This section provides a detailed explanation of the process for the addition of chemicals to Annex III of the Convention. The roles and responsibilities of the DNA(s) at the national level, as well as the Secretariat, Chemical Review Committee (CRC) and the Conference of the Parties (COP) and the various steps of the process are described. Figure 1 (page 11) provides a schematic diagram of the process and a simple summary of the different steps.
The information exchange provisions of the Convention apply broadly to any chemical that has been banned or severely restricted to protect human health or the environment by a Party, or to severely hazardous pesticide formulations causing problems under the conditions of use in developing a developing country Party or Party with an economy in transition. The provisions of the prior informed consent (PIC) procedure apply only to those chemicals listed in Annex III of the Convention.
Article 2 defines the following terms for the purposes of the Convention.
”CHEMICAL” means a substance whether by itself or in a mixture or preparation and whether manufactured or obtained from nature but does not include a living organism. It consists of the following categories: pesticides (including severely hazardous pesticide formulations) and industrial chemicals.
“BANNED CHEMICAL” means a chemical all uses of which within one or more categories, have been prohibited by final regulatory action in order to protect human health or the environment. It includes a chemical that has been refused approval for first-time use or has been withdrawn by industry, either from the domestic market or from further consideration in the domestic approval process, and where there is clear evidence that such action has been taken in order to protect human health or the environment.
“SEVERELY RESTRICTED CHEMICAL” means a chemical virtually all use of which, within one or more categories, has been prohibited by final regulatory action in order to protect human health or the environment, but for which certain specific uses remain allowed. It includes a chemical that has, for virtually all use, been refused approval or been withdrawn by industry either from the domestic market or from further consideration in the domestic approval process, and where there is clear evidence that such action has been taken in order to protect human health or the environment.
“SEVERELY HAZARDOUS PESTICIDE FORMULATIONS” (SHPF) mean a chemical formulated for pesticide use that produces severe health or environmental effects observable within a short period of time after single or multiple exposure, under conditions of use.
It is important to note that once a chemical is included in Annex III of the Convention, the obligations and responsibilities under the Convention are the same for all Parties.
Further clarification of the scope of the Convention is provided in Article 3 which specifies the sort of chemicals excluded from the Convention. These include: narcotic drugs and psychotropic substances; radioactive materials; wastes; chemicals used in chemical weapons; human or veterinary drugs; food additives and food itself. For the most part these items are the subject of other international agreements. For example, the Codex Alimentarius covers food additives and residues of pesticides in food, while the Basel Convention covers wastes.
The Convention does not cover small quantities of pesticide or industrial chemicals that are not likely to affect human health or the environment, provided that they are intended to be used for research or analysis or by a person for their own personal use. These quantities are not defined in the Convention. Some countries in implementing the Convention have set a level of 10 kilograms whereas others have set lower amounts. Whatever the amounts countries elect to apply, it is important to recognize that these should be small amounts compared to commercially traded quantities.
There are two principal means through which new chemicals are identified for inclusion in Annex III of the Convention. These are:
(1) notification by Parties of final regulatory actions to ban or severely restrict a chemical for health or environmental concerns;
The obligations of Parties and the process for submissions and review of notifications of final regulatory actions are contained in Article 5 of the Convention. Annex I of the Convention details the information requirements and. Annex II the criteria to be considered by the chemical review committee (CRC) in reviewing candidate chemicals for inclusion in Annex III of the Convention.
(2) a proposal from a Party which is a developing country or a country with an economy in transition that is experiencing human health or environmental problems with a severely hazardous pesticide formulation (SHPF) under the conditions of use in its territory;
The obligations of Parties and the process for the submission of proposals for SHPFs are contained in Article 6 of the Convention. Parts 1 and 3 of Annex IV of the Convention detail respectively the relevant supporting information required and the criteria considered by the chemical review committee (CRC) in reviewing candidate formulations for inclusion in Annex III of the Convention.
What is a banned chemical or a severely restricted chemical?
The terms “banned chemical” and “severely restricted chemical” are defined in Article 2 of the Convention. Simply put:
a ban is where all uses of the chemical as either a pesticide or an industrial chemical are prohibited;
a severe restriction is where virtually all use of a chemical within one or more categories has been prohibited but for which certain uses are still permitted.
These definitions include situations where a chemical is prohibited for further use, where the chemical has been refused registration (or approval) for first time use, where industry withdraws its application for approval prior to the government making a final decision on the application or where it is withdrawn by industry from the domestic market. To qualify for notification under the Convention, these bans or severe restrictions must have been made for health or environmental reasons.
It is relatively easy to determine when a final regulatory action is a ban but is sometimes more difficult to determine when a final regulatory action is a severe restriction. Where some uses have been prohibited, judgement is needed as to whether this constitutes a prohibition of virtually all use. If all but one or two uses of an extensive range of uses have been prohibited and those remaining are relatively small uses, then this clearly constitutes a severe restriction. However, where all but one or two uses of an extensive range of uses have been prohibited and those remaining are major uses, then this may not constitute a severe restriction, particularly if the prohibited uses were all moderate to minor uses.
