The traditional approach to food safety assurance was based on applying codes of Good Hygiene Practices (GHP) and Good Manufacturing Practices (GMP) in food processing. Confirmation of safety and identification of potential problems were obtained by end-product testing. Inspectors checked for compliance with the codes and sampled the foods for laboratory analysis. Although these actions are still essential parts of any foods control programme, they have certain limitations and shortcomings as pointed out in section 3.1.
In contrast, the HACCP system clearly identifies food safety problems and also where and how they can be controlled or prevented. To assure that these actions are executed regularly and consistently, they have to be described and people who are responsible for their execution have to be trained. A record-keeping system has to be developed to provide documentation for all actions and measurements.
|
HACCP is a system which identifies, evaluates and controls hazards which are significant for food safety (CAC, 2001) |
Originally, HACCP was developed and used by the private food industry. The concept was used by the Pillsbury Company in the late 60ies for the safety of food intended for the US Space Program. However, it took many years and endless discussions between regulatory agencies and the food industry on the value of end-product testing and microbiological standards for the food before the HACCP concept was generally accepted as the primary means to assure food safety. A few milestones in this development are shown below:
|
1971: |
The HACCP concept presented at the US National Conference on Food Protection |
|
1973: |
Comprehensive treatise on HACCP published by the Pillsbury Co. HACCP - with only three principles |
|
1980: |
WHO/ICMSF report on HACCP |
|
1983: |
WHO EUROPE recommends HACCP |
|
1985: |
National Academy of Sciences (NAS) (USA) recommends HACCP (Anon., 1985) |
|
1988: |
Book on HACCP by International Commission on Microbiological Specifications for Foods (ICMSF, 1988) |
|
1989: |
The National Advisory Committee on Microbiological Criteria for Foods (NACMCF), USA, approved the first major document on HACCP |
|
1992: |
NACMCF issues a revised document on HACCP (NACMCF, 1992). HACCP now has seven principles |
|
1993: |
Codex issues the first HACCP Guidelines which were adopted by the FAO/WHO Codex Alimentarius Commission |
|
1997: |
Based on a number of FAO/WHO Consultations, Codex issues a revised document (CAC, 2001). NACMCF issues the third revised document (NACMCF, 1997). The two revised documents from Codex and NACMCF are very similar. |
Integration of HACCP into the official regulations in the European Union (EU) and the United States (US) took place as follows:
|
1991: |
Council Directive no. 91/493/EEC (EC, 1991) which places the responsibility of product safety on the industry and introduces the concept of 'own checks' and Critical Control Points during processing |
|
1993: |
Council Directive no. 93/43/EEC (EC, 1993) on the hygiene of foodstuffs |
|
1994: |
Commission Decision 94/356/EEC (EC, 1994) detailing the rules for the application of the HACCP system |
|
1995: |
US-Food and Drug Administration (FDA, 1995) issues the Code of Federal Regulations on safe and sanitary processing and importing of fish and fishery products |
|
1996: |
US Department of Agriculture, Food Safety and Inspection Service adopts the final rule on the HACCP system (USDA, 1996). |
Although the HACCP system both in EU and US is based on the same seven principles, there are some differences between the two systems. These differences are mainly related to the prerequisite programmes, the way they are documented and verified, and the scope and content of the identification of hazards.
Until April 1995, acceptance of the work of Codex by the member governments was voluntary. However, with the establishment of the World Trade Organization (WTO) in April 1995 the situation has changed. According to two of the Agreements of the WTO (the Agreement on Sanitary and Phytosanitary measures (SPS) and the Agreement on Technical Barrier to Trade (TBT)), the work of Codex is recognised as the reference for international food safety requirement. This implies that in the future member states of WTO cannot reject food, which meets Codex recommendations and standards without providing justification based on risk assessment. Since the application of HACCP is recommended by Codex, this means that HACCP has become the international reference system for food safety assurance.
Many excellent books and articles on the principles and the application of HACCP have been published in recent years. Examples are: ILSI (1997), Mortimore and Wallace (1998), Corlett (1998), Dillon and Griffith (2001), Motarjemi and van Schothorst (1999), and National Seafood HACCP Alliance (1997).
These publications should be consulted for detailed information. The present Chapter is intended as a general introduction to HACCP giving sufficient information to the reader to understand the system and to enable him/her to apply or assess the system in practical food safety assurance programmes.
The HACCP system is science-based and uses a systematic approach to the identification of specific hazards and measures for their control or prevention to ensure the safety of food. The preventive measures must be described in detail and people who have to execute them must be trained. HACCP involves careful recording of all details and actions in order to provide documentation that the system is in operation and in full control of all hazards in food processing. The HACCP system consists of seven basic principles as outlined by CAC (1997) and NACMCF (1997):
|
Principle 1: |
Conduct a hazard analysis |
|
Principle 2: |
Determine the critical control points (CCPs) |
|
Principle 3: |
Establish critical limits |
|
Principle 4: |
Establish monitoring procedures |
|
Principle 5: |
Establish corrective actions |
|
Principle 6: |
Establish verification procedures |
|
Principle 7: |
Establish record-keeping and documentation procedures. |
Guidelines for the application of the HACCP system have been presented by CAC (1997). In these guidelines it is pointed out that, prior to application of HACCP to any food operation, this sector should be operating on the basis of a prerequisite programme as outlined in Chapter 7. Furthermore, it is essential that top-management is firmly committed to introduce the system. Many departments and different personnel from chiefs to line operators will be involved and responsible for part of the system, and their full support and cooperation will be needed.
