

Small-scale livestock producers often felt the dramatic impact of animal disease outbreaks and this meeting should strive to assist them with practical and achievable outcomes which would make a difference in their production capacities |
The third meeting of the FAO/International Office of Epizootics (OIE - World Organisation for Animal Health)/African Union (AU)/Interafrican Bureau for Animal Resources (IBAR)/International Atomic Energy Agency (IAEA) Consultative Group on Contagious Bovine Pleuropneumonia (CBPP) was held from 12 to 14 November 2003, at FAO headquarters in Rome and was attended by 39 participants from various research institutions, government departments, international organizations and FAO staff members and consultants.
The theme of the meeting was “Towards sustainable CBPP control programmes for Africa”, and it was formally opened by Ms Fernanda Guerrieri, Chief of the Emergency Operations Service of FAO (TCEO). In her opening remarks, Ms Guerrieri thanked the Animal Health Service (AGAH), for the excellent working relationship and the professionalism between AGAH and TCEO staff in providing technical assistance to member countries in the control of animal disease outbreaks. She stressed that small-scale livestock producers often felt the dramatic impact of animal disease outbreaks and this meeting should strive to assist them with practical and achievable outcomes which would make a difference in their production capacities. Special thanks were extended to Dr Yves Cheneau for his able leadership, untiring efforts and technical efficiency during his tenure as Chief of the Animal Health Service of FAO for nearly 12 years until his retirement at the end of November 2003. Ms Guerrieri declared the meeting open and wished all participants fruitful and productive deliberations.

Dr Juan Lubroth, Senior Officer of the Infectious Diseases/EMPRES group of FAO delineated the objectives and expected outcome of the meeting by reiterating the need for practical solutions and an innovative approach to the control of CBPP in Africa. He reminded participants that the consultative group meeting for CBPP was unique in character in that it brought together field veterinarians, laboratory diagnosticians, researchers, policy-makers and international partner institutions such as AU-IBAR, OIE and the Joint FAO/International Atomic Energy Agency (IAEA) Division for seeking solutions to the problem of CBPP control. Dr Lubroth said that the cross-fertilization of ideas, technical exchanges and forceful interpretation of the way forward in CBPP control have at times resulted in sterile debates and arguments that have unfortunately led to less than productive meeting outcomes. He hoped that this would not be the case at this important meeting.
Since 1990, 13 projects have been utilized in emergency and capacity-building operations to establish field and laboratory activities for CBPP surveillance and control |
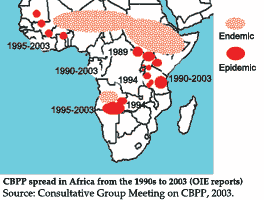
It was evident from the overview given by the FAO Animal Health Officer responsible for bacterial diseases, Dr William Amanfu, that CBPP was a major problem on the African continent and that it threatened uninfected areas of the continent from the focal regions of endemic disease in western, central and eastern Africa.
Such incursions were serious developments leading to urgent requests for assistance from governments and requiring emergency inputs in the form of Technical Cooperation Programme (TCP) projects delivered through FAO's EMPRES programme. Since 1990, 13 projects with an overall expenditure of over US$3 million have been utilized in emergency and capacity-building operations to establish field and laboratory activities for CBPP surveillance and control. The Joint FAO/IAEA Division in Vienna has also contributed significantly to the laboratory capability for CBPP diagnosis. These activities have collectively been a catalyst for synergism between member and donor communities and it is expected that such technical assistance, coupled with long-term strategic planning, could lead to sustainable strategies for CBPP control in Africa.
 Technical presentations on “CBPP control strategies” were made by Drs B. Kebkibah (AU/IBAR, Nairobi), F. Musisi (FAO/Subregional Office for Southern and East Africa [SAFR], Harare), J. Mariner (AU/IBAR, Nairobi) and M. Lesnoff (International Cooperation Centre of Agricultural Research for Development/CIRAD-EMVT, Debre Zeit, Ethiopia). There was a pressing need for the collection of more timely, geographic data for a better understanding of disease dynamics and for the development of control strategies. Diagnostically derived data mainly from serological testing were required for the assessment of prevalence to confirm zoning. Therefore, further diagnostic capacity building and longitudinal studies on CBPP disease prevalence in local communities could be advantageous. Models of CBPP transmission that took into account social structures in pastoral communities were also discussed and could be used to predict the progress and persistence of disease in herds. The models showed that CBPP could persist in relatively small herds and could propagate easily wherever cattle were gathered, particularly at kraals and watering holes. Although quarantine was essential to break the transmission cycle of the disease, the difficulty of implementing this strategy in Africa was highlighted. The models were used to predict the effects of vaccination, antibiotic treatment alone and combined application of antibiotic and vaccine. Simulations showed that vaccination alone could not eradicate the disease and that treatment with an effective antibiotic might reduce current losses attributed to it. More field data would be required to improve the accuracy of these models and they would need to be validated against the many and varied situations observed in the African pastoral systems. The significance of CBPP resurgence in some Southern African Development Community (SADC) member countries was explained and continuing threats to international exports were raised by the recent outbreak of CBPP in the East Caprivi strip of Namibia near the border with Botswana. A phased 16-year plan to reduce CBPP endemicity in southern African countries was presented.
