Tek Bahadur Gurung, Ash Kumar Rai1, Purusottam
Lal Joshi2, Agni Nepal3
Arun Baidya, Jaidev Bista and Sadhu Ram Basnet
Fisheries Research Centre, Pokhara, PO Box 274, Pokhara
1Fisheries Research Division, Godawary, Lalitpur, 2Fisheries
Research Centre, Tarhara
3Fisheries Research Center, Trishuli
ABSTRACT
Female golden mahseer (T. putitora) of 3-5 years old spawned without hormonal use when reared in ponds at the rate of 1 000 kg/ha with 30-40 percent crude protein supplementary feed. Males of more than 2+ years were maintained with similar feed. Ova from mature females were obtained by simple hand-stripping method. Out of 50 females only 4-6 percent released viable ova in April, August, September, October and November 2 000, while 12-16 percent of females responded in March and April 2001. Data on breeding of naturally mature brood collected from inlet streams of lakes revealed that golden mahseer could spawn in July, August, September and October. Breeding response studies of pond reared broodstock and data from the field (inlet streams of lakes) both showed that golden mahseer could breed most months of a year when water temperature ranged between 18.5 and 33°C. The present study indicates intermittent breeding characteristics of pond-reared golden mahseer. Females released 550 to 19 795 ova at single response. Most ova were successfully fertilized and hatched out. During bi-weekly breeder maturity examination in August-November 2000 and March-April 2001 much brood were found over-matured implying that breeders should be examined more frequently. We compared size of ova, larvae and fecundity (released ova/body weight of female) of golden mahseer with some cultivable carps. It seems that from the egg size and larval size point of view (energy investment in reproduction by the fish) fecundity is reasonable when compared to other species. This suggests that mass scale breeding of golden mahseer is possible by maintaining a reasonable number of broodstock for aquaculture development and restocking purposes.
Golden mahseer (Tor putitora), one of the well-known large freshwater game fish of mountain rivers and lakes of most Trans-Himalayan countries, is reported to be declining in their natural habitats due to overfishing, and chemical and physical alterations of their habitats. Attempts to culture and conserve Tor spp have been initiated in most Trans-Himalayan countries (Joshi, 1994; Shrestha, 1997).
In general, knowledge and methodological development of breeding techniques are an essential part of commercial fish production in a closed system, however, milk fish (Chanos chanos) and mullet (Mugil cephalus) industries were solely dependent on natural spawning for maintaining their stocks (Bardach et al., 1972). Most fish in nature are specific to certain feeding and breeding grounds, which restricts their distribution to certain agro-ecological zones (Maitland and Morgan, 1997). Generally, fish breeding in hatcheries is done for commercial production, however, it seems that loss of particular fish habitat, threat to species due to multiple factors, and limited ability of fish to acclimatize in a specific environment or habitat could also offer impetus for scientific intervention to breed specific fish to maintain diversity. Golden mahseer (T. putitora) at present is one of such fish in most Trans-Himalayan waters due to their habitat loss and over fishing (Shrestha, 1997; Nautiyal, 1994).
Aquaculture production mostly depends on mass seed production, however, it seems that advantages of hatchery breeding could also be used to conserve fish in nature. Fish conservation is improvement and strengthening of population by safeguarding and restoring endangered species and habitat, while fish farming is a production process using established technical methodologies from breeding to rearing which may be specific for a particular species. If the above categorization is true, species that multiply under human control may not need much effort to conserve, as they can be transferred easily into suitable habitats. But developing a farming methodology for some desired species is not simple. If it were then conservation and commercial production of desirable species would be an easy task.
T. putitora is reported from most Trans-Himalayan countries ranging from Afghanistan to Myanmar (Skene-Dhu, 1923; MacDonald, 1948; Day, 1958; Desai, 1994; Khan et al., 1994). Two species of mahseer, T. putitora and T. tor inhabit Nepalese torrential waters and lakes of mid hills (Shrestha, 1981; Shrestha, 1997). These can be differentiated by the longer head length in T. putitora than body depth (Shrestha, 1981). T. putitora is relatively abundant in lakes of Pokhara Valley. The body length of this species may reach more than 1.0 m and the weight about 45 kg (Shrestha, 1997), suggesting a high growth rate. Over the last two decades most Trans-Himalayan countries have reported declining populations of mahseer (Tor spp) due to overfishing, damming and degradation of the environment (Desai, 1994). This has led to efforts to conserve, manage and propagate the species (Pathani, 1981; Shrestha et al., 1990; Azadi et al., 1991; Shrestha, 1997), especially after the 1970s.