The Codex guidelines suggest that the introduction and application of the HACCP principles should follow a series of 12 steps in a logic sequence as described below:
Step 1: Assemble the HACCP team
Introduction of a HACCP system in large food factories is a complex process and requires a multidisciplinary approach by a team of specialists. The microbiologist is of paramount importance, and must advise the team on all matters related to microbiology, safety and risks. He must have an updated knowledge on these matters and also access to technical literature on the most recent developments in his field. In many cases, he will also need access to the use of a well-equipped laboratory if specific questions and problems cannot be solved by studying the technical literature. Examples are investigations of the microbial ecology of specific products, challenge tests and inoculation studies for evaluation of safety aspects.
Another important member of the HACCP team is the processing specialist. He must advise on production procedures and constraints, prepare the initial process-flow diagram, advise on technological objectives at various points in the process and on technical limitations of equipment.
Other technical specialists such as a food chemist, a food engineer as well as packaging technologists, sales staff, training and personnel managers can provide valuable information to the HACCP team and they should attend some of the meetings.
Key-members of the HACCP team (including the leader) must have an intimate knowledge of the HACCP system. Small and medium size industries are not likely to have qualified personnel on the payroll and must therefore buy assistance form outside consultants in order to implement the system. One person should be appointed as leader of the team.
When the HACCP team is assembled, the scope of the HACCP plan should be identified, describing which segment of the food claim is involved and addressed in the work.
Step 2: Describe product
A full and detailed description of the final production must be drawn up. The raw materials and ingredients used must be specified including the market name or Latin name of the fishery component. Details regarding hazards in the raw material will be included in the HACCP plan. All factors which influence safety such as composition, physical/chemical structure including water activity (aw) and pH must be described, and any microbiocidal/-static treatment such as heating, freezing, brining and smoking must be specified as well as packaging type, storage conditions and methods of distribution. The normal shelf life under specified condition should also be recorded as shown below.
|
Elements of the product description |
|
|
1 |
Product name |
|
2 |
Raw material and ingredients used |
|
3 |
Parameters influencing safety (aw, pH, salt%, etc.) |
|
4 |
Processing |
|
5 |
Packaging and packaging material |
|
6 |
Storage conditions and shelf life |
|
7 |
Conditions during distribution |
|
8 |
Intended use and consumer |
|
9 |
Labelling instructions |
Step 3: Identify intended use and consumer
The HACCP team will need to identify the intended use and consumer of the product. The intended use should be based on expected use by the consumer. The use and preparation before use greatly influence the safety of the product. Certain products may be contaminated or carry pathogenic organisms as a part of the natural flora. If the processing does not include a killing step, the only critical control point (CCP) which can render the product safe is adequate heat treatment during preparation.
The intended consumer may be the general public or a particular segment of the population such as infants or elderly. If the product is to be sold to hospitals or groups of the population with high susceptibility, more safety is required and critical limits need to be more strict.
Step 4: Construct flow diagram
The purpose of the flow diagram is to provide a clear simple description of all steps involved in the processing. Receiving and storage steps for raw materials and ingredients should be included. Time and temperature conditions during processing should be mentioned whenever there is a holding step e.g. in holding vats, buffer tanks or other areas, where this could be a potential delay in processing.
Step 5: On-site confirmation of flow diagram
The constructed flow diagram should be verified on-site for accuracy. The site should be inspected during all hours (night shifts, weekends) of operation to check for correctness and ensure that nothing crucial was overlooked.
Step 6: List all potential hazards associated with each step in the operation, conduct a hazard analysis and consider any measure to control identified hazards (Principle 1)
The words "hazard" and "hazard analysis" have been defined by Codex (CAC, 2001):
|
Hazard A biological, chemical or physical agent in, or a condition of, food with the potential to cause an adverse health effect (CAC, 2001) |
|
Hazard Analysis The process of collecting and evaluating information on hazards and conditions leading to their presence to decide which are significant for food safety and therefore should be addressed in the HACCP plan (CAC, 2001) |
Thus, the word hazard has a particular meaning. It refers to both a specific agent and/or a condition (e.g. elevated temperature) with the potential to cause harm. After having identified all potential hazards all the information available must be evaluated in order to decide, which ones of the hazards are significant and reasonably likely to cause illness if not effectively controlled.
The hazard analysis is the key to preparing an effective HACCP plan and serves three purposes (NACMCF 1997):
Providing a basis for determining CCPs (principle 2).
Examples of questions to be considered, when conducting a hazard analysis has been listed by NACMCF (1997) and includes[8]:
Raw materials and ingredients - do they contain any hazardous agents?
Intrinsic factors - will the food permit survival, multiplication of pathogens or toxin formation?
Processing conditions - are any pathogens destroyed, are there any possibilities for recontamination?
Packaging - does the packaging affect the microbial population?
Preparation and intended use - will the food be heated by the consumer?
Intended consumer - is the product for the general public or for consumption by a population with high susceptibility to illness?
A decision tree with a number of questions can be used to determine if potential hazards are "real" as demonstrated in Figure 8.1.
Figure 8.1 Hazard determination - Questions to be answered for each potential hazard at each step (based on ILSI, 1997).
