The transport and processing module describes the processing of broiler chickens, from the point of leaving the farm to the time the finished product leaves the slaughterhouse. The outputs of this step should be an estimate of (i) the prevalence of Salmonella-contaminated product, and (ii) the numbers of organisms per contaminated product unit.
Transport and processing steps
Overview
There are many different sub-modules within this stage, some of which increase or decrease the level of Salmonella contamination. Figure 6.3, from Eley (1996), summarizes the main steps of the process. This discussion focuses on transport, stun-and-kill, scalding, de-feathering (plucking), evisceration and chilling, although the other operations are also briefly mentioned.
Figure 6.3. A flow chart describing transport and processing of raw poultry meat (from Eley,1996)
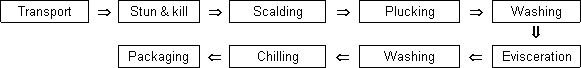
Many sources give detailed descriptions of the processing of poultry (e.g. Geornaras and von Holy, 1994; ACMSF, 1996). Each stage can potentially increase or decrease the prevalence of Salmonella in broilers, or increase or decrease the numbers of organisms on the exterior of the broiler chicken carcass, or a combination. Overall, it is probable that the stages will be similar in all regions of the world, although the changes in microbial load occurring at each step can differ, depending on the facilities, technologies and hygienic practices employed.
Transport
During transportation, birds are often stored in open crates that are placed on top of each other; thus, faeces can drop from an upper crate to a lower crate and cause cross-contamination. The stress of transport associated with factors such as vehicle conditions, length of journey, temperature and road conditions, will increase faecal excretion (and hence Salmonella excretion in Salmonella-positive birds) and therefore the possibility of cross-contamination is increased (ACMSF, 1996). There is an additional problem if the crates used are not thoroughly cleaned and disinfected between each collection of birds.
Stun and kill
Birds are stunned when their heads are submersed into water within which there is an electrical current. They are then killed by exsanguination. These procedures have not been identified as major cross-contamination steps. A second, more modern technique is using a mixture of gas, which is also unlikely to be a significant cross-contamination step.
Scalding
Scalding facilitates the removal of feathers. Birds are immersed in water, the temperature of which can depend on whether the bird is to be sold fresh or frozen. A scald tank with water that is too hot can cause discoloration of the skin, so broilers to be sold fresh are scalded at a lower temperature of 50-52°C (soft-scald), whereas birds to be sold frozen are scalded at higher temperatures, 56-58 C (hard-scald) (ACMSF, 1996). The temperatures have important implications of Salmonella. In particular, some Salmonella species may remain viable in the scald tanks for long periods (ICMSF, 1996). As a result, there is potential for cross-contamination.
The addition of chemicals to the scald tank water may reduce the potential for pathogen survival and hence cross-contamination. However, in certain areas of the world (e.g. Europe) regulations may not permit such practices due to the requirements to use only potable water and to demonstrate that no residues remain on the carcass.
There are a number of options for the mechanical system used for scalding, including spray systems, counter-current scald tanks and multi-stage scalding. More information from different areas of the world is required to assess the different systems used.
Plucking or de-feathering
During de-feathering, machinery mechanically removes the feathers from the birds using counter-rotating domes or discs that have rubber fingers mounted on them. De-feathering is regarded as a major site for contamination. In particular aerosol spread of microoganisms may occur as the feathers are removed (ACMSF, 1996). In addition, organisms can sometimes persist in machines due to inadequate cleaning.
Evisceration
Evisceration involves the removal of internal organs. Initially, the intestines remain attached so that they can be inspected. Due to this, the exterior of the bird may be contaminated if the intestines are damaged. Such damage can occur frequently since the machinery used for evisceration is not flexible with respect to the size of the bird. However, newer evisceration machines, which separate the carcass from the offal at the point where the offal becomes exposed, may overcome this problem.
