Stephen F. Siebert is Associate Professor
in the School of Forestry,
University of Montana, Missoula,
Montana, United States.
The article examines the viability of managed harvesting - with a focus on a study of the ecological effects associated with cane extraction in Central Sulawesi, Indonesia - considered against contrasting alternatives: clearing for cash crops, and rattan cultivation.
Rattan, one of the world's most important non-wood forest prod-ucts (NWFPs), offers a vivid example of the difficulties and uncertainties inherent in ascertaining sustainable extraction levels and impacts associated with harvesting. Rattan is collected almost exclusively from wild populations, and market demand for cane is strong. Indonesia supplies perhaps as much as 90 percent of the world's commercial rattan cane (Dransfield and Manokaran, 1994), and most is gathered from forests in which management has been largely absent or ineffective (Barr, 2000). There have been little or no monitoring or management of wild rattan harvesting and virtually nothing is known about ecological effects associated with extraction.
Mature Calamus zollingeri plant growing in Lore Lindu National Park, Central Sulawesi
- S.F. SIEBERT

Sustainable harvesting of NWFPs has been advocated as a means of simultaneously conserving forests and encouraging economic development (Anderson, 1990; Freese, 1997) and is now an integral component of most tropical forest conservation and management efforts (CIFOR, 1999). However, many ecologists contend that NWFP harvesting is neither ecologically sustainable nor economically viable (Kramer, van Schaik and Johnson, 1997; Rice, Gullison and Reid, 1997) and that the notion of sustainable extraction is an ecological oxymoron (Struhsaker, 1998). Is sustainable harvesting of wild rattan an oxymoron? If not, under what biophysical, socio-economic and institutional conditions might harvesting be viable?
This article documents an attempt to assess and monitor ecological effects associated with cane extraction in primary forests, based on five years of ongoing research by the author in Central Sulawesi, Indonesia. The study focused on Calamus zollingeri, a commercially important large-diameter rattan used in furniture manufacturing. The article focuses on only two among the many direct and indirect ecological effects examined in the study:
In addition, the article points out that possible adverse effects to other flora or fauna must be weighed against those of alternative economic activities (e.g. converting forests to annual or perennial cash crops). Finally, it examines the viability of managing wild rattan harvesting versus rattan cultivation.
A study to examine the ecological effects of cane harvesting was carried out in Lore Lindu National Park in Central Sulawesi. It focused on Calamus zollingeri, which is abundant in and around the park at altitudes below 1 150 m. C. zollingeri is a robust, clustering rattan found throughout Sulawesi, the Mollucas and other islands of eastern Indonesia and is one of the most important large-diameter canes in the furniture industry (Dransfield and Manokaran, 1994). Virtually all C. zollingeri cane is collected from unmanaged, wild populations in primary forests. Despite prohibitions, huge quantities of C. zollingeri cane are collected from Lore Lindu National Park and cane harvesting shows no signs of declining (author's observation). Indeed, given expanding export demand and the central Indonesian government's inability to enforce prohibitions against collecting, rattan harvesting is likely to accelerate.
One of the challenges in assessing ecological sustainability is determining and establishing monitoring protocols for the vast array of possible direct and indirect biological effects. Table 1 summarizes the primary direct and indirect effects associated with C. zollingeri cane harvesting in Central Sulawesi and the methods used to assess and monitor them in this study. Principal effects associated with rattan cane harvesting include:
TABLE 1. Potential effects of rattan cane harvesting and monitoring methods | |
Potential effect |
Monitoring methods |
Species level |
|
Plant (genet) survival |
Resampling of permanently marked plants |
Plant population structure |
Resampling of permanently marked plants and replicated sampling of random transects |
Ecosystem level |
|
Nutrient stocks |
Determination of nutrients in foliage and canes and of the volume of cane extracted per unit area |
Forest structure |
Long-term monitoring of sample plots |
Vertebrate food resources |
Biweekly sampling of marked plants |
Invertebrate use |
Not investigated in this study |
Other |
|
Cane transport (floater logs) |
Determination of weight of rattan extracted, tree species and volume used as floater logs and location of trees extracted |
Hunting |
Not investigated in this study |
The use of belt transects (three per subwatershed), each measuring 10 × 500 m or 10 × 1 000 m, randomly established perpendicular to the contour and located approximately 500 to 1 000 m apart, was found to provide an effective, quick and easy way to assess and monitor C. zollingeri populations. Belt transects can reliably sample the abundance and distribution of rattan and other lianas that tend to have patchy or clumped populations (Hegarty and Caballe, 1991).