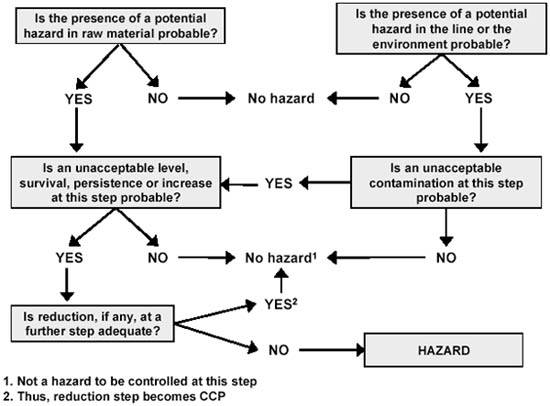
The questions in Figure 8.1 have to be asked at each step of the processing chain and all hazards must be considered.
An element of risk assessment is involved in the evaluation of potential hazards. Only these hazards which are likely to occur and which will cause a reasonably serious adverse health affect are regarded as significant as shown in Figure 8.2.
Figure 8.2 Determination of hazard significance (after Mortimore and Wallace, 1998).
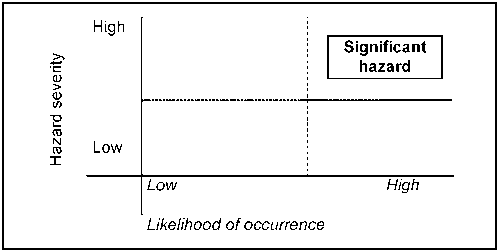
Thus, the basic procedures to use in conducting the hazard analysis are as follows:
based on the product description and the flow diagram, all the potential hazards associated with the product and at each processing step is determined and listed
Make a hazard evaluation:
o assess severity of health consequences if potential hazards are not controlled
o determine likelihood of occurrence of potential hazards if not properly controlled
o using information above, determine if this potential hazard is to be addressed in the HACCP plan
o describe control measures.
Control measure(s) is (are) any factor or activity, which can be used to prevent, eliminate or reduce a food safety hazard to an acceptable level. More than one control measure may be required to control a hazard.
Upon completion of the hazard analysis, the hazards associated with each step in the production should be listed along with any measure(s) that is (are) used to control the hazards. A "hazard analysis worksheet" can be used to organize and document the considerations in identifying food safety hazard. An example of a hazard analysis worksheet is shown in Appendix 2.
Step 7: Determine the critical control points (CCPs) (Principle 2)
Complete and accurate identification of all the CCPs is fundamental to controlling food safety hazards. To facilitate this identification, the use of a CCP decision tree can be of great help. Example of decision trees are found in NACMCF (1997), CAC (1997) and in the ILSI (1997) document. The latter is shown in Figure 8.3.
|
Critical Control Point (CCP) Is a step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level (CAC, 2001) |
Figure 8.3 Critical control point decision tree (ILSI, 1997).
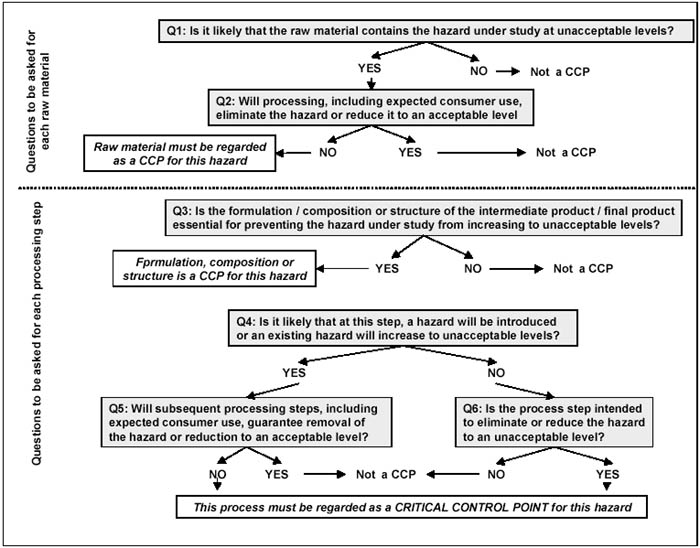
The first two questions in Figure 8.3 deal with the raw material. It is important to note, that if an identified hazard is eliminated or reduced at a later process step or by normal consumer use, the raw material is not a CCP. Question 3 deals with formulation or composition of the product. In Chapter 5 of this publication it has been pointed out, that in preventing multiplication of pathogens the pH or aw or presence of specific antibacterial compounds may be extremely important. Question 4 asks, if contamination, recontamination or even multiplication of pathogens can take place at this step. If the answer is 'No', question 6 thus has to be answered, but if the answer is 'Yes', the answer to question 5 will decide whether this step is a CCP or not.
Only points where truly significant hazards can be controlled should be designated CCPs. A tendency exists to control too much and to designate too many CCPs. This should be avoided as it will create confusion and divert attention from the true CCP.
Step 8 Establish critical limits (Principle 3)
The third HACCP principle deals with establishing one or more maximum or minimum critical limits that must be controlled at each CCP.
|
Critical limit is a criterion which separates acceptability from unacceptability (CAC, 2001) |
All critical limits should be scientifically based and refer to factors such as: time/temperature conditions, moisture level, water activity (aw), pH, titratable acidity, salt concentration, available chlorine, preservatives, organoleptic or sensory quality.
Microbiological limits should normally be avoided. This is because microbiological data can usually only be produced by a process, which may take several days. The monitoring of microbiological limits would therefore not allow you to take instant action when the process deviates.