Technical presentations on “CBPP control strategies” were made by Drs B. Kebkibah (AU/IBAR, Nairobi), F. Musisi (FAO/Subregional Office for Southern and East Africa [SAFR], Harare), J. Mariner (AU/IBAR, Nairobi) and M. Lesnoff (International Cooperation Centre of Agricultural Research for Development/CIRAD-EMVT, Debre Zeit, Ethiopia). There was a pressing need for the collection of more timely, geographic data for a better understanding of disease dynamics and for the development of control strategies. Diagnostically derived data mainly from serological testing were required for the assessment of prevalence to confirm zoning. Therefore, further diagnostic capacity building and longitudinal studies on CBPP disease prevalence in local communities could be advantageous. Models of CBPP transmission that took into account social structures in pastoral communities were also discussed and could be used to predict the progress and persistence of disease in herds. The models showed that CBPP could persist in relatively small herds and could propagate easily wherever cattle were gathered, particularly at kraals and watering holes. Although quarantine was essential to break the transmission cycle of the disease, the difficulty of implementing this strategy in Africa was highlighted. The models were used to predict the effects of vaccination, antibiotic treatment alone and combined application of antibiotic and vaccine. Simulations showed that vaccination alone could not eradicate the disease and that treatment with an effective antibiotic might reduce current losses attributed to it. More field data would be required to improve the accuracy of these models and they would need to be validated against the many and varied situations observed in the African pastoral systems. The significance of CBPP resurgence in some Southern African Development Community (SADC) member countries was explained and continuing threats to international exports were raised by the recent outbreak of CBPP in the East Caprivi strip of Namibia near the border with Botswana. A phased 16-year plan to reduce CBPP endemicity in southern African countries was presented.
Presentations that concerned novel vaccine technologies and vaccination strategies were given by Drs J. Frey (Institute of Veterinary Bacteriology, Bern, Switzerland), R. Nicholas (Veterinary Laboratories Agency, Weybridge, United Kingdom), H. Wesonga (Kenya Agricultural Research Institute, Nairobi, Kenya), A. Yaya (LANAVET, Garoua, Cameroon) and J. March (Moredun Research Institute, Edinburgh, United Kingdom). In this scientifically oriented session, the work resulting from field trials of new improved vaccine formulations, dose response and apparent reversion to virulence of the current vaccine strain and the molecular basis of virulence were presented. CBPP is caused by the small colony variant of Mycoplasma mycoides subsp. mycoides (MmmSC). No toxins have been described for this mycoplasma. Instead, it probably produces disease through adverse interactions with the host's immune system and cellular metabolism. Several candidate molecules of MmmSC were described and evidence of their role as pathogenic factors was demonstrated. Some of these, especially those that were antigenic membrane proteins, could be targeted for vaccine development. It was shown that inoculation with whole saponized MmmSC vaccine exacerbated CBPP, as did pure preparations of a membrane lipoprotein Q (LppQ). It has been known for some time that CBPP vaccines sometimes lead to unpredictable adverse reactions at the site of inoculation. Organisms isolated from one such reaction site were shown to be capable of producing this reaction consistently in other inoculated bovines. These isolates were found to be biochemically different from the parental T1 44 vaccine strain.
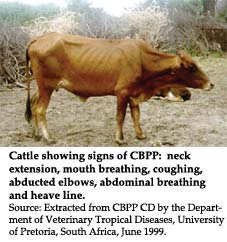 The significance of this in the field is not clearly understood. The failure of some CBPP vaccines was also investigated and provisionally attributed to insufficient dosage. CBPP vaccine is a live attenuated vaccine and therefore its stability under field conditions and its correct administration are very critical to vaccine efficacy. Recent studies with diluents containing magnesium sulphate have shown that they lower the pH of the vaccine, resulting in the death of mycoplasma cells in the vaccine preparation. Moreover, they are stable for only two hours under these conditions. Options for alternative diluents that do not alter pH, or provide better buffering formulations and the addition of pH indicators in the vaccine preparation to assess deterioration under field conditions, were described. Better protocols and formulation of vaccine batches were presented to solve some of these problems. Discussions on the requirement of new vaccines came to the conclusion that current vaccines could be improved in their formulations, thermal stability and quality assurance. Basic research was needed to continue towards realization of better vaccines that produce higher protective immunity and allow differentiation between vaccination immunity and field-acquired immunity.