In the early 1960s Tor spp represented one of the major species in the commercial fishery in lakes of Pokhara Valley (Shrestha and Gurung, 1990). But at present they contribute negligibly to the total catch (Swar and Gurung, 1988; Wagle and Bista, 1999). It is possible that effects of overfishing, environmental degradation, sedimentation, destruction of spawning grounds, non-conventional fishing methods, or all of these factors have caused the low yields (Shrestha, 1997). In Nepal, since mahseer is present in cool waters of mid hills and the inner Terai region of the country (Gurung and Wagle, 2000), conservation, management, and propagation were carried in water bodies close to two research centers: Pokhara and Trishuli, located in mid-hills. Shrestha (1994, 1997) contributed substantially to the knowledge on T. putitora in Nepal.
To enhance fishery and aquaculture, over a number of years investigations for developing a propagation method for T. putitora have been carried out in most of the Trans-Himalayan countries. This has led to a better knowledge of spawning biology, ecological aspects and behavior of T. putitora in its natural habitats (Tripathi, 1978; Masuda and Bastola, 1987; Shrestha, 1994; Kulkarni, 1980; Joshi, 1994; Nautiyal, 1994). While the natural, semi-natural, and stimulatory spawning techniques of T. putitora are known, there is no standardised breeding method for mass seed production of the species in captivity. MacDonald (1948) was the first to mention that ".... Mahseer is said to spawn three times in a season". Similarly, Desai (1994) noted breeding of mahseer from July to March in the Narmada River, Madhya Pradesh, India. Pathani (1983) suggested at least four spawning events on the basis of four types of egg diameter and stages of development in mature ovary of mahseer. Most authors agreed that mahseer is a partial spawner due to the low number of released eggs during a single spawning event (Joshi, 1994; Shrestha, 1997; Shrestha et al., 1990). The partial or intermittent spawning could be used to conserve and develop fishery of the species by restoring its natural habitat as fish sanctuaries, while successes in captive breeding could be useful for aquaculture production of the species.
This paper discusses the breeding of pond reared mahseer and its intermittent spawning, summarizing the results of many years experience with breeding of this species in Nepal.
Preliminary work on breeding of the species has been carried out in Pokhara and elsewhere in Nepal since the early 1960s. Spawning of golden mahseer (T. putitora) collected as mature broodfish from lakes started around 1982 in Lake Phewa with the objective of maintaining pure strains. Later this work was followed by Nakamura (1987), Shrestha et al. (1990), Morimoto et al. (1995) and Baidya et al. (2000) using different methods of mahseer breeding.
At the beginning mature males (0.3-3.4 kg body weight) and females (3-6.1 kg body weight) were collected at night at inlet streams using gill nets (50-125mm mesh, knot-less monofilament and trammel nets) in Lake Phewa and Begnas during mahseer upward migration in the pre- to post-monsoon season (June-October). Mature females were stripped and fertilized with milt obtained from mature males. Fertilized eggs were transported to hatcheries for incubation.
2.1 Study area and habitats
Study area is the lakes of Pokhara Valley in Kaski, Nepal (Fig. 1). Most field experiments were conducted near inlet streams of Lake Phewa and Lake Begnas. Indoor experiments were conducted in the Fisheries Research Centre, Pokhara.
Fig. 1 - Map showing lakes in Pokhara Valley: natural spawning sites of mahseer (Tor spp)
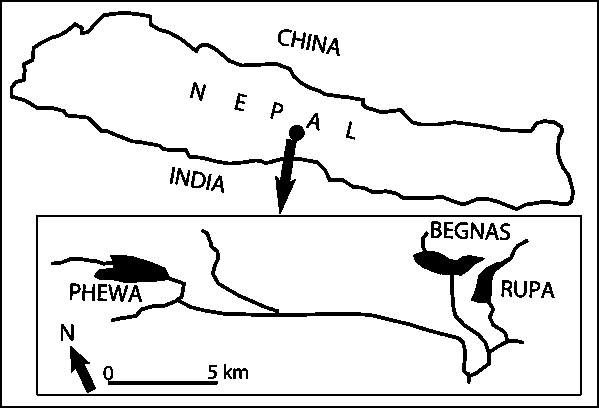
Lake Phewa: Lake Phewa (742 m a.s.l.) is the largest (523 ha) and deepest (max. depth 24m) among all lakes in Pokhara Valley. Surface area, water volume, maximum and average depths of the lake are 5.23 km2, 39.32 x 106 tons, 24 m and 7.5 m, respectively. However, the water surface area of the lake has shrunken and an island near the inlet stream has appeared due to silt deposition. Harpan Khola is the main perennial inlet stream feeding the lake. Harpan Khola is one of the best-known spawning spots of mahseer (Tor spp). Annual water temperature in this lake fluctuates between 14-29°C.