Local rattan collectors were actively involved in the project and helped to identify relevant research questions and appropriate field sampling methods. With three experienced rattan collectors sampling upslope in 10 m segments, an average of 500 m of transects were sampled per day. Workers on the project recorded the identity of all rattan species, the number of canes per plant, cane lengths and evidence of harvesting within 5 m of either side of the transect line. At the same time, data on slope, elevation, light regime, soil, canopy height and dominant tree species were gathered along each transect.
To monitor potential harvesting effects on individual plants, more than 100 mature C. zollingeri plants encountered along the transects were permanently marked (with flagging and metal tags) in 1996, and the number of canes, cane lengths, evidence of cane harvesting and associated environmental conditions were recorded (as noted above). This article summarizes data from marked plants located in primary forests at altitudes below 1 000 m that were in well-drained sites and not affected by tree felling (n = 74). In addition, three permanent 1 × 1 m sample plots were established around each plant to monitor the effects of cane harvesting on understorey vegetation (i.e. extent and persistence of trampling).
A mature Calamus zollingeri plant with numerous vegetative sprouts and canes, marked with flagging and metal tags for monitoring of potential harvesting effects on individual plants
- S.F. SIEBERT

The marked plants and associated plots were resampled annually for four years. Marked plants were monitored for flowering and fruiting phenology and for evidence of their use by birds and mammals on a biweekly basis for two years. Any evidence of damage to forest vegetation along the transects (i.e. branches broken or trees cut to harvest cane) was noted each year and the persistence of damage was monitored for four years (e.g. natural and cut tree falls, trampling while harvesting cane).
Leaf and cane nutrient contents and losses associated with harvesting were assessed in paired leaf and cane samples (Siebert, 2001). Impacts of using logs to transport cane to market were investigated by identifying the species, volume and location of trees harvested to float cane downriver over a two-year period.
Throughout the duration of this study, rattan (including marked plants) was harvested as desired by local collectors. In general, long canes (i.e. greater than 20 m) that could be readily pulled from supporting vegetation were preferred and no canes shorter than 10 m were cut.
During the first sampling in 1996, an average of 149 C. zollingeri plants with a total of 1 431 canes of which 66 were of harvestable length (i.e. greater than 10 m) were recorded per hectare (Table 2). In 2000, the study recorded an average of 143 plants with 1 595 canes and 46 harvestable canes per hectare in the same watershed. Populations of C. zollingeri exhibited extremely patchy distribution; massive plants (i.e. with more than 20 canes) dominated the understorey and canopy along some portions of the transects, while no C. zollingeri were observed in other areas. This variance is reflected in and largely explains the high standard deviations of the mean figures in Table 2.
TABLE 2. Differences in Calamus zollingeri plant and cane populations over four years (based on 100 m2 sample plots along three random transects) | ||||
Component surveyed |
1996 (n = 205) |
2000 (n = 150) | ||
Mean no./ha |
Standard deviation |
Mean no./ha |
Standard deviation | |
Plants |
149 |
±103 |
143 |
±118 |
Canes |
1 431 |
±1 402 |
1 595 |
±1 437 |
Harvestable canes (>10 m) |
66 |
±120 |
46 |
±84 |
C. zollingeri occurred in both high-light (e.g. canopy gaps) and densely shaded environments, but was absent in poorly drained or seasonally flooded sites. No evidence of mortality or dieback to C. zollingeri plants was observed, irrespective of the frequency or number of canes harvested. For example, on marked plants no cane harvesting-induced mortality was recorded even though approximately 33 percent were harvested each year and in 2000 canes were frequently cut on reaching 10 m in length.
