diameter and 25 mm high attached to filter papers (35 mm or 42.5 mm diameter) or small flat-bottom evaporating dishes are recommended as containers in which to confine insects during exposure to fumigant. Stainless steel gauze discs should be placed over fumigation cages when dosing species that may fly.
3. Gas-tight syringes. A range of gas-tight syringes (e.g. Hamilton gas-tight syringes) is required. The range chosen will be influenced by the volumes of the fumigation chambers but would probably include 50, 100 and 250 microlitre syringes together with 1-, 2.5- and 10- millilitre syringes. (It is not good practice to dispense small volumes from a large volume syringe.)
4. Magnetic strirrer. A magnetic stirrer is recommended for stirring gas mixtures within fumigation chambers. For this purpose a 45-mm PYCcoated follower with a rim is placed in the bottom of each fumigation chamber. Where necessary a magnetic stirrer may be made by fitting a suitable magnet to the shaft of a small electric motor. Speed control is not necessary.
5. Fumigant storage vessels. A 25- or 35-ml vial fitted with a screw cap and a Mininert valve provides a satisfactory method for storing methyl bromide (Figure 1). From this vessel, dose volumes of methyl bromide vapour may be obtained with a gas-tight syringe. Alternatively a tube fitted with a septum and side-arm with stopcock, as shown in Figure 1, may be used as a means of providing a source of methyl bromide vapour.
For phosphine, a glass tube fitted with a septum together with a gas jar or measuring cylinder is required. A diagram of this apparatus together with accessory items is shown in Figure 2.
Collection of specimens and rearing
For identification of the various species see Appendix 2. The correct identification of species is particularly important and the tests have been designed to permit workers to identify species after discriminating tests have been conducted. It is essential that all identifications be checked at this time, particularly of survivors, and if there is any doubt as to the identity of specimens these should be submitted to an appropriate expert.
Figure 1. Methyl bromide storage/dose-source vessels.
Preparation of gas sources
Methyl bromide. All handling of methyl bromide should be carried out in a fume cupboard or well ventilated area. The Maximum Allowable Concentration (MAC)4 for methyl bromide is 20 ppm.
The container of methyl bromide, the storage vessel (dose-source vessel, see Figure 1) and a small glass funnel should be cooled to about 0°C beforehand. Liquid methyl bromide is then transferred to the storage vessel until it is about three-quarters full, following which the cap of the storage vessel is firmly replaced to ensure a gastight seal.
Generally, sufficient methyl bromide will be vaporized during transfer to the storage vessel to flush out most of the air. To expel remaining air from the space above the liquid, the septum should be removed from the Mininert valve when the liquid is at room temperature and the valve opened for a short time allowing gas to escape. If the alternative storage vessel is used, the stopcock in the side-arm is opened for a short time. When not in use, both vessels should be stored in a refrigerator or cool place or in a fume cuphoard. Although both vessels should be completely gas-tight the possibility of leakage should not be overlooked.
Phosphine. All handling of phosphine should be carried out in a fume cuphoard. The MAC value for phosphine is 0.3 ppm.
Phosphine is conveniently obtained for laboratory dosing purposes from aluminium phosphide according to the equation:
A1P + 3HOH - A1(OH)3 + PH3
Some commercially available aluminium phosphide formulations contain ammonium carbamate as well, which also reacts with water liberating ammonia carbon dioxide.
Commercially available pellets containing a phosphine equivalent of approximately 0.2 9 are recommended as the most suitable source of phosphine for the present method.
The apparatus (Figure 2) for generating phosphine is prepared by filling the gas jar, collection tube and rinsing tube with water. All air must be removed from the collection tube and rinsing tube. Approximately half the water in the gas jar is siphoned out and replaced by 10 percent (v/v) aqueous sulphuric acid solution. The contents of the gas jar and collection tube should be thoroughly stirred. Mixing within the collection tube may be achieved by forcing a jet of liquid through the rinsing tube and repeating this procedure a few times. A pellet containing aluminium phosphide is then placed in the gas jar with the aid of the stainless steel wire, the funnel is lifted slightly and placed over the pellet. Liberation of phosphine commences almost immediately.