The significance of this in the field is not clearly understood. The failure of some CBPP vaccines was also investigated and provisionally attributed to insufficient dosage. CBPP vaccine is a live attenuated vaccine and therefore its stability under field conditions and its correct administration are very critical to vaccine efficacy. Recent studies with diluents containing magnesium sulphate have shown that they lower the pH of the vaccine, resulting in the death of mycoplasma cells in the vaccine preparation. Moreover, they are stable for only two hours under these conditions. Options for alternative diluents that do not alter pH, or provide better buffering formulations and the addition of pH indicators in the vaccine preparation to assess deterioration under field conditions, were described. Better protocols and formulation of vaccine batches were presented to solve some of these problems. Discussions on the requirement of new vaccines came to the conclusion that current vaccines could be improved in their formulations, thermal stability and quality assurance. Basic research was needed to continue towards realization of better vaccines that produce higher protective immunity and allow differentiation between vaccination immunity and field-acquired immunity.
In the session on the “Use of antibiotics and diagnostic tests”, presentations were made by Drs F. Thiaucourt (CIRAD-EMVT, Montpellier), R. Gieger (IAEA, Vienna), J.†Regalla (National Laboratory of Veterinary Science [LNIV], Lisbon), A. Catley (Pan African Programme for the Control of Epizootics [PACE]/Community-based Animal Health and Participatory Epidemiology Unit [CAPE]/IBAR, Nairobi), M. Rweyemamu (Advanced Veterinary Information System [AVIS], London), G. Thompson (PACE/IBAR, Nairobi) and F. Mbithi (International Livestock Research Institute [ILRI], Nairobi). Dr M. Rweyemamu presented a new Web-based product from AVIS on the current information on CBPP; it was demonstrated and opened for comment from participants.
The use of antibiotics in the treatment of CBPP has long been a source of heated debate and controversy |
The use of antibiotics in the treatment of CBPP has long been a source of heated debate and controversy. Their use to treat lung sickness has been illegal in many countries. The reality in the field is that tetracycline is often used to treat CBPP. A trial showed that it reduced local inflammation and reduced the overall disease pathology in the animals but did not kill the pathogen. It could therefore not be recommended for use in eradication campaigns. Clearly more research into the antimicrobial activity of antimycoplasma antibiotics needs to be conducted urgently.
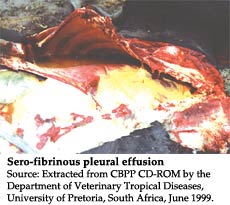 The complement fixation test (CFT) developed in 1953 has been the linchpin of CBPP serological diagnosis. Recently, a competition ELISA (cELISA) was developed and a five-year coordinated research project was conducted by the FAO/IAEA Joint Division to validate the test in the field. This study was completed in 2003. The strengths of this technique were its ability to detect infected animals over a longer period of the infectious cycle than the CFT, its ease of use and the provision of internal quality control systems. However, it did not consistently detect animals at the early stages of infection as did the CFT. It exhibited a relative sensitivity of 73 percent compared to the CFT and a relative specificity of about 98 percent. Based on these data, the test has been recommended to the OIE for inclusion in the prescribed tests for CBPP diagnosis. It was surmised that no single diagnostic method was suitable for definitive serological diagnosis of CBPP but CFT and cELISA could be conducted in tandem to increase the overall performance of serological diagnosis. CFT followed by the immunoblotting test (IBT) was used consistently in Portugal during its CBPP eradication campaign. The IBT was capable of confirming the results of CFT and distinguishing false positive results that were sometimes obtained with CFT. It also remained positive over a longer period of infection than the CFT. The control measures of zoning after serological prevalence studies, abattoir surveillance and movement control coupled with CFT, mycoplasma isolation, histopathology, and the polymerase chain reaction (PCR) test were adopted in the effort that eventually eradicated CBPP from Portugal, culminating in the country's declaration of freedom from CBPP by the OIE in 2003.