TABLE 3.Calamus zollingeri cane production and growth over four years on marked plants (n = 74) | ||
Parameter |
1996 |
2000 |
Mean number of canes/plant |
12.4** |
15.6** |
Mean number of harvestable canes/plant (canes >10 m) |
1.0* |
0.7* |
Mean cane length (m) |
22.4** |
11.4** |
Mean length of harvestable canes (canes >10 m) (m) |
26.0** |
17.3** |
Total length of harvestable cane (all plants) (m) |
1 953 |
880 |
** Significantly different at P = 0.005 based on paired sample t test. | ||
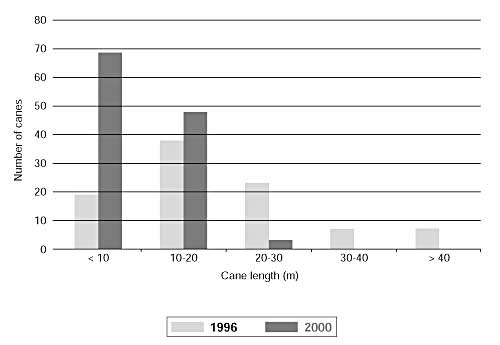
On marked C. zollingeri plants the mean number of sprouts per plant was significantly greater in 2000 than in 1996 (Table 3). However, the mean number of harvestable canes per plant (i.e. canes longer than 10 m) was significantly lower in 2000 than in 1996, as was the mean length of harvestable canes and the total amount (i.e. length) of harvestable cane. In fact, in 2000 the amount of harvestable cane was less than half that recorded on the same plants in 1996. The impact of intensive cane harvesting is readily apparent from a comparison of cane length distribution classes in 1996 and 2000 (see Figure). For example, 37 canes longer than 20 m were recorded on marked C. zollingeri plants in 1996, but only three canes longer than 20 m were found on the same plants four years later.
These data have several important implications for the management of wild rattan. First, repeated cane harvesting appears to stimulate the production of new canes. Indeed, an average of 3.5 new canes per plant and an average of 4.7 m of cane growth were observed on marked plants one year after harvesting. The production of new sprouts or canes and rapid cane growth suggest that large numbers of canes could continue to be available in the future. Second, repeated cane harvesting significantly reduces mean cane length, which in turn reduces returns to labour and requires collectors to travel further into the forest.
In addition, cutting all mature canes may preclude sexual reproduction; this is supported by the absence of flowering and fruiting on marked rattan during two years of biweekly monitoring. While C. zollingeri is clearly capable of vigorous vegetative growth, sexual reproduction is important for maintaining species vigour and diversity over the long term.
Distribution of Calamus zollingeri cane lengths on marked plants in 1996 and 2000
The use of rivers to transport rattan, timber and other forest products is common throughout Asia. Transporting rattan to market in the southern region of Lore Lindu National Park entails dragging rattan to the Lariang river, cutting and bundling the cane, cutting small trees for flotation and then floating the bundles down the river for 2 to 14 days to a roadside access point. Tree cutting has been reported to be a serious threat to biological diversity and forest conservation in the park (BCN, 1996; Schweithelm et al., 1992).
The study found that eight tree species were regularly used to float rattan (Artocarpus teysmannii, Evodia latifolia, Grewia multiflora, Horsfieldia sp., Macaranga hispida, Macaranga triloba, Pterospermum celebicum and Trema orientalis). Not surprisingly, floater trees were lightweight, fast-growing pioneer species. Floater logs averaged 25 cm in diameter and 3 m in length and typically floated a 50 to 60 kg bundle of cane. Canes were cut to 4 m lengths before bundling; the number of canes per bundle varied with cane diameter. The study recorded an average of 135 tonnes of rattan extracted each year from the watershed (from October 1996 to October 1998). Based on a conservative estimate of 50 kg per rattan bundle, a total of 2 350 logs were required to float cane down the river each year from the case study watershed.
In the southern region of Lore Lindu National Park, rattan is transported by lashing to floater logs and floating down the Lariang River
- S.F. SIEBERT

Floater logs were harvested almost exclusively from fallowed shifting cultivation fields and, to a lesser extent, from naturally disturbed riparian floodplains along the Lariang river. Over the four-year study period, no evidence was found of floater log cutting in primary forests in this region of Lore Lindu National Park. According to rattan collectors, floater logs are rarely harvested from primary forests because suitably sized light woods are uncommon there and because primary forests are further from the river than fallowed swiddens. Thus, there is little evidence to support the claim that cutting small, early successional trees to float cane threatens primary forests or biological diversity in Lore Lindu National Park, at least within this case study watershed.