Washing
Washing a carcass (in any form) should decrease the numbers of Salmonella residing on the exterior, although many studies have highlighted the attachment of Salmonella to the skin of broiler chickens during processing (e.g. Notermans and Kampelmacher, 1974, 1975). Depending on the method of washing, the prevalence of Salmonella may increase or decrease. For example, if washing takes place in an immersion tank, although Salmonella will be washed off those carcasses contaminated on their exterior, these organisms may then cross-contaminate an initially Salmonella-free carcass.
Chilling
The two most common methods of chilling are the immersion chiller and the air chiller. Different countries may use different chilling methods. For example, in the United States of America, immersion chilling is generally used, while in Europe immersion chilling can only be used for frozen poultry products. With immersion chilling, a counterflow current can be used such that a carcass is always moving towards cleaner water. Note that counterflow immersion chilling is a requirement of the EU, but it is not necessarily used in other parts of the world. Chlorine in the form of hypochlorite or chloride dioxide has been shown to reduce levels of cross-contamination within immersion chillers. Addition of chemicals to the chill tank is country dependent and, as with scalding, may depend on regulations. In the United States of America, in 1992, a decision was made to include chlorine in the chill tank (Waldroup et al., 1992).
Portioning and packaging
Portioning and packaging of broiler chicken products can also potentially cause cross-contamination, but it is not considered to be significant. Briefly, a chicken can be portioned either by personnel from the processing plant or by machinery. The usual order of removal is neck skin, wings, breast, backbone, thighs and drumsticks (ACMSF, 1996). Manual handling by workers during inspection for cosmetic defects in de-boned meat, such as chicken breast, can also increase the level of cross-contamination.
Data requirements
Data requirements for modelling transport and processing fall into two categories. First, data are needed to describe how the prevalence of contaminated birds, carcasses and products changes during each sequential step, and, second, data are needed to describe the corresponding changes in numbers of the pathogen per contaminated bird, carcass or product at each stage.
Change in prevalence and numbers during transport and processing will be variable in nature, due to varying conditions, handling practices and temperatures. In addition to variability, it is likely that there will be an extensive amount of uncertainty associated with each step. Therefore, ideally, data to quantify both variability and uncertainty would be useful to characterize these steps.
Many studies that have investigated the effect of processing on Salmonella contamination of broiler chicken only consider a single step or a few of the sequential steps. Consequently, if combining data to generate estimates of the magnitude of change, details of the sampling methods and tests used and the associated sensitivity and specificity is important. Several different methods have been employed by various researchers to determine the presence or numbers of Salmonella, and samples may range from carcass rinse fluids and carcass swabs, to neck skin, or intestinal contents for direct testing.
Summary of data available
Information collected for pathogen prevalence and concentration changes in and on birds during transport is limited. Studies in the late 1970s by Rigby et al. (1980b) indicated that Salmonella could be isolated from debris in live-haul trucks and crates before live poultry was loaded, after unloading, and after washing. In the United States of America, Jones et al. (1991a) reported that debris from 33.3% of live-haul trucks and crates were positive for Salmonella, and similar levels were reported by Carraminana et al. (1997) in Spain. However, these data do not provide sufficient quantitative information to use for risk modelling.
Tables 6.5 to 6.13 provide a summary of data collected for individual steps during processing, and give a snapshot of the Salmonella situation at the various processing steps. However, they do not monitor change directly. In Table 6.9, some data is included that shows changes that occur during one of the processing steps.
In general, most studies consider prevalence of positive birds or carcasses. Further, the extent of contamination in the surrounding environment is often investigated, such as the knife used for slaughter (Table 6.5), the scald tank water (Table 6.6), the de-featherer (Table 6.7) and the chill water (Table 6.9). Environmental data can be used to give an indication of the extent of cross-contamination and, in theory, could also be used to predict prevalence levels or numbers of organisms at a particular point. Such predictions would require appropriate mathematical techniques and might require a number of assumptions relating to, for example, the rate of transfer of organisms at different sites. However, the limited amount of available data would mean that any predictions would be very uncertain and thus should be undertaken with caution.