Authoritative critical limit information is available from sources such as the "Fish and Fisheries Products Hazards and Control Guide" (FDA, 1998) or may be found in scientific publications or obtained from regulatory agencies, universities or export groups or institutions.
When critical limits have been established, they should be entered on the "HACCP PLAN FORM". An example of a HACCP plan form is shown in Appendix 3.
Step 9: Establish monitoring procedures (Principle 4)
Monitoring of CCPs serves three purposes (NACMCF, 1997):
to determine if there is a loss of control and a deviation occurs at a CCP. Appropriate action must then be taken
monitoring keeps check on the operation and provides information whether there is a trend towards loss of control and action can be taken to bring the process back into control before a deviation occur
provides written documentation for use in verification and audit. All records must be signed.
|
Monitoring is the act of conducting a planned sequence of observations or measurements of control parameters to assess whether a CCP is under control (CAC, 2001) |
To be effective, all monitoring must be done rapidly and results must be evaluated by a designated person with knowledge and authority to carry out corrective actions. Typically, monitoring methods are:
sensory quality.
Thus, in planning the monitoring procedures there are typically four questions to be answered (CAC, 2001):
|
Planning monitoring procedures · What - usually a measurement or
observation |
As already stated, the main purpose of monitoring is to determine if there is loss of control or deviation.
|
Deviation is failure to meet a critical limit (CAC, 2001) |
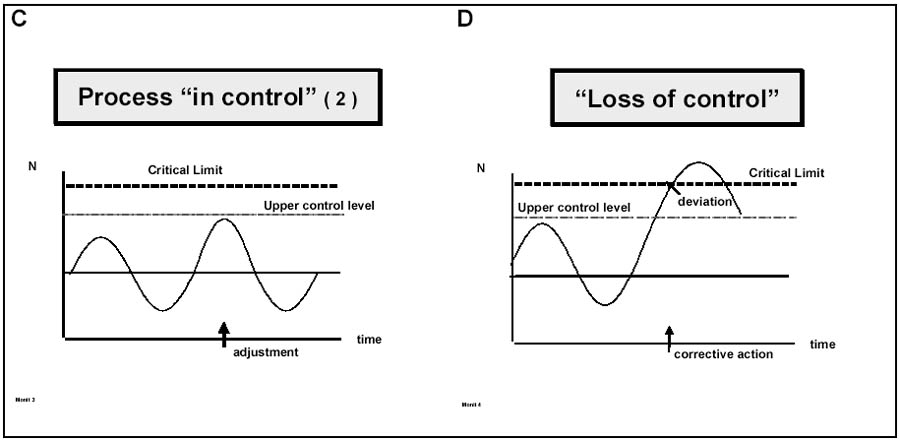
An example of a process being in control and out of control (deviation) has been illustrated by Motarjemi and van Schothorst (1999) as shown in Figure 8.4.
Step 10: Establish corrective actions (Principle 5)
|
Corrective Action is any action to be taken when the results of monitoring at the CCP indicate a loss of control (CAC, 2001) |
Whenever there is a deviation from established critical limits a corrective action must be instituted to ensure that defective products do not reach the consumer. These actions should include the following (NACMCF, 1997):
record the corrective action taken.
Figure 8.4 Monitoring: A: small fluctuations always occur around a target level, B and C: the process is under control but adjustment is needed in situation C as abnormal fluctuations are noted, D: a deviation occurs and corrective action is needed (from Motarjemi and van Schothorst,1999).
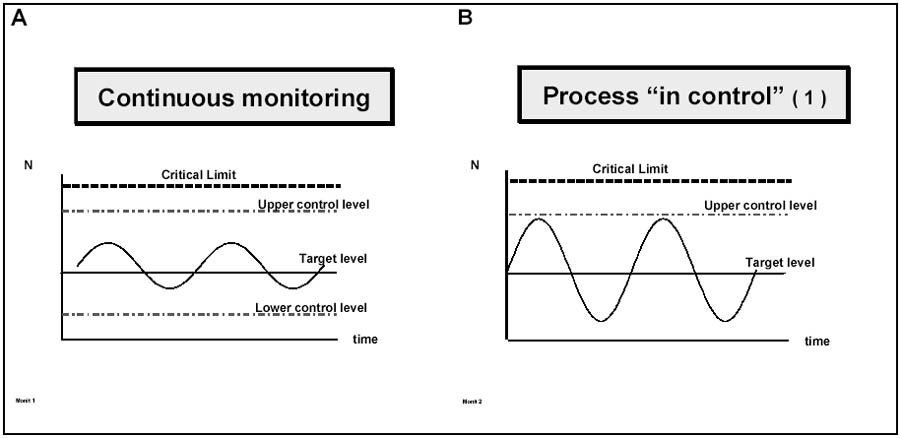
Options for disposition of products placed on hold include:
use as by-product (animal feed).
Corrective action procedures should be developed by the HACCP team in advance and specified in the HACCP plan. Any action should be recorded on the HACCP Plan Form (Appendix 3). If necessary, a more detailed corrective action report should be elaborated including the following information (National Seafood HACCP Alliance, 1997):
name of the individual responsible for taking action.
Step 11: Establish verification procedures (Principle 6)
|
Verification is the application of methods, procedures, tests and other evaluations, in addition to monitoring to determine compliance with the HACCP plan (CAC, 2001) |
The purpose of the HACCP plan is to prevent food safety hazards from occurring. Verification activities must provide a level of confidence that the HACCP plan is working properly and is adequate to control hazards. The NACMCF (1997) document is providing guidance on what elements should be included in the verification activities:
Verification of the CCP-monitoring
o CCP-record review
o calibration of instruments
o targeted sampling and testing
o microbiological testing
Comprehensive HACCP system verification.