Rattan is floated down the river to a roadside access point, where it is loaded on trucks for delivery to cane processing facilities
- S.F. SIEBERT

As conservation biologists note (e.g. Struhsaker, 1998), it is probably impossible to ascertain reliably all the possible ecological effects associated with NWFP extraction. The monitoring methods employed in this study, while time-consuming and reasonably rigorous, were not comprehensive and certainly did not assess all possible effects associated with wild rattan harvesting. For example, no information was gathered on invertebrate use of C. zollingeri or hunting by rattan collectors. It should also be emphasized that monitoring was carried out for only four years. Repeated harvesting of all mature cane could, over a longer period, adversely affect plant vigour or cane production and growth. Thus, this study does not prove that C. zollingeri cane harvesting is ecologically sustainable or that it is without adverse ecological effects. Nevertheless, the use of randomized and replicated sampling methods over a four-year period is biometrically rigorous (Wong, 2000) and can provide the basis for assessing ecological impacts and initiating adaptive management practices.
Even if harvesting wild rattan appears to be ecologically viable, socio-political and institutional aspects of cane extraction and declining wild rattan supplies may make it necessary to cultivate rattan. A few large-scale efforts (e.g. those of the Sabah Forest Development Authority [SAFODA], Malaysia) have shown that large-diameter, furniture-quality rattans can be grown in large plantations, while the long history of cultivating small and medium-sized rattan (Calamus caesius and Calamus tracycoleous) in swidden fallows in Kalimantan, Indonesia (Weinstock, 1983) (Editor's note: see also article by Belcher in this issue.) suggests that large canes could be grown by small-scale farmers as well.
In assessing opportunities and constraints to rattan harvesting and cultivation, policy-makers must consider the following key questions.
Although Indonesia has attempted to manage its forest resources, albeit with limited success (Barr, 2000; Peluso, 1996; Sunderlin, 1999), the country has made few efforts to regulate and manage rattan cane harvesting. In some areas of Indonesia and elsewhere in Southeast Asia, rattan exploitation exemplifies what can occur under unregulated, open-access resource extraction conditions. Since the 1970s, wild rattan supplies have drastically declined because of logging, forest conversion, overharvesting and forest fires. Even selective timber harvesting is likely to have adverse effects on rattan resources because of incidental mortality during felling and skidding and the widespread collection of rattan and other NWFPs by loggers. Some premier, large-diameter species such as Calamus manan, a solitary rattan that does not reproduce vegetatively, are now nearly extinct in the wild (Dransfield and Manokaran, 1994).
Considered in this light, the loss of rattan resources may simply reflect the political and economic choices of the state and private industrial élite groups to ignore customary resource tenure and forest management practices among local ethnic minorities in the rush to exploit timber or convert forests to agricultural plantations. The "legal" destruction of wild and cultivated rattan through commercial logging and for plantation agriculture is well documented among the Dayak of Kalimantan (Fried, 2000; Belsky, 1992) and has led to the loss of traditional rattan production and management systems that had operated for generations. Such smallholder rattan agroforestry systems appear to have been economically viable, compatible with community economic, social and cultural well-being and capable of producing large quantities of cane reliably and perhaps sustainably (Mayer, 1989; Dransfield, 1988; Godoy and Feaw, 1988; Weinstock, 1983).
Given this history, is managed harvesting of wild rattan possible? And, if so, by whom and under what property rights arrangements? Throughout Southeast Asia there is sufficient evidence to suggest that NWFPs, including rattan, have been successfully managed as a common property resource by traditional forest-dwelling peoples for centuries (Lynch and Talbott, 1995; Peluso and Padoch, 1996). In general, common property resource management may succeed where groups are relatively small and stable; where resource management perspectives and issues of access and control are shared; and where enforcement is simple and inexpensive (Ostrom, 1990). However, historic common property resource management systems have been suppressed or usurped by colonial and post-colonial authorities in Indonesia and other Southeast Asian countries (Peluso, 1996). Consequently, communal management of wild rattan now faces tremendous institutional challenges in many regions.