Differences in prevalence resulting from different practices are considered in several studies. In particular, differences between tanks (with and without additives) has been investigated for both scalding (Humphrey and Lanning, 1987) and chilling (Surkiewicz et al., 1969; Lillard, 1980; Campbell et al., 1983; Dougherty, 1974). The studies that look at the addition of chemicals show, in general, a reduction in prevalence (Table 6.10). In addition, variation during a day of processing is investigated for scalding (Abu-Ruwaida et al., 1994), plucking (Rigby et al., 1980a) and chilling (Rigby et al., 1980a). Also in relation to time, variation from day to day and from year to year is investigated for scalding (Abu-Ruwaida et al., 1994), evisceration (Baumgartner et al., 1992) and chilling (Rusul et al., 1996). Finally, plant-to-plant variation is considered for plucking (Chambers et al., 1998) and chilling (Lillard et al., 1990). Few of the studies on individual processing steps consider the number of organisms per bird. In fact, the only results relate to chilling (Surkiewicz et al., 1969; Dougherty, 1974; Waldroup et al., 1992). Although data for prevalence and numbers of organisms are available for individual processing steps, using these data to estimate levels of change requires additional assumptions because the data have been generated from different studies and thus there is no baseline value from which to commence estimation (Table 6.11).
Data relating to changes in prevalence and numbers of organisms are given in Table 6.9 and Table 6.11. Most of this data focuses on changes in prevalence; only one considers changes in numbers (Campbell et al., 1983). Of these studies, Abu-Ruwaida et al. (1994) and Lillard (1990) consider changes throughout the significant stages of processing. Abu-Ruwaida et al. (1994) also consider day-to-day variation, but their results give 100% prevalence at all points and thus would not be suitable for modelling change. The remaining studies in Table 6.11 commence later in processing and thus the problem of no baseline information from which to start, again arises. For example, the investigations by James et al. (1992a, b) commence after defeathering and so the prevalence level at the point of entry into the processing plant is unknown. These studies could, however, be used to look at change from one point to the next.
General conclusions on changes could be made from this data, but much of it is old and thus would require careful consideration within an exposure assessment. In particular, the effect of changes in practices and regulations would have to be investigated. Finally, Table 6.12 presents data on prevalence of Salmonella on finished products, at the end of processing. It is evident that it is difficult to combine these data for a risk assessment, as the different studies have used different sample types and analytical methods. Very few studies have quantified the numbers of Salmonella, and these are shown in Table 6.13 for whole carcass.
Table 6.5 Data collected at stun and kill processing stage.
|
Sample |
No. tested |
% Salmonella-Positive |
Enumeration (average of positive samples |
Reference (Country) |
|
|
Throat-cutting knife |
20 |
50 |
|
Carraminana et al., 1997 (Spain) |
|
|
Feathers |
|
|
|
Kotula and Pandya, 1995 (USA) |
|
| |
Breast |
40 |
75 |
7.2 "0.2 log CFU/g |
|
|
Thigh |
40 |
53 |
6.5 "0.2 log CFU/g |
|
|
|
Drum |
40 |
55 |
6.5 " 0.2 log CFU/g |
|
|
|
Skin |
|
|
|
Kotula and Pandya, 1995 (USA) |
|
| |
Breast |
40 |
45 |
6.3 "0.2 log CFU/g |
|
|
Thigh |
40 |
30 |
5.9 "0.2 log CFU/g |
|
|
|
Drum |
40 |
27 |
5.8 "0.2 log CFU/g |
|
|
|
Foot |
40 |
55 |
5.8 "0.2 log CFU/g |
|
|
Table 6.6 Data collected at scalding processing stage.