Thus, the verification procedures include verification of both the individual CCP and the overall HACCP plan. An essential component of verification is validation.
|
Validation is obtaining evidence that the elements of the HACCP plan are effective (CAC, 2001) |
In validation of the HACCP plan it needs to be established that the plan is scientifically and technically sound. This means that scientific validation includes review of each part of the HACCP plan from the hazard analysis through to each CCP. The needed information can be obtained from expert advice, scientific studies and literature, in-plant observations and measurements.
|
Validation |
· are the right things done? |
|
Verification |
· are the things done right? |
Apart from the initial validation, subsequent validation as well as verification must take place whenever there is a change in raw materials, product formulation, processing procedures, consumer and handling practices, new information on hazards and their control, consumer complaints, recurring deviations or any other indication, that the system is not working. Figure 8.5 shows where validation fits into the process of HACCP implementation.
Figure 8.5 HACCP validation and verification (based on ILSI, 1999).
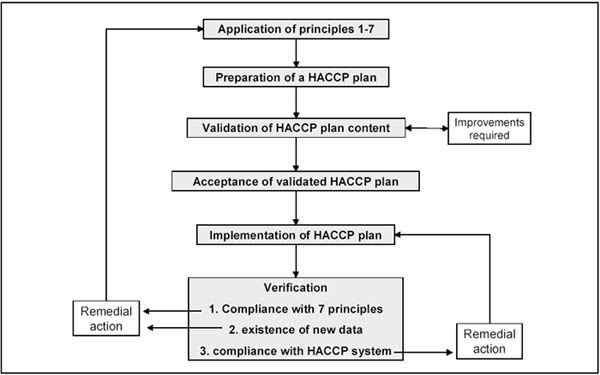
A periodic comprehensive verification of the HACCP system should be conducted yearly by an unbiased, independent authority. This should include a review of the HACCP plan for completeness, confirmation of the flow diagram, review of all records and validations, sampling and testing to verify CCPs (NACMCF, 1997).
Verification is the responsibility of the producer or food handler. However, where regulatory agencies are conducting audits or sampling end-products the results can be used by industry as part of the verification programme.
Verification procedures should be entered on the HACCP Plan Form (Appendix 3) and results into special verification records.
Step 12: Establish record-keeping and documentation procedures (Principle 7)
|
Record keeping ensures that the information resulting from the HACCP study and implementation of the resulting HACCP plan is available for validation, verification, review, auditing and other purposes (ILSI, 1997) |
Records and documentation are vital for the verification and auditing to determine, if the HACCP system in operation is in compliance with the HACCP plan and operating correctly. Also records of support documents must be kept such as data used to establish critical limits, reports from consultants or experts, a list of the HACCP team and their responsibilities and the preliminary steps taken before development and implementation of the HACCP plan. Examples of HACCP records are shown in NACMSF (1997). The CAC (1997) publication mentions the following examples of documentation:
critical limit determination
and as examples of records:
modifications of the HACCP system.
It is generally accepted that responsibility for producing safe food is in the hands of the producer. It is therefore the responsibility of the producer to ensure the development and application of a proper HACCP plan.
It is of paramount importance, that senior management of a company needs to understand and support the implementation of HACCP in the processing facilities. They need to understand the benefits as well as the cost and the resources needed.
While the HACCP team is conducting the HACCP study, it is advisable to initiate training of key-personnel. No plan will work if the people who have to implement it are not trained. People who have to develop the plan as well as people responsible for implementation and maintenance may all need training.
When the HACCP plan has been developed, it needs to be approved by senior management. During the development of the HACCP plan including the prerequisite programme, it often becomes clear that improvements may be needed in construction or layout of facilities, or utensils need to be replaced. This could involve considerable costs and run into budgetary constraints. However, modifications, which are essential to food safety, should always be executed immediately, while a timetable for less necessary modifications should be made.
Although implementation of HACCP is the responsibility of the industry, government (that is regulatory fish safety and quality control authorities) also have a role to play. Government authorities can play three roles: they can act as facilitators, enforcers or trainers (Motarjemi and van Schothorst, 1999):
as facilitators they can help industries understand the goals and scope of HACCP and provide expertise during the establishment of a HACCP plan or its verification
as enforcers their task is to assess the correct application and implementation of the seven HACCP principles
they can provide training courses and also participate in training courses organized by or for the industry.
Thus, it is a key role of government agencies to show leadership by promoting and facilitating the implementation of HACCP. However, the government has also a strategic role (i.e. a plan how to achieve a pre-set goal) as well as an ongoing role in assessing the HACCP systems applied in the industry.
With regard to the actual assessment of HACCP, government agencies also play an important role in providing guidance on the assessment process needed to be developed and provided to officials for its uniform and acceptable application. This guidance should be developed by government agencies in collaboration with, when possible, food control officials and industry (see also section 8.5).
Proper implementation of HACCP may also need the support of other institutions such as academia and research, trade associations, private sector etc. A consequence of the WTO/SPS agreement is that food safety criteria such as FSOs, performance criteria and microbiological end product criteria have to be based on scientific evidence, and where appropriate on a risk assessment. Scientific results produced lege artis and published in international literature is therefore the background for the HACCP plan and provides the transparency required by WTO/SPS.