The potential ecological effects associated with C. zollingeri cane harvesting need to be considered in light of the likely alternative land use practices. It is also important to ask how resident people would secure their livelihoods if rattan harvesting were effectively prohibited. It is likely that the loss of rattan resources would exacerbate pressures to convert forests to agricultural use. In Central Sulawesi, as in much of the tropical world, domestic and international market forces make it lucrative to cultivate export cash crops such as oil-palm, cocoa and coffee (Collier, Mountjoy and Nigh, 1994; Sunderlin, 1999). When weighed against forest conversion to agriculture, the potential ecological effects associated with rattan harvesting appear relatively benign.
A narrow consideration of economic costs and benefits suggests that returns from rattan gathering and cultivation compare poorly with those from perennial cash crops. In addition, coffee and cocoa begin to yield four and three years after planting, respectively, while large-diameter rattan is not likely to produce cane in less than 12 to 15 years. However, it is important to remember that rattan remains a primary or secondary source of cash income for tens of thousands of forest-dwelling people throughout Southeast Asia (DeBeer and McDermott, 1989) and is an important source of emergency income for thousands more (Siebert and Belsky, 1985). Furthermore, rattan cultivation by smallholders, either in swidden fallows or as an intercrop in traditional agroforestry systems, may provide important socio-economic and environmental benefits that are ignored in narrow cost-benefit analyses. Foremost among these benefits are:
Farmers and rattan collectors in the case study region expressed interest in intercropping rattan in rustic coffee and cocoa agroforests. On-farm trials involving C. zollingeri yielded 96 percent survival rates and excellent growth 18 months after transplanting (Siebert, 2000).
Given the relatively low financial returns from wild rattan harvesting and the long period of yield deferral in the cultivation of large-diameter canes, whether by small farmers or on large estates, significant private investment in rattan management or cultivation is unlikely without subsidies (state, international or non-governmental). Taylor and Zabin (2000) argue that support of such forms of community resource management should be viewed not as a subsidy, but rather as a payment for the goods and services provided by intact forests (e.g. carbon sequestration, functional watersheds and biodiversity conservation). Directed external funding of this sort could provide sufficient financial incentive for small farmers and rattan collectors to cultivate and manage rattan.
A Calamus zollingeri seedling transplanted in a rustic coffee farm; rattan intercropped with cash crops may provide important socio-economic and environmental benefits, and on-farm trials have given excellent survival and growth results
- S.F. SIEBERT

Declining supplies and strong market demand suggest that rattan resources, particularly large-diameter canes, will become increasingly scarce. Two general approaches could be pursued to increase rattan supplies: management of wild populations and/or smallholder or estate cultivation. Both strategies entail significant challenges, particularly regarding the unfavourable financial returns (narrowly calculated) of rattan in comparison with cash crop alternatives. The two approaches could also have profoundly different effects on different sectors of society, particularly as concerns smallholder versus estate cultivation.
Efforts to manage wild rattan or to cultivate rattan in small farms or plantations should concentrate on the following areas:
Both managed harvesting of wild rattan and rattan cultivation are likely to require significant long-term financial assistance, as well as technical and marketing support. It is essential that the amount and type of support complement the institutional capabilities of local resource managers (i.e. resident people). Policy-makers should pay particular attention to providing small farmers and rattan collectors with adequate economic incentives, particularly vis-à-vis perennial cash crop alternatives, and to developing secure, stable and enforceable resource management institutions and property rights. Private, state and international support for rattan management and cultivation may be justified as compensation for the public benefits provided by natural forests and diverse agro-ecosystems and for the loss of historic resources by forest dwellers living in and around forests.
Bibliography
Anderson, A., ed. 1990. Alternatives to deforestation. New York, Columbia University Press.
Barr, C. 2000. Will HPH reform lead to sustainable forest management? Questioning the assumptions of the "sustainable logging" paradigm in Indonesia. Bogor, Indonesia, CIFOR.
BCN. 1996. Biodiversity Conservation Network annual report: Asia/Pacific Region. Washington, DC, World Wide Fund for Nature (WWF), Biodiversity Conservation Network (BCN).
Belsky, J. 1992. Balancing forest and marine conservation with local livelihoods in Kalimantan and North Sulawesi. Final report to the Indonesia Natural Resource Management Project. Jakarta, Indonesia, United States Agency for International Development (USAID)/ARD Inc.
CIFOR. 1999. The world heritage convention as a mechanism for conserving tropical forest biodiversity. Bogor, Indonesia, Center for International Forestry Research (CIFOR).