Lake Begnas: This is a medium-sized lake of approximately 323 ha situated at 700 m a.s.l. The main breeding ground of mahseer (Tor spp) is known to be in Syangkhudi Khola, which is perennial inlet stream of the lake. Water temperature in this lake ranges from 14.5 to 31.0°C.
2.2 Breeding of pond-reared broodstock
To examine breeding responses of golden mahseer in captivity we carried out experiments with artificial diet (Baidya et al., 2000). For response studies T. putitora of F2 generation (F1 is considered those mature fish collected from Begnas Lake) were grown in controlled condition. For this, females of approximately 700-2 500g-body weight and 3-5 years age were stocked at the rate of 1 000 kg ha-1 in 2 ponds of 0.05 ha water surface area each. Broodstock fish were fed with 30-40% protein diets. The feeding rate was 4% of total body weight (Table 1). In broodstock ponds temperature and dissolved oxygen were measured every morning by YSI oxygen meter.
Table 1
Stocking density and feeding rate for broodstock of T. putitora in ponds
|
Description |
Pond 1 |
Pond 2 |
|
Area (m3) |
500 |
500 |
|
Stocking density |
1 000 kg/ha |
1 000 kg/ha |
|
Stocking No & BW |
25, |
25, |
|
Feeding rate (of BW) |
4% |
4% |
|
PH |
7-9 |
7-9 |
|
DO (mg/L) |
3-9 |
3-8.4 |
|
Depth (cm) |
70-90 |
70-90 |
|
Transparency (cm) |
30-50 |
30-50 |
From March to November 2000, female breeders were checked at biweekly intervals for maturity by applying gentle pressure by hand near the genital opening after partial anesthetization in 50 ppm Benzocain solution. Females releasing ova on slightest pressure were transported inside hatcheries, then anaesthetized and stripped gently to receive ova in a clean and dry bowl.
Milt from healthy males was gently mixed by feather with ova for dry fertilization. Eggs were weighed and counted, then washed with clean water as is commonly performed with other carps. This process of washing was repeated several times. Eggs were incubated for hatching in mesh trays in running water. The fertilized eggs in incubation trays were covered with black plastic screen. The rate of water flow in incubation trays was maintained at 4-5 L/minute. Dead eggs were counted and removed every day with a wooden fork to protect the eggs from fungal infection. After 3-5 days, hatching started and was completed within 48-72 hours. Early hatched larvae possessed large yolk sacs and settled down in corners of incubation trays. After attaining free-swimming stage the larvae were transferred into a tank of 2.5m x 0.40m x 0.30m dimension. Supplementary feed (microbinded feed) was fed to hatchlings after yolk sac absorption. Advanced fry were fed with zooplankton screened through a plankton net.
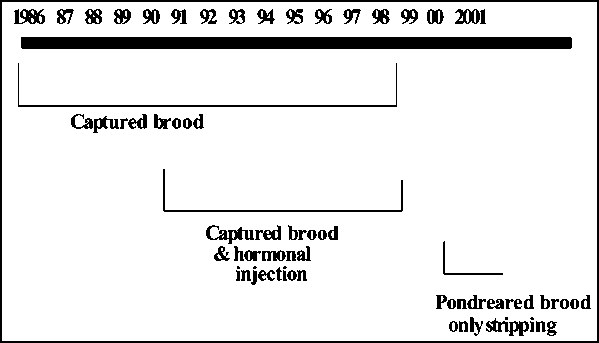
The techniques of T. putitora breeding gradually developed from stripping naturally mature brood collected in lakes to rearing of broods in pond conditions (Fig. 2). The use of sex hormone to induce spawning was also tested (Tripathi, 1978). The compilation of spawning data showed that golden mahseer could breed in most months of the year (Fig. 2). Since pond reared females were not examined in December and January, it is not yet clear whether they could breed in these months or not. Results showed that T. putitora could spawn from March to November at a water temperature ranging from 19.5 to 35°C (Fig. 3 and 4).