The complement fixation test (CFT) developed in 1953 has been the linchpin of CBPP serological diagnosis. Recently, a competition ELISA (cELISA) was developed and a five-year coordinated research project was conducted by the FAO/IAEA Joint Division to validate the test in the field. This study was completed in 2003. The strengths of this technique were its ability to detect infected animals over a longer period of the infectious cycle than the CFT, its ease of use and the provision of internal quality control systems. However, it did not consistently detect animals at the early stages of infection as did the CFT. It exhibited a relative sensitivity of 73 percent compared to the CFT and a relative specificity of about 98 percent. Based on these data, the test has been recommended to the OIE for inclusion in the prescribed tests for CBPP diagnosis. It was surmised that no single diagnostic method was suitable for definitive serological diagnosis of CBPP but CFT and cELISA could be conducted in tandem to increase the overall performance of serological diagnosis. CFT followed by the immunoblotting test (IBT) was used consistently in Portugal during its CBPP eradication campaign. The IBT was capable of confirming the results of CFT and distinguishing false positive results that were sometimes obtained with CFT. It also remained positive over a longer period of infection than the CFT. The control measures of zoning after serological prevalence studies, abattoir surveillance and movement control coupled with CFT, mycoplasma isolation, histopathology, and the polymerase chain reaction (PCR) test were adopted in the effort that eventually eradicated CBPP from Portugal, culminating in the country's declaration of freedom from CBPP by the OIE in 2003.
In Africa, where veterinary services have weakened, and field and laboratory resources have diminished, conventional basic epidemiological parameters such as prevalence that are essential for zoning are rarely available. Therefore, new techniques that draw on the local expert knowledge of livestock owners and handlers have been used to collect essential epidemiological data. These participatory methods have been used by PACE/CAPE to gauge the extent of CBPP, to map the extent of livestock movements and assess the impact of the disease within a community. Unlike conventional methods, participatory epidemiology (PE) is a comparative, proportional approach within which random sampling, standardization and error checking can be used. It is also “an insider's view” where private and sensitive information can be gathered that considers social factors while obtaining ground truth information.
The main mandate of PACE is policy development and to that end, it requires accurate epidemiological data, impact assessments and a deeper understanding of the tools available for CBPP control. Activities to generate these data were outlined over the PACE projects' lifespan that may come to an end in October 2004. The overall aim was to persuade the authorities to adopt regionally integrated policies of control and surveillance in the management of livestock diseases. Interim policies and the draft policy document were mentioned, but better impact measurement studies were required to secure their basis. The alternative vaccination strategies offered were based on a public-private partnership of “elective” vaccination and effective antibiotic treatment, neither of which is officially endorsed. The feasibility of this strategy would require liberalization of vaccine availability for farmers, acceptance of antibiotic therapy and training of appropriate personnel. The principle of elective vaccination was strongly debated but no firm decisions were taken.
 The situation of CBPP in various regions and countries was presented in the session “Country-specific control strategies”. The presenters, Drs B. Seck (Central Veterinary Laboratory, Bamako, Mali), F. Fasanmi (Abuja, Nigeria), J. Sim„o (Luanda, Angola), O.†Huebchele (Central Veterinary Laboratory, Windhoek, Namibia), D. Bangoura (Director, Veterinary Services, Conakry, Guinea) and P. Mangani (Deputy Director, Department of Research and Specialist Services, Lusaka, Zambia), gave an updated insight into the extent of the disease in west and central Africa. It was apparent that in west, central and southern Africa, where extensive pastoral and nomadic livestock husbandry is practised and where north to south cattle trade movement is very important, CBPP has an adverse impact as shown by mortality and morbidity data. However, it was not possible to assess the full impact of the disease because many countries in this region did not offer consistent reports of CBPP outbreaks. A phased approach to CBPP control was suggested, but the most significant factor in the implementation of such a strategy was the presence of political will and commitment. Other factors such as a good preparatory phase and the opportunity to develop and maintain institutional capacity and coordination among regions were likewise considered important. Some countries such as Nigeria cited the lack of consistent funding and sustainability coupled with the lack of a policy framework as the major constraints. In Angola, it was impossible to construct a physical barrier between infected and non-infected areas as has been done south of the border in Namibia. Instead, well-defined policies, good vaccine and vaccination campaigns, political will and international support were deemed necessary for effective CBPP control. An alarming report of a new outbreak in the East Caprivi strip in Namibia north of the Botswana border exemplified the threat of CBPP to free areas in the SADC region. In Zambia, the incursion of CBPP from the west and its movement northwards into previously free areas were shown. Control efforts in Guinea included zonation, public awareness via the popular press, training through workshops and the provision of handbooks, and legislation to ensure the traceable identity of animals with government and international financial support in CBPP disease control.