|
Sample |
Number tested |
% Salmonella-positive |
Enumeration (average of positive samples) |
Reference (Country) |
|
Tank Water |
15 |
100 |
13.9 "13.4 MPN/100 ml |
Humphrey and Lanning, 1987 (UK) |
|
Tank Water + NaOH |
15 |
27 |
3.0 "2.3 MPN/100 ml |
|
|
Tank Water - Entry |
4 |
NS(1) |
2.9 log CFU/ml |
Abu-Ruwaida et al., 1994 (Kuwait) |
|
Tank Water - Middle |
4 |
NS |
2.3 log CFU/ml |
|
|
4 |
NS |
2.1 log CFU/ml |
|
|
|
Tank Water - Exit |
4 |
NS |
2.3 log CFU/ml |
|
|
4 |
NS |
2.3 log CFU/ml |
|
|
|
Tank Water |
20 |
75 |
|
Carraminana et al., 1997 (Spain) |
|
Carcass, 52°C scald |
NS |
|
3.0 log MPN per carcass |
Slavik, Jeong-Weon and Walker, 1995. |
|
NS |
|
3.17 MPN per carcass |
|
|
|
NS |
|
3.09 MPN per carcass |
|
|
|
Carcass, 56°C |
NS |
|
3.16 MPN per carcass |
|
|
NS |
|
3.17 MPN per carcass |
|
|
|
NS |
|
3.34 MPN per carcass |
|
|
|
Carcass, 60°C |
NS |
|
3.50 MPN per carcass |
|
|
NS |
|
3.48 MPN per carcass |
|
|
|
NS |
|
3.36 MPN per carcass |
|
Note: (1) NS = not stated
Table 6.7 Data collected at de-feathering processing stage.
|
Sample |
Number tested |
% Salmonella-positive |
Reference (Country) |
|
|
De-featherer swabs |
|
|
Rigby et al., 1980a (Canada) |
|
| |
Before start-up |
3 |
33.3 |
|
|
Coffee break |
3 |
100.0 |
|
|
|
End of shift |
3 |
66.7 |
|
|
|
Crop swabs |
273 |
2.2 |
Chambers et al., 1998 (Canada) |
|
| |
(post-de-feathering) |
362 |
5.8 |
|
|
De-feathered carcass rinse |
6 |
83.3 |
Fuzihara, Fernades and Franco, 2000 (Brazil) |
|
Table 6.8 Data collected at evisceration processing stage.
|
Sample |
Number tested |
% Salmonella-positive |
Reference (Country) |
|
|
Carcass swabs |
|
|
Morris and Wells, 1970 (USA) |
|
| |
Pre-Evisceration |
203 |
23.6 |
|
|
Post- Evisceration |
212 |
17.9 |
|
|
|
Neck skin, 10-g sample(1) |
|
|
Goren et al., 1988 (Netherlands) |
|
| |
Carcasses |
3 099 |
11.7 |
|
|
Flocks (25 birds each) |
124 |
62.9 |
|
|
|
Neck skin, 50-g sample(1) |
|
|
Baumgartner et al., 1992 (Switzerland) |
|
| |
Carcasses |
485 |
19.2 |
|
|
Flocks (5 birds each) |
97 |
47.4 |
|
|
NOTES: (1) Sampled post-evisceration.
Table 6.9 Data collected at chilling.
|
Sample |
Number tested |
% Salmonella-positive(1) |
Enumeration (average of +ve samples) if available |
Reference (Country) |
|
|
Carcass rinse |
|
|
|
Lillard, 1990 (USA) |
|
| |
Pre-chill A(2) |
40 |
13 |
|
|
|
Post-chill A |
40 |
28 |
|
|
|
|
Pre-chill B(3) |
40 |
10 |
|
|
|
|
Post-chill B |
40 |
38 |
|
|
|
|
Pre-chill |
48 |
100 |
|
Izat et al., 1989 (USA) |
|
|
Post chill |
103 |
58 |
|
|
|
|
Carcass rinse |
|
|
|
Campbell et al., 1983 (USA) |
|
| |
Entry final wash |
108 |
22 |
1-30 MPN - 17 samples |
|
|
Entry chill tank |
108 |
6 |
1-30 MPN - 5 samples |
|
|
|
Exit chill tank |
215 |
12 |
1-30 MPN - 24 samples |
|
|
|
Chill water 1st tank |
71 |
20 |
< 1.1 MPN/ml - 14 samples) |
Campbell et al., 1983 (USA) |
|
|
Final tank |
71 |
3 |
>1 MPN/ml - 2 samples |
|
|
NOTES: (1) Percentages rounded. (2) Inside/outside bird washer used in facility. (3) Outside bird washer only
Table 6.10 Data collected at chilling processing stage: effects of chlorine addition (Lillard, 1980).