Fig. 2 - Methodological progression of obtaining mahseer broodstock from lakes and rivers and breeding it in ponds
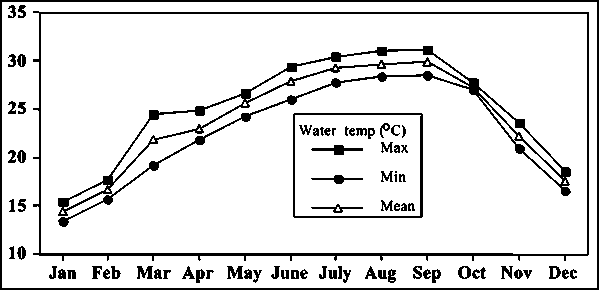
Water temperature in ponds, where golden mahseer were reared, varied from 14.5 to 33°C at 6-7 am (Fig. 4). However, water temperature in summer reached up to 37°C, and pH values ranged 6.6 to 8.8 and dissolved oxygen from 4 to 8 mg/L (Table 1).
The spawning size of female mahseer ranged from 780 to 1985 g in body weight. These released 535-19 795 yellowish brown ova depending on size and maturity stages when hand-stripping method was used (Fig. 5). During maturity examination 90-95% population was seined from experimental ponds. Usually, most females were found over-matured and only a few were at the right stage for releasing viable ova. Over-mature females were identified as those releasing degenerated ova, which turned opaque soon after fertilization, or releasing orange fluid at slight pressure on abdomen. Orange fluid turned white when it came in contact with water.
Fig. 3 - Response of mahseer (Tor putitora) in different months showing intermittent breeding
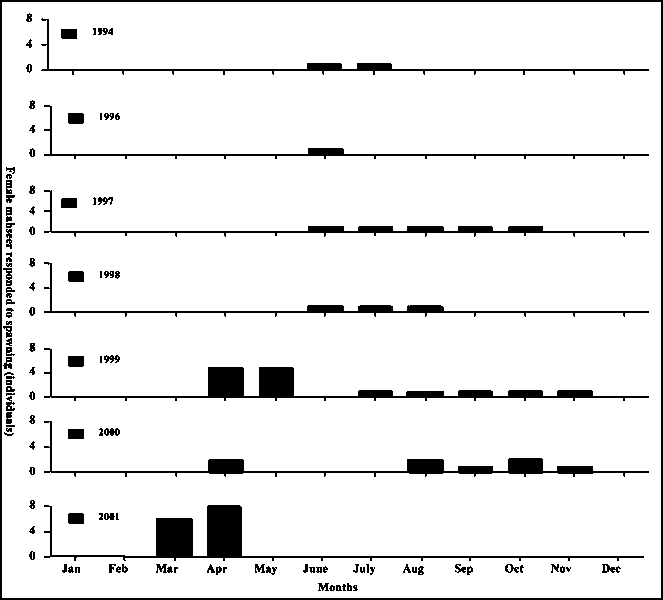
Fig. 4 - Seasonal changes in water temperature in golden mahseer rearing pond
Eggs were incubated in Atkin's incubators by allowing one layer of eggs on single mesh screen in flow through system. Water flow was maintained at a discharge rate of 6-8 L/min. Dead eggs were removed using forceps without touching other eggs. The dead eggs if not removed became infected with fungus (Saprolegnia) which could spread rapidly to the adjacent healthy eggs.
Hatchability of eggs ranged from 0 to 98% (Fig. 5). Fertilized eggs of mahseer also swelled slightly. The diameter of fertilized eggs ranged from 2.8 to 3.02 mm. Incubation period was 45-125 hours at water temperature between 19 and 28°C showing that hatching time is dependent on water temperature.