It is not uncommon for industry to withdraw applications for approval for uses or to withdraw already approved uses when it becomes apparent that there is a problem with these uses. In such circumstances, it may be difficult to determine whether this action has been taken because of commercial reasons or whether it is because industry is aware of the health or environmental concerns.
|
Obligations and the Process for Submission of Notifications of Final Regulatory Actions to ban or severely restrict Chemicals Obligations under Article 5 of the Convention Under Article 5 of the Convention Parties have the following obligations with respect to notifying the Secretariat of their final regulatory actions to ban or severely restrict a chemical for health or environmental reasons.
|
Some countries may ban or severely restrict a chemical included in Annex III of the Convention. Parties are still obliged to submit notifications of these regulatory actions. One reason for this is because the basis for a country’s regulatory action on a chemical may not be the same as that which led to the inclusion of the chemical in Annex III of the Convention. For example:
the original reason for inclusion may have been related to environmental concerns whereas the more recent regulatory action to ban may be based on human health concerns;
certain severely hazardous formulations of a chemical may be included in the Convention. Subsequent regulatory actions by Parties may, for example, ban all formulations of the pesticide. This could lead to a broader scope of the chemical subject to the Convention (e.g. monocrotophos);
if the chemical had uses as both an industrial chemical and a pesticide then it may have been listed for one category and the new regulatory action applies to the other category.
Process for submission of a notification of a final regulatory action
As set out in Article 5 of the Convention notifications of final regulatory actions must contain the information specified in Annex I of the Convention, where it is available. The notification should describe the scope of the regulatory action inter-alia the categories and/or uses to which the action applies, the chemical concerned and details of the regulatory decision. The notification should also include the reason a decision was taken and whether it was based upon a risk or hazard evaluation.
To facilitate the preparation and submission of these notifications of final regulatory action a detailed form has been developed that meets the information requirements of Annex I of the Convention. Copies of the Notification of Final Regulatory Action form and instructions on how to complete the form are included in Annex 7.5.1 of this guide or can be downloaded from the Rotterdam Convention web site (www.pic.int).
What is a Severely Hazardous Pesticide Formulation (SHPF)
The term “severely hazardous pesticide formulations” is defined in Article 2 of the Convention. Simply put they are formulations that cause problems (severe health or environmental effects observable within a short period of time after single or multiple exposures) under the conditions of use in developing countries or countries with economies in transition.
This provisions in Article 6 were included in the Convention in recognition that in some developing countries and countries with economies in transition the conditions are such that certain pesticide formulations cannot be used safely. These same formulations may be used safely in developed countries and as a result are not subject to regulatory actions to ban or severely restrict their use and thus would not be identified as candidates for inclusion under Article 5 of the Convention.
|
Obligations and the Process for the Submission of Proposals for a Severely Hazardous Pesticide Formulation (SHPF) Obligations under Article 6 of the Convention Under Article 6, any Party that is a developing country or country with an economy in transition that is experiencing problems caused by a SHPF, either due to human health or environmental problems in its territory, may propose to the Secretariat the inclusion of the formulation in the Convention. The proposals must contain the information specified in Part 1 of Annex IV of the Convention and be submitted by the DNA of that country to the secretariat. In preparing such proposals, the DNA may draw upon technical expertise from any relevant source. |
Process for the submission of a Proposal for a SHPF
To facilitate the development and submission of proposals in support of severely hazardous pesticide formulations two incident report forms have been developed, one for environmental incidents and a second for human health incidents.
These forms consist of two parts, Part A and Part B. Part A (Transmittal Form) is to be used by the DNA to transmit an incident report form to the Secretariat. Part B (Pesticide Incident Report Form) has been developed to meet the information requirements of Part 1 of Annex IV of the Convention. It is intended to provide a clear description of the incidents related to the use of the pesticide formulation, any adverse effects and the way in which the formulation was used. The use of these forms is purely voluntary; other forms/formats used in a country for collecting pesticide incident reports may be used to replace Part B provided that those submissions meet the information requirements of Part 1 of Annex IV of the Convention.
Copies of the incident report forms and instructions on how to complete them are included in Annexes 7.5.3 and 7.5.4 of this guide or can be downloaded from the Rotterdam Convention web site (www.pic.int).
Role of the Secretariat
Verification of Notifications of Final Regulatory Action by the Secretariat
When the Secretariat has received a notification for a final regulatory action to ban or severely restrict a chemical, it must verify that the notification meets the information requirements of Annex I of the Convention. The Secretariat completes this review with the assistance of a detailed checklist. If the notification meets the information requirements, a draft summary is prepared. The notifying country is informed that their notification was complete and is invited to review the draft summary. The summaries of the verified notifications are published in Appendix I of the PIC Circular within six months of their being received.
If a notification does not meet the information requirements of Annex I of the Convention, the Secretariat sends a letter to the DNA of the notifying country with a completed checklist detailing the missing information. The DNA is invited to submit the missing information so that the notification might be verified as complete and a summary prepared for publication in the PIC Circular.