Finally, consumers and consumer advocate groups have a counter-balancing role to ensure that safety and quality are not undermined by political and socio-economical considerations when drafting legislation or implementing safety and quality policies.
Many fish producing, exporting and/or importing countries have undertaken a thorough evaluation and reorganization of fish inspection and control systems with the aim to improve efficiency, rationalize human resources and introduce risk analysis-based approaches. The HACCP principles play a pivotal role in these preventive approaches. Their application is the responsibility of the fish industry, whereas government control agencies are responsible for monitoring and assessing their proper implementation.
Many inspection agencies have developed approaches and procedures for carrying out HACCP compliance auditing. These approaches and modalities have used the terminology and basic requirements of the ISO 10011 standards (ISO, 1993a,b) that were adapted to the specificities of HACCP and to the countries regulations. Information regarding these procedures will not be reviewed here in details as it is widely accessible, especially via internet. This Chapter will rather attempt to demystify the issues and advise on how to achieve practical HACCP auditing.
Audit is a systematic and independent examination to determine whether activities and results comply with the documented procedures; also whether these procedures are implemented effectively and are suitable to achieve the objectives (ISO, 1993a,b). In HACCP terms, achieving the objectives means managing the production and distribution of safe fish products through the use of an HACCP based approach.
The outcome of the audit is to have established whether the manufacturer has
the necessary support (or prerequisite) programmes in place to assess adherence to Good Hygienic and Good Manufacturing Practices (GHP/GMP).
The audit will encompass assessment of the management commitment to support the system and assessment of the knowledge, competency and decision-making capabilities of the HACCP team members to apply the system and maintain it. Four types of HACCP audits can be envisaged:
An internal HACCP audit to establish the effectiveness of the HACCP system using the company's own human resources or by bringing in an external HACCP assessor.
An external HACCP audit of suppliers of critical raw materials or of packed finished products to establish whether they have robust HACCP systems in place. This includes regulatory HACCP auditing
Audit of the customers HACCP system. This may be important where the customer is responsible for the distribution and sale of a high risk (e.g. a chilled ready meal) product which bears the brand of the manufacturing company
An investigative audit can also be conducted to analyse a specific problem area. This may be used for example when a CCP regularly goes out of control and more studies are needed to investigate the real cause in order to take corrective action, or where a previously unknown problem has arisen.
An HACCP audit needs to be properly prepared. Figure 8.6 describes the steps generally required in a HACCP audit. This guidance is useful for independent (third-party) audits as well as for internal or compliance audits. It should be adapted to the particular circumstances of the firm being audited.
Figure 8.6 Steps in HACCP auditing (Mortimore and Wallace, 1998).
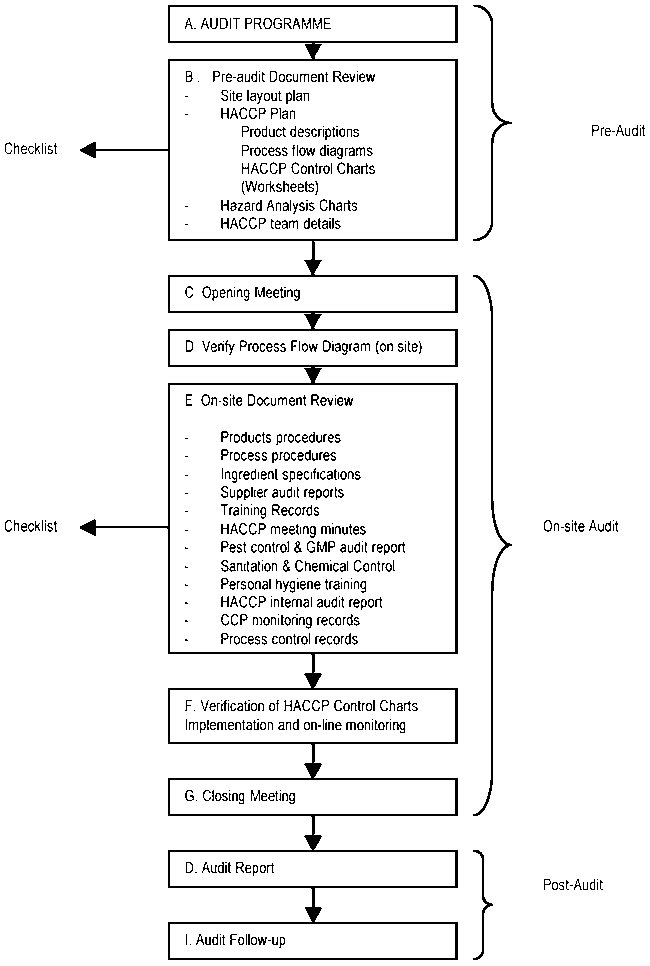
Pre- audit
A preparatory phase is necessary to elaborate the schedule and the definition of the scope of the audit. All the personnel required during the audit should be notified to ensure that they are available. Also the necessary documentation should be made available for the audit.
This starts with a "desktop assessment" of the HACCP system, to review all of the documentation relating to the scope of the audit such as the flow diagram layout, the time/temperature and other technological information, the hazard analysis, etc.
The pre-audit document review can be done as an initial scan to get a feel for who carried out the HACCP study, its style, its completeness, and also familiarisation with the site being audited and the products and process itself. It will give an opportunity for the auditor to carry out some research before the assessment. At this stage, it is important to build up knowledge of the product/process technology concerned. Literature searches of the technology, fish contamination outbreaks and legislative controls should be included. Guides and other support documents can be useful.