Fig. 5 - Number of ova, hatchlings produced and percentage hatchability in pond-reared golden mahseer (T. putitora) in different breeding events
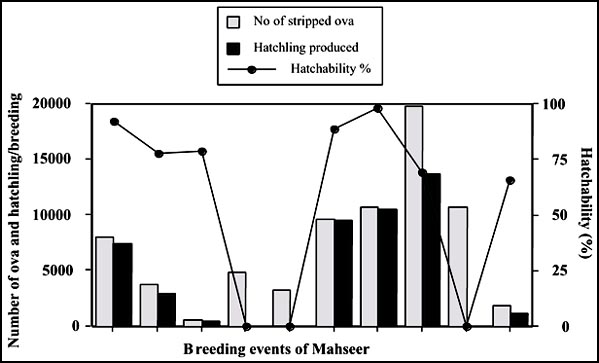
Fig. 6 - Relationship between hatching period and incubation water temperature
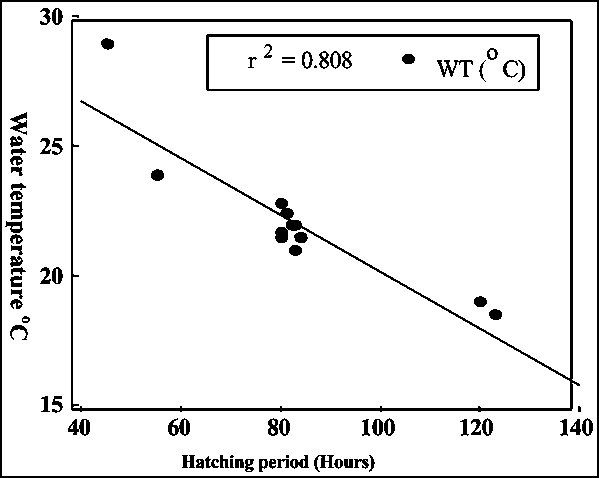
Even with the small set of the present data set the relationship between water temperature and hatching time (Fig. 6) showed substantial correlation (r2=0.808).
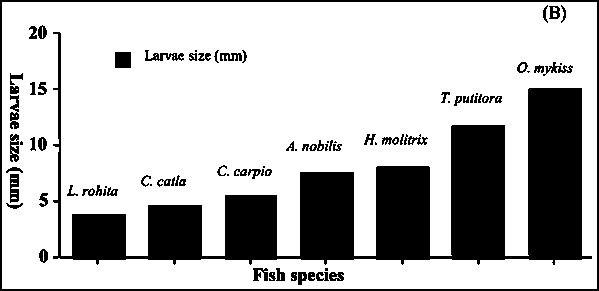
The hatched larvae hatched on mesh net had a large yolk sac. Usually absorption of the yolk sac took 3-4 days depending on water temperature. Hatched larvae remain on the same mesh net until complete absorption of the yolk sac and attaining free swimming stage. At this stage mean larval length was 12.5 mm (Fig. 7).
The present study demonstrates that golden mahseer (T. putitora) do not breed only in pre- to post- monsoon period, but also in March and November when water temperature reaches 19°C. This implies that mahseer could breed in most months of the year. The present study suggests the possibility of mass seed production of T. putitora, though they might breed intermittently. The breeding responses between March-November imply that at least single brood might respond twice in a year. Giving support to this is the multiple spawning of the common carp (Cyprinus carpio) 3-5 times a year (Horvath 1978; Gurung et al., 1993). Recent studies have also shown that grass carp (Ctenopharyngodon idella) can breed twice a year, and Catla catla four times a year (Rath et al., 1999).
This study showed that pond reared golden mahseer could breed at 19.5-33°C from March to November by simple hand stripping without any hormone use and brood loss. This also demonstrated that golden mahseer brood could be reared like other carps such as C. carpio, Chinese and Indian major carps (Table 1). Interestingly, surface water temperature in experimental ponds exceeds 37°C in summer. Successful breeding in such waters without any apparent negative effects suggests their high acclimatization capabilities.
Spawning of mahseer mostly was dependent on collection of naturally mature broodstock from lakes (Morimoto et al., 1995). However, attempts were also made to study the possibilities of reproduction in captivity by using sex hormones (Shrestha et al., 1990; Baidya et al., 2000) as it is practiced with the Chinese and Indian major carps (Chondar, 1994). Several studies reported successful breeding of mahaseer (Tor spp) by stimulating breeders by use of sex hormones (Tripathi, 1978; Shrestha, 1990; Joshi, 1994) showing possibilities of maturation of gonads by using sex hormones. However, the present study revealed that to breed mahseer the use of hormone is unnecessary, if breeders are managed using present methods.
At the beginning of our investigations mahseer broodstock collected from lakes (F1 generation) were wild, shy and hesitant to take supplementary feed. However, these problems were overcome by mahseer of F2 generation onwards. At present golden mahseer consume supplied feed as voraciously as C. carpio in captivity, suggesting that wild stock have been domesticated in captive conditions.