|
Concentration of ClO2 (ppm) |
Time of day |
Number tested |
% Salmonella-positive(1) |
MPN/ml |
Number tested |
% Positive(1) |
MPN/g |
|
0 |
a.m. |
30 |
43 |
<0.4-15.8 |
28 |
21 |
< 0.4-48 |
|
p.m. |
30 |
40 |
|
28 |
7 |
|
|
|
3 |
a.m. |
24 |
29 |
<0.4 |
24 |
4 |
< 0.4 |
|
p.m. |
24 |
21 |
|
24 |
0 |
|
|
|
5 |
a.m. |
24 |
0 |
0 |
48 |
0 |
< 0.4 |
|
p.m. |
24 |
0 |
|
48 |
2 |
|
|
|
20 |
a.m. |
26 |
15 |
<0.4 |
26 |
4 |
< 0.4 |
|
p.m. |
26 |
19 |
|
26 |
0 |
|
|
|
34 |
a.m. |
22 |
0 |
0 |
22 |
9 |
< 0.4 |
|
p.m. |
22 |
0 |
|
22 |
0 |
|
NOTES: (1) Percentages have been rounded.
Table 6.11 Summary of data collected for changes during processing.
|
Sample and site |
No. positive out of no. tested (%)(2) |
Reference (Country) |
|
|
Cloacal and pericloacal swabs, 5 pooled |
|
Carraminana et al., 1997 (Spain) |
|
| |
Post-picking |
11/20 (55%) |
|
|
Post-vent cutting |
9/20 (45%) |
|
|
|
Post-evisceration |
12/20 (60%) |
|
|
|
Post-spray washing |
7/10 (70%) |
|
|
|
Post-air chilling |
12/20 (60%) |
|
|
|
Overall change |
|
|
|
|
Neck skin |
|
Abu-Ruwaida et al., 1994 (Kuwait) |
|
| |
Bleed (pre-scald) |
11/11 (100%)(1) |
|
|
De-feathering |
11/11 (100%) |
|
|
|
Carcass rinse |
|
Dougherty, 1974 (USA) |
|
| |
Pre-evisceration |
39/60 (65%) |
|
|
Final product |
28/60 (47%) |
|
|
|
Carcass rinse |
|
Fuzihara, Fernades and Franco, 2000 (Brazil) |
|
| |
Post-de-feathering |
5/6 (83%) |
|
|
Post-evisceration |
4/6 (66%) |
|
|
|
Post-immersion 1 |
5/6 (83%) |
|
|
|
Post-immersion 2 |
5/6 (83%) |
|
|
|
Carcass rinse |
|
Lillard, 1990 (USA) |
|
| |
Pre-scald |
16/84 (19%) |
|
|
Post-scald |
10/84 (12%) |
|
|
|
Post-pick |
10/84 (12%) |
|
|
|
Post-evisceration |
12/84 (14%) |
|
|
|
Pre-chill (after wash) |
12/84 (14%) |
|
|
|
Post-chill |
31/84 (37%) |
|
|
|
Carcass rinse |
|
James et al., 1992A (USA) |
|
| |
Pre-evisceration: |
93/160 (58%) |
|
|
Pre-chill |
77/160 (48%) |
|
|
|
Post-chill |
114/158 (72%) |
|
|
|
Post-cut |
119/154 (77%) |
|
|
|
Carcass Rinse |
|
James et al., 1992b (USA) |
|
| |
Pre-evisceration |
33/99 (33%) |
|
|
Pre-chill |
21/50 (43%) |
|
|
|
Post-chill |
23/50 (46%) |
|
|
|
Carcass Rinse |
|
James et al., 1992c (USA) |
|
| |
Pre-evisceration: |
24/99 (24%) |
|
|
Pre-chill |
28/99 (28%) |
|
|
|
Post-chill |
24/49 (49%) |
|
|
|
Carcass Rinse |
|
Jones et al., 1991b |
|
| |
After chilling |
6/57 (11%) |
|
|
At packaging |
3/14 (21%) |
|
|