Collier, G., Mountjoy, D. & Nigh, R. 1994. Peasant agriculture and global change. BioScience, 44: 398-407.
DeBeer, J. & McDermott, M. 1989. The economic value of non-timber forest products in South-East Asia. Amsterdam, the Netherlands, Netherlands Committee for the World Conservation Union (IUCN).
Dransfield, J. 1988. Prospects for rattan cultivation. Advances in Economic Botany, 6: 90-200.
Dransfield, J. & Manokaran, N., eds. 1994. Rattans. PROSEA Handbook No. 6. Bogor, Indonesia, Plant Resources of South-East Asia.
Freese, C. 1997. The "use it or lose it" debate. In C. Freese, ed. Harvesting of wild species, p. 1-48. Baltimore, Maryland, USA, John Hopkins University Press.
Fried, S. 2000. Tropical forests forever? A contextual ecology of Bentian rattan agroforestry systems. In C. Zerner, ed. People, plants and justice: the politics of nature conservation, p. 204-233. New York, Columbia University Press.
Godoy, R. & Feaw, T. 1988. Smallholder rattan cultivation in southern Borneo, Indonesia. Cambridge, Massachusetts, USA, Harvard Institute for International Development.
Hegarty, E. & Caballe, G. 1991. Distribution and abundance of vines in forest communities. In F. Putz and H. Mooney, eds. The biology of vines, p. 313-335. Cambridge, UK, Cambridge University Press.
Kramer, R., van Schaik, C. & Johnson, J., eds. 1997. Last stand: protected areas and the defense of tropical biodiversity. Oxford, UK, Oxford University Press.
Lynch, O. & Talbott, K. 1995. Balancing acts: community-based forest management and national law in Asia and the Pacific. Washington, DC, World Resources Institute (WRI).
Mayer, J. 1989. Rattan cultivation, family economy, and land use: a case from Pasir, East Kalimantan. In Forestry and forest products. GFG Report No. 13. Samarinda, Indonesia, German Forestry Group.
Menon, K. 1989. The rattan industry: prospects for development. Jakarta, Indonesia, UN/FAO.
Ostrom, E. 1990. Governing the commons: the evolution of institutions for collective action. Cambridge, UK, Cambridge University Press.
Peluso, N. 1996. Rich forests, poor people: resource control and resistance in Java. Berkeley, California, USA, University of California Press.
Peluso, N. & Padoch, C. 1996. Changing resource rights in managed forests of West Kalimantan. In C. Padoch and N. Padoch, eds. Borneo in transition: people, forests, conservation and development. Kuala Lumpur, Malaysia, Oxford University Press.
Rice, R., Gullison, R. & Reid, J. 1997. Can sustainable management save tropical forests? Scientific American, 276(4): 44-49.
Schweithelm, J., Wirawan, N., Elliott, J. & Khan, J. 1992. Sulawesi parks program land use and socio-economic survey: Lore Lindu National Park and Morowali Nature Reserve. Jakarta, Indonesia, Nature Conservancy.
Siebert, S. 2000. Survival and growth of rattan intercropped with coffee and cacao in the agroforests of Indonesia. Agroforestry Systems, 50: 5-102.
Siebert, S. 2001. Nutrient levels in rattan (Calamus zollingeri) foliage and cane and implications for harvesting. Biotropica. (in press)
Siebert, S. & Belsky, J. 1985. Forest-product trade in a lowland Filipino village. Economic Botany, 39: 522-533.
Struhsaker, T. 1998. A biologist's perspective on the role of sustainable harvest in conservation. Conservation Biology, 12: 930-932.
Sunderlin, W. 1999. Between danger and opportunity: Indonesia and forests in an era of economic crisis and political change. Society and Natural Resources, 12: 559-570.
Taylor, P. & Zabin, C. 2000. Neoliberal reform and sustainable forest management in Quintana Roo, Mexico: rethinking the institutional framework of the Forestry Pilot Plan. Agriculture and Human Values, 17: 141-156.
Weinstock, J. 1983. Rattan: ecological balance in a Borneo rainforest swidden. Economic Botany, 37: 58-68.
Wong, J. 2000. The biometrics of non-timber forest product resource assessment: a review of current methodology. London, Department for International Development (DFID).