As the reaction proceeds, the liquid disphlaced from the collection vessel is removed by means of the siphon, thus maintaining the level in the gas jar more or less constant. Throughout the procedure the liquid level in the gas jar should be maintained above that in the collection tube thus exerting slight positive pressure on the gas collected.
When the collection tube has been filled with gas, the walls of the tube may be rinsed with the "rinsing tube" to remove adhering pellet residue. This step is not absolutely necessary. However, frequently liquid remains in the neck of the collection tube and this may be displaced by a jet of liquid from the rinsing tube.
The gas mixture obtained in this manner from a "fresh" pellet contains approximately 86 percent phosphine (1.195 hg/ul) and is sufficient to provide a source of phosphine for dosing purposes over several weeks. The inclusion of sulphuric acid in the liquid contents of the gas jar and collection tube is primarily to absorb ammonia but it should also prevent the production of diphosphine (P2H4) which is spontaneously inflammable.
Measuring volume of desiccator
The volume of each desiccator must be determined so that dose volumes may be calculated. Thus each desiccator should be fully assembled to simulate operating conditions and then completely filled with water. The weight of water, corrected for the temperature of the water, provides a satisfactory estimate of volume. At 25°C 1 gramme of water occupies 1.003 cc.
Prefumigation procedures
The following procedures are carried out on the day prior to dosing.
Preparation of insect cages. Filter-paper circles are fixed to the bottom of glass rings with a water soluble adhesive (a film of adhesive in a petri dish facilitates this operation) and the adhesive allowed to dry, preferably in an oven. If the flatbot tomed dishes are used, a filterpaper circle of suitable size should be placed in the bottom of the dish so that insects may walk normally.
When testing species that are able to climb a vertical glass surface, rings or dishes should be dipped in liquid Fluon to produce a film approximately 5 mm wide on the upper portion of the inner surface. With dishes it is necessary to provide a ventilation tube to permit displacement of air from inside the inverted dish.
Preparation of insects. A complete test comprises two replicates of 50 insects for each of five concentrations puls two control replicates, i.e. 600 insects in 12 glass rings for each strain tested. Adult beetles are counted in 12 batches of 50 into small vials or directly into the glass rings, progressively placing a maximum of ten insects in each vial or ring until each has the required number. The 12 batches are then assigned at random to the six desiccators, i.e. five treatments plus one control.
When testing species that are inclined to fly, the rings or dishes are covered with a square of stainless steel mesh. The squares of mesh are held in place by pushing down and banding the four corners over the ring or dish.
The insects thus prepared are held overnight in the desiccators, with lids removed, in a controlled temperature environment at 25°C and 70 percent relative humidity.
Determination of dose volumes. The five concentrations to be used in the test should be selected. The data of Appendixes 3 and 5 are intended as a guide to likely dose ranges of susceptible strains. A dose equal to the discriminating dose should be selected as the highest dose of the test.
Having selected the concentrations, the required dose volumes (i.e. gas volumes) are determined (see Appendix 4).
Fumigation procedures
Preparation of chambers. Replace the lids on desiccators within the controlled temperature environment in which the desiccators and insects were held ovemight. The atmosphere of the chambers is thus conditioned at atmospheric pressure to 70 percent relative humidity at 25°C. Transfer the desiccators to the dosing area.
Dosing chambers with methyl bromide. If the storage vessel (dose-sorec vessel) has been stored in a refrigerator, remove the vessel well before dosing is to commence to allow vessel and contents to reach ambient temperature.
Withdraw required gas volumes from the dose source vessel with the appropriate gasligkt syringe. Flush the syringe, before taking the first dose, by withdrawing small volumes of gas and expelling syringe contents into the fume cupboard. Do not allow heat from the hands to warm the contents of the syringe. Inject the dose into the desiccator and record the time of dosing. Immediately stir the atmosphere of the desiccator ÷ith a magnetic stirrer for one to two minutes.