It is also important to gauge the level of commitment of the management and the competency of the HACCP team members by asking for their training and experience.
If the pre-audit indicates obvious inadequacies, it may be advisable to stop the assessment at this point prior to the on-site audit. The deficiencies should be discussed with the HACCP team, who can then review their HACCP system and implement any required corrective measures.
On-site audit
An opening meeting is useful to present the team of auditors, the scope and the tentative timetable and to identify the personnel and documentation required.
At this stage, the accuracy of the process flow diagram will be carefully checked, followed by a full review of operational procedures for CCP monitoring, CCP monitoring records, training records, etc. The prerequisite GMP and hygiene maintenance records, pest control and also the HACCP team meeting minutes can be reviewed. In the latter case, it may be helpful to use this to get an idea of the decision making process, who attended the meetings on each occasion and whether difficulties were encountered. The review will also include previous audit records where non-compliances may have been found. The assurance of the effectiveness of any corrective actions taken must be sought. Other quality and safety related data for review will include customer complaints and customer audit reports.
It is often useful to use checklists during the audit. An example of a checklist is presented in Table 8.1. The "considerations" column can be completed during the document review step of the process, and the "auditors' findings" column during the audit itself.
During a closing meeting, the overall assessment findings are presented and an overall view of the proceedings is given. Non-compliances should be discussed together with supporting evidence and a schedule for the corrective actions agreed. The auditor must ensure that identified deficiencies are clearly understood and that the recommended corrective actions are feasible and agreed by a senior manager.
Table 8.1 Example of a checklist for assessing HACCP implementation (Ababouch, 2000).
|
Component to assess |
Compliance, considerations, points to raise on site |
Findings of the auditor |
|
|
(1) Commitment of the management |
|
|
|
|
Financial commitment |
|
|
|
|
Awareness/support |
|
|
|
|
(2) HACCP team |
|
|
|
|
The HACCP team leader has effective power of decision |
|
|
|
|
The HACCP team members are qualified |
|
|
|
|
(3) Composition of products |
|
|
|
|
Fish composition is properly described |
|
|
|
|
Any modification is recorded and taken into account for HACCP revision |
|
|
|
|
(4) Intended use |
|
|
|
|
Valid description of the intended use |
|
|
|
|
Any modification is recorded and taken into account for HACCP revision |
|
|
|
|
(5) Process flow diagram(s) |
|
|
|
|
The flow diagram is correct |
|
|
|
|
Any modification is recorded and taken into account for HACCP revision |
|
|
|
|
(6) Hazard analysis |
|
|
|
|
All control measures are correctly implemented, eventually validated |
|
|
|
|
Personnel in charge of control measures are identified and qualified |
|
|
|
|
New hazards, introduced because of changes in product, process,... were taken into consideration |
|
|
|
|
Control measures have been identified for these new hazards |
|
|
|
|
(7) Critical control points (CCPs) |
|
|
|
|
CCPs are properly identified (e.g. using the decision tree) |
|
|
|
|
Introduction of new hazards has resulted in CCP analysis to implement proper control measures |
|
|
|
|
(8) Critical limits |
|
|
|
|
Critical limits are properly identified and eventually validated |
|
|
|
|
Introduction of new hazard has resulted in the revision of the critical limits |
|
|
|
|
(9) Monitoring procedures |
|
|
|
|
Monitoring procedures are properly identified |
|
|
|
|
The reliability of the monitoring procedures has been validated |
|
|
|
|
Personnel in charge of monitoring is well identified and trained |
|
|
|
|
All necessary modifications have been made to take into account the introduction of new control measures |
|
|
|
|
(10) Corrective actions |
|
|
|
|
Corrective actions are properly identified and eventually validated |
|
|
|
|
Personnel in charge of corrective actions has been identified and trained |
|
|
|
|
All necessary modifications have been made to take into account the introduction of new control measures |
|
|
|
|
(11) Verification of the HACCP system |
|
|
|
|
The method and frequency of verification are appropriate |
|
|
|
|
The validity of the verification method has been confirmed |
|
|
|
|
Personnel in charge of verification is identified |
|
|
|
|
Changes of products, processes, standards, regulations,.... were taken into consideration |
|
|
|
|
(12) Record-Keeping System |
|
|
|
|
Forms are appropriate and complete |
|
|
|
|
Forms are up to date for recording the following: |
|
|
|
| |
· Monitoring results, |
|
|
|
· Corrective actions, |
|
|
|
|
· Modifications of the HACCP system |
|
|
|
|
· HACCP Verification/revision results |
|
|
|
|
Some records have been tampered with |
|
|
|
Post- audit
Audit reports should provide evidence of the findings of the assessment - primarily what deficiencies have been found in the HACCP system, the non-compliance notes, the recommended corrective measures and the timetable to implement them.
During the audit follow-up, the auditor should ensure that the non-compliances are closed off. The effectiveness of corrected non-compliances should be verified as soon as the corrective action has been taken and reviewed during subsequent audit to ensure that the corrective actions taken have been effective on an ongoing basis
The frequency of HACCP audit should be based on:
the reputation of the fish company: previous safety and quality records, HACCP manual and implementation classification, training and qualification.
An HACCP audit exercise should lead to an audit report which should state whether the system provides enough assurance to control fish safety and quality. However, fish processors look for a formal recognition (validation, certification). It should be stressed that although this is legitimate, an HACCP audit is a snapshot punctual evaluation and any recognition should not lead to false assurance. It is a temporary recognition and audit should be as frequent as seen fit.