|
Swab - post-scalding |
Day 1: 0% |
Patrick, Collins and Goodwin, 1973 (USA) |
|
|
Day 2: 0% |
|
||
|
Day 3: 0% |
|
||
|
Day 4: 4% |
|
||
|
Day 5: 16% |
|
||
|
Swab - after de-feathering |
Day 1: 12.5% |
Patrick, Collins and Goodwin, 1973 (USA) |
|
|
Day 2: 0% |
|
||
|
Day 3: 0% |
|
||
|
Day 4: 4% |
|
||
|
Day 5: 16% |
|
||
|
Swab - after chilling |
Day 1: 19% |
Patrick, Collins and Goodwin, 1973 (USA) |
|
|
Day 2: 4% |
|
||
|
Day 3: 8% |
|
||
|
Day 4: 4% |
|
||
|
Day 5: 32% |
|
||
|
Carcass rinse/caeca cutting |
129/330 (39%) |
McBride et al., 1980 |
|
| |
Before scalding |
|
|
|
After inspection |
59/330 (18%) |
|
|
|
After chilling |
73/330 (22%) |
|
|
|
Not stated |
|
Rigby et al., 1980b |
|
| |
Unloading |
311/331 (94%) |
|
|
After chilling |
11/25 (44%) |
|
|
Table 6.12 Prevalence of Salmonella on finished carcasses and portions.
|
Country & year of sampling if known |
Sample |
Number sampled |
Percentage positive |
Data source |
|
|
Argentina |
Carcass surface swab |
96 |
31.3 |
Terisotto et al., 1990 |
|
|
Argentina |
Carcass rinse |
86 |
2.3 |
Argentina - Call for data by FAO/WHO |
|
| |
1994-98 |
Carcass rinse |
39 |
15.4 |
|
|
Austria |
NS(1) |
1342 |
3.7 |
EC, 1998 |
|
|
Austria |
NS |
124 |
2.4 |
EC, 1998 |
|
|
Austria - 1998 |
Skin samples |
1207 |
22.2 |
EC, 1998 |
|
|
Austria - 1997 |
|
80 |
62.5 |
|
|
|
Austria - 1996 |
|
3485 |
20.9 |
|
|
|
Belgium |
NS |
127 |
28.4 |
EC, 1998 |
|
|
Brazil |
25 g of meat+skin |
60(2) |
42.0 |
Fuzihara, Fernandes and Franco, 2000 |
|
|
Canada - 1985-86 |
Carcass rinse |
205 (46)(3) |
80.5 (89.2)(4) |
Lammerding et al., 1988 |
|
| |
1984-85 |
|
180 (47)(3) |
80.6 (76.6)(4) |
|
|
1983-84 |
|
140 (41)(3) |
70.0 (68.3) (4) |
|
|
|
Denmark |
Neck skin |
4985 |
11.1 |
EC, 1998 |
|
|
Finland - 1998 |
NS |
384 |
0.52 |
EC, 1998 |
|
| |
1997 |
|
611 |
3.1 |
|
|
Ireland - 1998 |
NS |
2 695 |
16.6 |
EC, 1998 |
|
| |
1997 |
|
2 218 |
22.6 |
|
|
1996 |
|
1 632 |
22.2 |
|
|
|
Malaysia |
Carcass rinse - Plant A (5) |
12 |
91.7 |
Rusul et al., 1996 |
|
| |
12 |
75 |
|
||
| |
20 |
75 |
|
||
|
Malaysia |
Carcass rinse - Plant B (5) |
20 |
30 |
Rusul et al., 1996 |
|
| |
20 |
0 |
|
||
| |
20 |
55 |
|
||
|
Netherlands - 1997 |
Neck skin |
NS |
53.4 |
EC, 1998 |
|
| |
1998 |
|
NS |
41-50 |
|
|
Netherlands |
10 g fillet6 |
10 10 10 10 10 10 10 |
0 1 90 80 10 80 60 |
EC, 1998 |
|
|
Norway - 1998 |
Neck skin |
7 112 |