Following dosing, the desiccators should be held at a temperature of 25°C throughout the exposure period. The exposure period required is five hours. This period hould be adhered to strictly in normal testing experiments.
Dosing chambers with phosphine. It is advisable to flush syringes to be used for dispensing phosphine doses in a stream of oxygen-free£nitrogen immediately before use.
Before taking the first dose with a particular syringe, flush the syringe with small quantities of the phosphine source by withdrawing small volumes of gas and expelling these into the fume cuphoard. When drawing phosphine into the syringe move the plunger slowly at all times. A sudden reduction in pressure within the syringe may ignite the phosphine spontaneously. As a further precaution ensure that at all times the liquid level in the gas jar is above that in the collection vessel thus exerting slight pressure on the phosphine gas mixture.
Withdraw the required dose volumes and inject these into the appropriate desiccator. recording the time when each dose is applied. Immediately stir the atmosphere of the desiccator with a magnetic stirrer for one to two minutes. Do not allow heat from the hands to warm the contents of the syringe during the dosing procedure. Between doses syringe needles should be capped with a rubber plug.
Following dosing, the desiccators should be held at a temperature of 25°C throughout the exposure period. The exposure period required for hosphine tests is 20 hours. This period should be adhered to exactly in normal testing experiments.
Post-fumigaticn procedures
Termination of exposure. At the end of the required exposure period the lids of desiccators are removed, insect cages extracted and the desiccators ventilated. All insects are transferred to a small quantity of medium in a suitable container and held at 25°C and 70 percent relative humidity.
Mortality assessment. Mortality should be assessed 14 days from the end of the exposure period. This post-treatment holding period allows time for end-point mortalities to be reached for both fumigants.
Numbers responding, i.e. dead, should be taken to include those insects that are in fact dead together with those showing only slight twitching of appendages when prodded. If death is recorded in the controls, the percentage responding to all test levels should be corrected by Abbott's formual. Results should be discarede and the test repeated if the percentage allected in controls is greater than 10.
Interpretation, calculation and reporting
The mortality figures are plotted on logarithmicprobability paper and the dosage-mortality relationship fitted by eye or by appropriate calculations. The LD50 and LD99.9 values are then determined from these lines. Values are expressed in concentration of fumigant (mg/l) or as concentration x time products (c x t) expressed in milligramme hours per litre (mg h/l). when reporting these values both methods of expression should be prefaced (e.g. in table headings) be a statement of the exposure period since this is the fixed variable of dosage in the tests described.
The above procedures serve to establish baseline data for susceptible reference strains of beetles. Some results obtained by this method are referred to in Appendix 3. Testing of field strains may also be carried out in the foregoing manner. However, when testing field strains a full series of replicates of the susceptible reference strain should be included. Thus chemical estimation of gas concentrations is not necessary. Abnormal gas concentrations during tests will be revealed by the abnormal response of the reference strain. Nevertheless, a detailed evaluation of strains showing increased tolerance may require gasconcentration measurements.
Testing resistant strains
Procedures similar to the above allow the determination of LD50 and LD99.9 values for resistant strains. It will be necessary to increase dosages to obtain an equivalent range of response. When testing strains resistant to methyl bromide, dosages may be increased by increasing either the concentration or the exposure time. Strains resistant to phosphine, however, should be tested by increasing the fixed period of exposure, e.g. from 20 to 48 hours, and using the same range of concentrations. Small increases in concentrations can be made. At high concentrations of phosphine, however, insects become narcotized, the effect of which is to produce nonlinear probit lines, apparent resistance in susceptible strains and exaggerated resistance factors in resistant strains.
LD50 and LD99.9 Values obtained for resistant strains may be compared with those obtained for the susceptible reference strain and resistance factors calculated.