Most breeders showed over-mature ova during routine checks, which implied that broodstock should be examined more frequently. Over mature females were recognized as those releasing ova with 100% mortality soon after fertilization; or degenerating ova, which become thick orange watery fluid oozing through the vent when slightly pressed. Such over-matured females were obtained even when fish were examined biweekly during the peak season in March-April. Usually such over matured breeders releases more ova. Breeding of golden mahseer was examined in December and January when water temperature in ponds ranged from 16 to 18°C.
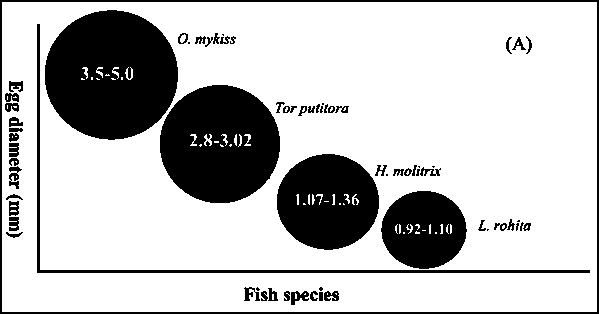
Fig. 7 - Egg diameter (A) and larval size (B) of different fish
(A)
(B)
(Source: Bardach et al. (1972); Jhingran and Pullin (1985); Huet (1972); Jhingran (1991)
Male mahseer larger than 100g could release milt throughout the year, indicating that males could be used for breeding more frequently, if mature female could be found. Such trends in other exotic carps such as bighead carp and silver carp are not recorded. They release milt only for a short time and soon loose their secondary sexual characters such as roughness of their pectoral fins and body after the breeding period is over.
It is yet to be demonstrated how many times a single female mahseer could attain sexual maturity in one year. The present study has reconfirmed that golden mahseer is a partial breeder as pointed out by Skene-Dhu (1923) seven decades ago. When we considered intermittent characteristics of golden mahseer, and compared the number, size, incubation period of eggs and larval size in relation to other species (Fig. 7A, B), the general trend suggests that fish possessing fewer eggs release relatively large ova; for example rainbow trout (Oncorhynchus mykiss) possess largest ova and lowest fecundity among all compared presently. In contrast, smaller ova bearers produce numerous ova, such as the Indian major carps Labeo rohita and Cirrhinus mrigala. Generally, incubation time for hatching and temperature were also related to egg size. Smaller eggs require relatively shorter incubation period but higher water temperature, while larger eggs require long incubation time but low water temperature. When golden mahseer is evaluated, the size of eggs, larvae, time for incubation and water temperature fall into intermediate category: fish spawning only once in a year release few ova if large, and numerous if they are small. Ova of mahseer are of moderate size therefore it seems that fecundity of golden mahseer is justified from energy investment in ova production.
In the present study stocking density of golden mahseer in ponds was kept lower than usual. For example carp broods are usually kept at a density of 2 000 kg/ha (Jhingran and Pullin, 1985). Mahseer broods were well fed with 30-40% protein-containing feed, thus it is possible that they had opportunity to consume natural food in ponds due to their low density. Thus abundant nutritious diet might have caused repeated sexual maturity in mahseer and intermittent breeding in pond condition. It is usually recommended that for successive development of reproductive cells it is essential to provide nutritious feed to brood fish (Horvath, 1972; Gurung et al., 1993). In the present response study male and female mahseer were reared separately. It could be that separation of sexes could lead to an increased potency in mahseer for breeding. Such trend is known for Cyprinus carpio where with onset of summer male and female common carp are separated, which facilitates deposition and development of ova in female breeders (Gurung et al., 1993). If male and female common carp are reared together usually such broods lack zest for breeding. It could be that naturally collected golden mahseer breeders responded to breeding only once in the monsoon season.
Mahseer have been well studied for their sport fishery values (Skene-Dhu, 1923; McDonald, 1942). Naturally these fish prefer fast flowing rivers and mountain lakes with clean water, high transparency and high dissolved oxygen content. In Nepalese rivers mahseer inhabit mid hills and inner Terai (Shrestha, 1997). Shrestha (1997) was breeding golden mahseer using mature male and female collected from the Tadi River at Trishuli.