0.0 |
ARZN, 1998 |
|
| |
1997 |
|
7 591 |
0.0 |
|
|
Portugal |
Swabs of surface and abdominal cavities |
300 |
57 |
Machado and Bernardo, 1990 |
|
|
Sweden - 1998 |
NS |
1 138 |
0.0 |
EC, 1998 |
|
|
1997 |
|
723 |
0.0 |
|
|
|
1996 |
|
581 |
0.0 |
|
|
|
Sweden |
Neck skin |
4 010 |
0.02 |
EC 1998 |
|
|
Thailand |
Chicken meat (7) |
353 |
181 (51%) |
Jerngklinchan et al., 1994 |
|
|
USA |
|
NS |
3-4% |
Lillard, 1989a |
|
|
USA |
Cloacal swabs, giblets, whole carcasses and parts |
247 |
4.0% |
Harris et al., 1986 |
|
|
USDA-FSIS |
Carcass rinse |
1 297 |
20% (8) |
USDA-FSIS, 1996 |
|
| |
|
11.6% (MPN) |
|
||
|
USA |
Carcass rinse(6) |
14 |
21.4 |
|
|
|
Venezuela |
|
45 |
49 |
Rengel and Mendoza, 1984 |
|
NOTES: (1) NS = Not stated. (2) Sampled from 60 individual small poultry slaughterhouses (<200 birds per day). (3) Number of lots sampled, with 5 carcasses per lot. (4) Percentage of lots positive; one or more positive carcasses. (5) Samples not specified - some pre-chill, others post-chill;. (6) Sampled prior to packaging. (7) 25 g sample of raw chicken muscle. (8) Recovered using enrichment media.
Table 6.13 Numbers of Salmonella on finished carcasses.
|
Number of samples |
% |
MPN per carcass(1) |
Source |
|
136 |
79.5 |
< 1 |
Surkiewicz et al., 1969 |
|
28 |
16.4 |
1- 30 |
|
|
1 |
0.6 |
30 -300 |
|
|
6 |
3.5 |
> 300 |
|
|
112 |
25.9 |
0.108 ±0.279 |
Waldroup et al, 1992 |
|
112 |
32.1 |
0.172 ±0.363 |
|
|
112 |
77.3 |
0.736 ±0.672 |
|
|
112 |
38.2 |
0.188 ±0.259 |
|
|
112 |
30.4 |
0.085 ±0.226 |
|
|
109 |
41.9 |
< 12 |
USDA-FSIS, 1996 |
|
118 |
45.4 |
12 - 120 |
|
|
24 |
9.2 |
121 - 1200 |
|
|
6 |
2.3 |
1201 - 12000 |
|
|
3 |
1.2 |
>12000 |
|
|
99 |
60.7 |
< 12 |
CFIA, 2000 |
|
60 |
36.8 |
12 - 120 |
|
|
2 |
1.3 |
121 - 1200 |
|
|
1 |
0.6 |
1201 - 12000 |
|
|
1 |
0.6 |
>12000 |
Notes: (1) MPN per carcass calculated from reported values (MPN per millilitre rinse fluid) × 400 ml total rinse fluid for USDA-FSIS and CFIA results.
Data gaps
The main data gaps for processing are:
There is limited public information on the processing practices followed by different countries of the world (for example, scalding or chilling methods, including addition of chemicals).
Quantitative data (i.e. numbers of organisms) are limited for several processing steps.
Many studies are old, so more recent information on changes in prevalence and numbers would be beneficial.