Monitoring for resistance
For routine monitoring to detect the inintial appearance of resistance in wild populations of stored product beetles, it is convenient to use a discriminating dose which is expected to kill all susceptible specimens. The dose chosen is that corresponding to slightly above the LD99.9 obtained from the regression line for susceptible beetles allowing for, in the case of phosphine, what appears to be inherent variability of response. Some discriminating concentrations are given in Appendix 1 Susceptible reference strains must always be included in discriminating tests.
When using a discriminating test with fumigants it is advisable always to make provision for abnormal concentrations. If a concentration is obtained that is less than the discriminating concentration this will be revealed by abnormal survival in the susceptible reference strain. Abnormally high concentrations may be revealed by the inclusion in the tests of a reference strain (or species) with slightly greater tolerance to the fumigant than the susceptible reference strain on which the discriminating dose is based, approximately x 1.5 for methyl bromide tests and x 2.5 for phosphine tests. An alternative approach to this is to use three concentrations, one at the discriminating concentration, one at the approximate LC90 level and the other at an equivalent level above the discriminating concentration.
In regular monitoring for resistance, it is desirable that it may be detected when only a small proportion of resistant individuals is present. For such purposes a minimum of 100 insects in two batches of 50 should used per sample.
Limited numbers of insects may not be sufficient to detect low levels of resistance. There-fore, additional samples should be obtained if possible. If, however, there is suspicion of serious resistance (e.g. from failure of treatments) a test with small numbers (10 to 20) may provide early valuable indication.
The insects are exposed to the discriminating dose for the appropriate period, in the usual way. If all of them are dead at the end of the post-treatment holding period, the sample can be classified as "no resistance detectable", and the medium in which they were held is put into a hot-air oven to destroy the culture. On the other hand, the presence of unaffected insects at the end of the test should be regarded as prima facie evidence of resistance, requiring further investigation.
Confirming resistance when a few Insects are unaffected
The appearance of unaffected insects in a discriminating test could be due to the presence of unusually tolerant individuals from a normal population. Provided that the conditions of exposure, the physiological state of the insects and the dosages are consistent, the probability of a single insect in a batch of 100 being unaffected due to chance is less than 0.1 (i.e. Iess than once in ten tests). It is important to determine whether incomplete response is due to such causes or to genuine resistance. This can be checked in the following manner:
1. By repeating the test, using further samples from the same field population. The chances of adventitious failure to respond by a single individual in each of successive tests decline progressively (less than 0.01, 0.001, 0.0001 and so on). Survival of two or more individuals throughout is even less likely. Therefore, the continued appearance of a proportion of unaffected individuals can be considered as proof of resistance.
2. Alternatively, the insects which were unaffected in the discriminating test may be kept and used for breeding a further generation. should their reaction be truly due to resistance, it will be found that a substantially larger proportion of their progeny will fail to respond to the discriminating concentration.
Appendix 1 Some normal susceptibility data obtained for methyl bromide and phosphine together with discriminating concentrations
| LC50 | LC99.9 | Discriminating concentration | |
mg/l |
|||
| Methyl bromide | |||
| (Exposure period 5 hours) | |||
| Sitophilus oryzae | 3.6 | 4.8 | 6 |
| S. zeamais | 3.2 | 5.4 | 6 |
| S. granarius | 5.1 | 7.5 | 9 |
| Rhyzopertha dominica | 4.0 | 7.4 | 7 |
| Tribolium castanceum | 8.4 | 11.7 | 12 |
| T. confusum | 8.6 | 11.2 | 13 |
| Oryzaephilus surinamensis | 5.8 | 8.5 | 9 |
| O. mercator | 5.8 | 8.5 | 9 |
| Phoshpine | |||
| (Exposure period 20 hours | |||
| Sitophilus oryzae | 0.011 | 0.039 | 0.04 |
| S. zeamais | 0.007 | 0.013 | 0.04 |
| S. granarius | 0.013 | 0.041 | 0.07 |
| rhyzopertha dominica | 0.008 | 0.028 | 0.03 |
| Tribolium castaneum | 0.009 | 0.028 | 0.04 |
| T. confusun | 0.011 | 0.029 | 0.05 |
| Oryzaephilus surinamensis | 0.012 | 0.036 | 0.05 |
| O. mercator | 0.011 | 0.034 | 0.05 |
Appendix 2 Calculations of dose volumes and concentrations
The method requires the selection of concentrations, following which dose volumes of gas are determined. The following examples show the calculations and steps required to determine dose volumes and actual concentrations for a temperature of 25°C (298°K).