The collection of naturally gravid broodstock and their spawning success showed that golden mahseer in Central Nepal breed during monsoon in inlet streams of lakes, when water temperature ranges from 26-30°C. Their collection is rigorous and labour intensive. The declining population of mahseer in natural habitats is a concern in most of the Trans-Himalayan countries. While it is important to have a good breeding technology, in-situ conservation and restoration of natural populations is also important. In rivers and lakes wherever mahseer spawn, their stocks could be enhanced by strict regulation of fishing and by restocking through community participation. In those countries where breeding technology has not yet been fully developed, habitat conservation of mahseer should be a priority.
Acknowledgements
This paper is dedicated to planners, scientists, technicians and administrators who have helped to promote mahseer in the past several years, and directly or indirectly assisted with the present study. We are thankful to all the staff at the Pokhara Fisheries Research Centre and the Trishuli Fisheries Research Centre for their kind cooperation for providing up-to-date information on mahseer. We are indebted to JICA for providing facilities and support to studies on mahseer.
References
Azadi, M.A., M. Shafi and M.A. Islam, 1991. Studies on the age and growth of Mahseer Tor tor (Ham.) from the Kaptai Lake, Bangladesh. Bangladesh J. Zool. 19: 47-54.
Baidya, A.K., T.B. Gurung and B. Shrestha, 2000. Study on the propagation of sahar (Tor putitora) in relation to hormones and nutritional management. In: Annual Technical Report ARS (Fishery), Pokhara 1999-2000 (T.B. Gurung, ed.): 44-48.
Bardach, J.E., J.H. Ryther and W.O. McLarney, 1972. Aquaculture: The farming and husbandry of freshwater and marine organisms. John Wiley and Sons. 867p.
Chaturvedi, S.K., 1974. Spawning biology of tor mahseer, Tor tor (Ham.). J. Bom. Nat. Hist. Soc. 12: 63-73.
Chondar, S.L., 1994. Induced carp breeding. CBS Publishers and Distributors, New Delhi, India.133p.
Day, F., 1958. The fishes of India; being a Natural History of the Fishes known to inhabit the Seas and Fresh Waters of India, Burma, and Ceylon, Volume 1. London. William Dawson and Sons Ltd, London. 564p.
Desai, V.R., 1994. Ecostatus of Mahseer in River Narmada (Madhya Pradesh). In: Mahseer: The Game Fish (Natural History, Status and Conservation Practices in India and Nepal). (Compiled and edited by P. Nautiyal). Rachna, Garhwal UP, India.
Gurung, T.B., U. Silwal, L.S. Lama and K.B. Khatry, 1993. A study on multiple use of common carp (Cyprinus carpio, Lin.) in a year cycle. Annual Report. Fisheries Research Centre, Godawary. Pp 5-16.
Gurung, T.B. and S.K. Wagle, 2000. Importance of water quality for optimizing fish yield in Nepal: a review. Paper presented at the Technical Seminar on Information Sharing in Aquaculture (18-19 October, 2000). Fisheries Development Directorate, Kathmandu, Nepal. 27p.
Horvath, L., 1978. Experiences in propagation of the common carp out od spawning season. Aquacultura Hungarica (Szarvas) 1: 66-72.
Huet, M., 1972. Breeding and cultivation of salmonids. In: Textbook of Fish Culture Breeding and Cultivation of Fish: 59-110. Fishing News Books, Surrey, England.
Jhingran, V.G. and R.S.V. Pullin, 1985. A hatchery manual for the Common, Chinese and Indian major carp. Asian Development Bank. International Center for Living Aquatic Resources Management, Manila, Philippines. 177p.
Jhingran, V.G., 1991. Fish and Fisheries of India, revised and enlarged third edition. Hindustan Publishing Corporation (Delhi), India.
Joshi, C.B., 1994. Conservation of Tor putitora: hatchery practices. In: Mahseer The Game Fish (Natural History, Status and Conservation Practices in India and Nepal). (Complied and edited by P. Nautiyal): D15-D25. Published by Rachna, Garhwal UP, India.
Kaushal, D.K., M.D. Pisolker and Y.R. Rao, Observation on the food habits of Tor putitora (Hamilton) from Gobindsagar Reservoir, Himachal Pradesh. J. Inland Fish. Soc. India 12: 138-139.