In international fish trade, there is a danger of duplication of HACCP audit efforts. This can be alleviated by the development of an internationally recognized equivalency system, for example through the Codex Committee on import/export inspection and certification systems.
Furthermore, third party certification can complement the work of government inspectors in assessing HACCP. However, certifying bodies should demonstrate proper qualifications and integrity in HACCP development and verification. This may require the establishment of a certification system for third party HACCP assessors.
Proper assessment of HACCP requires demonstrated knowledge and qualifications in different areas of science and technology pertinent to the products and processes of interest, in addition to confidentiality, objectivity and experience and skills in auditing and communication (ISO, 1993b). These qualifications are acquired through training and experience. It should be stressed that any training activity should provide evidence of satisfactory completion through examination. Also, the training programs and examinations should be harmonized to allow for easy recognition and equivalency between countries.
References
Ababouch., L. 2000. The role of government agencies in assessing HACCP. Food Control 11, 137-142.
Anonymous 1985. An Evaluation of the Role of Microbiological Criteria for Foods and Food Ingredients. National Research Council, National Academy Press. Washington, DC, USA.
CAC (Codex Alimentarius Commission) 2001. Food Hygiene Basic Texts. 2nd ed. Food and Agriculture Organization / World Health Organization, Rome, Italy.
Corlett, D.A. 1998. HACCP Users Manual. A Chapman and Hall Food Science Title. Aspen Publishers Inc., Gaithersberg, Maryland, USA.
Dillon, M. and C. Griffith 2001. How to HACCP. 3rd ed. M.D. Associates, 32a Hainton Avenue, Grimsby, North East Lincolnshire DN 329 BB, UK.
EC (European Commission) 1991. Council Directive 91/493/EEC of 22 July 1991 laying down the health conditions for the production and the placing on the market of fishery products. Official Journal of the European Community L268, pp.15-34.
EC (European Commission) 1993. Council Directive 94/43/EEC of 14 June 1993 on the hygiene of foodstuffs. Official Journal of the European Communities L175, pp. 1-112.
EC (European Commission) 1994. Commission Decision 94/356//EC of 20 May 1994 laying down detailed rules for the application of Council Directive 91/493/EEC, as regards own health checks on fishery products. Official Journal of the European Communities L156, pp. 50-57.
FDA (US Food and Drug Administration) 1995. Procedures for the Safe and Sanitary Processing and Importing of Fish and Fishery Products; Final Rule. Code of Federal Regulations, Parts 123 and 1240. Volume 60, No 242, 65095-65202.
FDA (US Food and Drug Administration) 1998. Fish and Fisheries Products Hazards and Controls Guide. 2nd ed. Washington, USA.
ICMSF (International Commission on Microbiological Safety of Foods) 1988. Microorganisms in Foods 4: Application of the Hazard Analysis Critical Control Point (HACCP) to ensure microbiological safety and quality. Blackwell Scientific Publications, London, UK.
ILSI (International Life Sciences Institute) 1997. A simple Guide to understanding and applying the Hazard Analysis Critical Control Point Concept ILSI Europe, Brussels, Belgium.
ILSI (International Life Sciences) 1999. Validation and verification of HACCP. ILSI Europe, Brussels, Belgium.
ISO (International Standards Organization) 1993a. ISO 10011-1. Guidelines for auditing quality systems. Part 1: auditing. 8 pages. Geneva. Switzerland.
ISO (International Standards Organization) 1993b. ISO 10011-1. Guidelines for auditing quality systems. Part 2: Qualification criteria for quality systems auditors. 6 pages. Geneva. Switzerland.
Mortimore, S. and C. Wallace 1998. HACCP, A practical approach. A Chapman and Hall Food Science Book. Aspen Publishers Inc., Gaithersberg, Maryland, USA.
Motarjemi, Y. and M. van Schothorst 1999. HACCP, Principles and Practice. In Jongeneerl, S. (ed) Teacher's Handbook. A WHO/ICD Training Manual in Collaboration with FAO. World Health Organization, Geneva, Switzerland.
NACMCF (National Advisory Committee on Microbiological Criteria for Foods) 1992. HAZARD Analysis and Critial Control Point System. International Journal of Food Microbiology 16, 1-23.
NACMCF (National Advisory Committee on Microbiological Criteria for Foods) 1997. HAZARD Analysis and Critial Control Point Principles and Application Guidelines. Journal of Food Protection 61, 762-775.
National Seafood HACCP Alliance 1997. HACCP: Hazard Analysis and Critical Control Point Training Curriculum. 2nd ed. (Chairman editorial committee: Donn Ward). Publication UNC-SG-96-02, North Carolina State University, Raleigh, North Carolina.
USDA (US Department of Agriculture, Food Safety and Inspection Service) 1996. 9 CFR Pathogen reduction; hazard analysis and critical control point (HACCP) systems: final rule. US Federal Register, 61, 38806-38989, 25 July.
|
Web Sites relevant to seafood HACCP FDA-Center for Food Safety & Applied Nutrition-Seafood HACCP Manual - Food Safety Canada NOAA Fisheries-National Marine Fisheries Service SeafoodNIC Home Page Seafood NIC Home Page - Compendium of Fish and Fishery Products Processing
Methods, Hazards and Controls Seafood NIC Home Page - HACCP Plans |
|
[8] Conditions covered by
the prerequisite programme have been excluded from the list |