The situation of CBPP in various regions and countries was presented in the session “Country-specific control strategies”. The presenters, Drs B. Seck (Central Veterinary Laboratory, Bamako, Mali), F. Fasanmi (Abuja, Nigeria), J. Sim„o (Luanda, Angola), O.†Huebchele (Central Veterinary Laboratory, Windhoek, Namibia), D. Bangoura (Director, Veterinary Services, Conakry, Guinea) and P. Mangani (Deputy Director, Department of Research and Specialist Services, Lusaka, Zambia), gave an updated insight into the extent of the disease in west and central Africa. It was apparent that in west, central and southern Africa, where extensive pastoral and nomadic livestock husbandry is practised and where north to south cattle trade movement is very important, CBPP has an adverse impact as shown by mortality and morbidity data. However, it was not possible to assess the full impact of the disease because many countries in this region did not offer consistent reports of CBPP outbreaks. A phased approach to CBPP control was suggested, but the most significant factor in the implementation of such a strategy was the presence of political will and commitment. Other factors such as a good preparatory phase and the opportunity to develop and maintain institutional capacity and coordination among regions were likewise considered important. Some countries such as Nigeria cited the lack of consistent funding and sustainability coupled with the lack of a policy framework as the major constraints. In Angola, it was impossible to construct a physical barrier between infected and non-infected areas as has been done south of the border in Namibia. Instead, well-defined policies, good vaccine and vaccination campaigns, political will and international support were deemed necessary for effective CBPP control. An alarming report of a new outbreak in the East Caprivi strip in Namibia north of the Botswana border exemplified the threat of CBPP to free areas in the SADC region. In Zambia, the incursion of CBPP from the west and its movement northwards into previously free areas were shown. Control efforts in Guinea included zonation, public awareness via the popular press, training through workshops and the provision of handbooks, and legislation to ensure the traceable identity of animals with government and international financial support in CBPP disease control.
Summary of recommendations
The recommendations of the meetings were to gather data to conduct impact, cost-benefit and prevalence studies in order to formulate strategic and progressive control methodologies that were regionally targeted |
Three working groups to consider control strategies, tools for CBPP control, vaccines, and the use of antibiotics and diagnostic tests were formed to consider the current status and provide workable solutions that would aid CBPP control in Africa. The recommendations of the meetings were to gather data to conduct impact, cost-benefit and prevalence studies in order to formulate strategic and progressive control methodologies that were regionally targeted. The use of PE and modelling were seen as useful tools to this effect. Quality assured vaccines with improved formulations and improved thermal stability and the use of antibiotics that were clinically proven to produce bacteriological sterility from MmmSC were identified as the main requirements for CBPP therapeutic control. It was noted that combined therapy with these could be a useful regimen for this purpose. The Pan African Veterinary Vaccine Centre (PANVAC) should be re-established and maintained by AU/IBAR for vaccine production and quality assurance. Continuing research efforts into the basic pathobiology of CBPP with a view towards vaccine improvement and robust diagnostic tests that were capable of distinguishing between vaccinated and field-acquired immunity or disease were required. However, the current serological tests of CFT and cELISA were deemed adequate for herd diagnoses and epidemiological studies. Thus, laboratory capacity building for these and other diagnostic tests such as mycoplasma identification by culture and the confirmatory IBT were also required.
There is a wealth of knowledge available on CBPP in the scientific literature and increasingly in Web-based information packages (links to some of these may be found on the FAO EMPRES Web site (http://www.fao.org/ag/AGA/AGAH/EMPRES/index.asp).
After this very successful consultative group meeting that assembled knowledge from wide areas of regulatory, field, scientific and technical disciplines, consensus was obtained that CBPP was difficult to control in Africa and innovative approaches may eventually be necessary to control the disease. The recommendation of establishing pilot projects to evaluate alternatives was made.
ALAIN PROVOST The second consultative group meeting on CBPP was held in Rome from 24 to 26 October 2000. Dr Alain Provost, then representing the OIE, was the session Chair for the meeting. Sadly, Dr Provost, affectionately referred to as the “Pope” of CBPP by his colleagues and peers, passed away on 24 November 2002. In a brief citation before the posthumous award of a silver medal of recognition from FAO in honour of Dr Provost, Dr Yves Cheneau acknowledged his scientific achievements and contributions to the control of CBPP in Africa. He said that “Dr Alain Provost's research work on CBPP will forever remain part of the foundation of fundamental research in the control of CBPP in the world, particularly his scientific contributions in the development of current CBPP vaccines”. |