Example 1. Methyl bromide
Step 1. Determine the volume, d1(ml) of methyl bromide vapour at 25°C required to obtain a concentration of x1(mg/l) in a desiccator of volume V1(l)
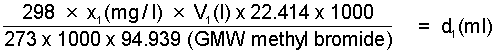
i.e. x1(mg/l) x V1(l) x 0.2577 = d1(ml)
Step 2. Select nearest whole division on appropriate syringe to give actual dose volume D1(ml) of vapour and recalculate concentration, i.e. concentration X1(mg/l) actually applied.
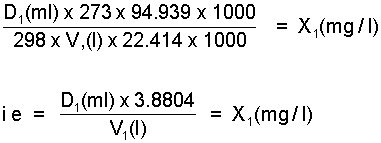
Example 2. Phosphine
Step 1. Determine the volume d1(ul) of the 86% phosphine gas source at 25°C required to obtain a concentration of x1(mg/l) in a desiccator of volume V1(l).
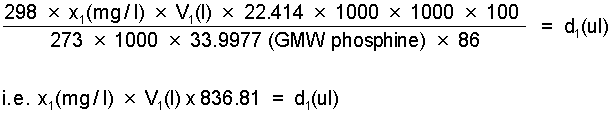
Step 2. Select nearest whole division on appropriate syringe to give actual dose volume D1(ul) of the 86% phosphine source and recalculate concentration, i.e. concentration X1(mg/l) actually applied.
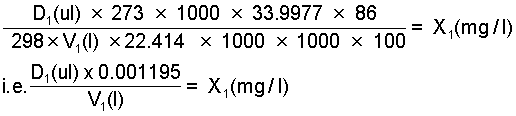
An 86% phosphine source is equivalent to a concentration of 1.195 ug/ul. Thus if concentration is expressed in terms of (ug/l) the equivalent of steps 1 and 2 in the foregoing is given by
Step 1 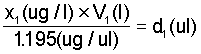
Step 2 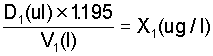
Appendix 3 Suggested concentration ranges for tests on susceptible beetles
Table 1. Optimal Temperature Ranges of some common stored products insects.
| Insect Species | Common Name | Optimum Range (°C) |
| 1. Species thriving at moderate grain temperatures: | ||
| Acaros siro | Flour mite | 21 - 27 |
| Ptinus tectus | Australian spider beetle | 23 - 25 |
| Sitophilus granarius (L.) | Granary weevil | 26 - 30 |
| 2. Species requiring moderate - warm temperatures: | ||
| Sitophilus oryzae (L.) | Rice weevil | 27 - 31 |
| Acanthoscelides obtectus (Say) | Bean weevil | 27 - 31 |
| Sitotroga cerealella (L.) | Angoumois grain moth | 26 - 30 |
| 3. Species fequiring high temperatures: | ||
| Tribolium confusum Duv. | Confused door beetle | 30 - 33 |
| Zabrotes subfasciatus (Boh.) | Tropical bean weevil | 29 - 33 |
| Oryazephilus surinamensis (L.) | Saw-Toothed grain beetle | 31 - 34 |
| 4. Species thriving in a hot climate: | ||
| Rhyzopertha dominica (Fab.) | Lesser grain borer | 32 - 35 |
| Tribolium castaneum (Herbs".) | Rost-red door beetle | 32 - 35 |
| Trogoderma granarium Everts | Khapra beetle | 33 - 37 |
| (max 41°C) |
Source: Howe (1965).