Khan, H.A., M.S. Joshal, K.K. Tandon, G.S. Sandhu, S.S. Pathani, C.V. Kulkarni, S.N. Ogale, P. Nautiyal, D. Gupta, Rachna Preeti, D.R. Khanna, S.P. Badola, M.P. Gusain and O.P. Gusain, 1994. The Himalayan or Putitor Mahseer Tor putitora (Hamilton). In: Mahseer: The Game Fish (Natural History, Status and Conservation Practices in India and Nepal). (Compiled and edited by P. Nautiyal). Published by Rachna, Garhwal UP, India.
Kulkarni, C.V., 1980. Eggs and early development of Tor tor mahseer. J. Bom. Nat. Hist. Soc. 77: 70-75.
Lewis, R., 1988. Fish: new focus for biotechnology, genetically engineered fish are expected to be used first in aquaculture. BioScience 4(38): 225-227.
MacDonald, A. St. J., 1948. Simple Natural History of the Mahaseer. In: Circumventing the mahaseer and other sporting fish in India and Burma. Natraj Publishers, Dehradun, India. 16p.
Masuda, K. and K.R. Bastola, 1987. Breeding of Sahar (Tor putitora, Hamilton) using naturally matured broods in Tadi River of Central Nepal. A Report submitted to the Fisheries Development Division, HMG/Nepal.
Maitland and N.C. Morgan, 1997. Conservation and Management of Freshwater Habitats: Lakes, Rivers and Wetlands. Chapman and Hall. 233p.
Morimoto, N., K. Sakai and S.R. Basnet, 1995. Basic research study of mahaseer (Tor putitora) in Pokhara Fisheries Research Center, Nepal, Natural Water Fisheries Development project, FRC, Pokhara, ARCC, Pokhara, Nepal, 30p.
Nakamura, M. 1987. A study on age and growth of sahar (Tor putitora, Hamilton) in Phewa Lake, western Nepal. 7p.
Nautiyal, P., 1994. Mahseer: The Game Fish (Natural history, Status and Conservation Practices in India and Nepal). (Compiled and edited by P. Nautiyal). Rachna, Gaarhwal U.P., India.
Pathani, S.S., 1981. Fecundity of mahseer Tor putitora. Proceeding of the Indian Academy of Sciences 90:253-260.
Pathani, S.S., 1983. Studies on the spawning ecology of Kumaon Mahseer Tor tor and Tor putitora (Ham.). J. Bom. Nat. Hist. Soc. 79(3): 525-530.
Rath, S.C., S.D. Gupta and S. Dasgupta, 1999. Non-stripping induced spawning and double spawning of grass carp in a hatchery system with foliage-free brood diet. Veterinarski Archiv 69:7-15.
Shrestha, B.C., A.K. Rai, T.B. Gurung and K. Mori, 1990. Successful artificial induced spawning of Himalayan Mahaseer (Tor putitora) in Pokhara Valley, Nepal. In: The Second Asian Fisheries Forum (R. Hirano and I. Hanyu, eds). Asian Fisheries Society, Manila, Philipines.
Shrestha, B.C. and T.B. Gurung, 1988. Declining fishing of sahar (Tor sp.) from Lake Begnas, Pokhara, Nepal. Paper presented at the National Conference on Science and Technology 25-29 April 1988, Kathmandu, Nepal. 8p.
Shrestha, J., 1981. Fishes of Nepal. Curriculum Development Centre, Tribhuvan University, Kathmandu, Nepal.
Shrestha, T.K., 1994. Development of Mahseer culture towards ranching. In: Mahseer: The Game Fish (Natural History, Status and Conservation Practices in India and Nepal).(Compiled and edited by P. Nautiyal): D26-D41. Rachna, Garhwal UP, India.
Shrestha, T.K., 1997. The Mahseer in the rivers of Nepal disrupted by dams and ranching strategies. R.K. Printers, Teku, Kathmandu, Nepal. 259p.
Skene-Dhu, 1923. The Angler in India or The Mighty Mahseer. Natraja Publication, India. 786p.
Swar, D.B. and T.B. Gurung, 1988. Introduction and cage culture of exotic carps and their impact on fish harvested in Lake Begnas, Nepal. Hydrobiologia 166: 277-283.
Tripathi, Y.R., 1978. Artificial breeding of Tor putitora (Ham). J. Inland Fish. Soc. India 9:161.
Wagle, S.K. and J.D. Bista, 1999. Catch efforts and capture fishery in lakes of Pokhara Valley: status and management perspective. Paper presented at the 3rd National Workshop on Livestock and Fisheries held at Lumle on 21-23 June 1999.