Once the Secretariat has received two notifications for the same chemical verified as complete from at least two PIC Regions, it requests the notifying countries to submit the supporting documentation referenced in their notification. The notification of final regulatory action and the supporting documentation are forwarded to the CRC for consideration.
Verification of Proposals for SHPFs
When the Secretariat receives a proposal for a SHPF, it verifies that it includes the information specified in Part 1 of Annex IV of the Convention. If the submitted proposal meets the information requirements a draft summary is prepared by the Secretariat. The proposing country is informed that their proposal was complete and invited to review the draft summary. The summaries of the verified proposals are published in Appendix II of the PIC Circular within six months of their being received.
At the same time, the Secretariat initiates collection of relevant information relating to the formulation as set out in Part 2 of Annex IV of the Convention. This includes information from other Parties, international organizations, non-governmental organizations or other relevant sources on handling restrictions or incidents related to the formulation and other formulations of the pesticide in question in other States, and risk or hazard evaluations. The proposal and the additional information collected by the Secretariat are forwarded to the CRC for consideration.
It is important to note that unlike notifications of final regulatory actions a proposal from one Party is all that is required to initiate review of a SHPF by the CRC.
Role of the Chemical Review Committee (CRC)
After a proposal or notification and relevant supporting data are submitted to the CRC for their consideration, the process for the addition of a chemical to the Convention is the same. The primary difference remaining in the operation of the Committee is the criteria used to assess the different submissions. For a banned or severely restricted chemical the relevant criteria are contained in Annex II, whereas the criteria for a severely hazardous pesticide formulations are contained in Part 3, Annex IV of the Convention.
|
Banned or severely restricted (BSR) chemicals In reviewing notifications for BSR chemicals, the CRC establishes that:
|
|
Severely Hazardous Pesticide Formulations In reviewing the proposal for a SHPF, the CRC considers whether:
For both BSR chemicals and SHPFs, the Convention states that intentional misuse (for example, deliberate ingestion as a means of suicide) does not constitute adequate reason to list a chemical In Annex III. |
FIGURE 1. PROCESS FOR ADDING CHEMICALS TO THE ANNEX III OF THE CONVENTION
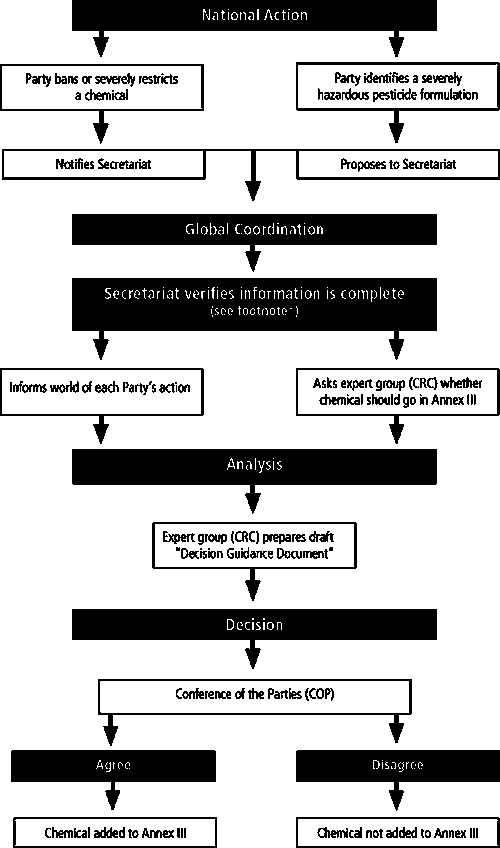
* To start the process – it requires one party to propose a severely hazardous pesticide formulation; two parties from two PIC regions to ban or severely restrict a chemical
If the CRC considers that the information in support of a BSR chemical or a SHPF meets the relevant information requirements and criteria set out in the Convention, it will recommend the inclusion of the chemical in Annex III of the Convention to the COP and initiate preparation of a draft DGD (for the process on developing a DGD see section 3).
Role of the Conference of the Parties (COP)
In line with Article 7 of the Convention, the Conference of the Parties (COP) will decide whether or not to include a chemical in Annex III of the Convention and, if so, to approve the draft DGD. Once a decision to include a chemical in Annex III of the Convention is made, the Secretariat will circulate the decision and the approved DGD to all Parties with a request that they provide a decision on future imports of the chemical.
Article 9 of the Convention addresses the removal of chemicals from Annex III of the Convention. A chemical may be considered for removal if a Party submits information which was not available when a decision was made to include a chemical in the Convention and that information indicates that the basis for inclusion of the chemical is no longer in accordance with the relevant criteria. The Secretariat will forward the information to the CRC, which will review the information according to the relevant criteria. For each chemical that the CRC decides to recommend to the COP for removal, it shall prepare a revised draft DGD. The COP then will decide upon the recommendation. If the COP decides to remove the chemical from Annex III of the Convention and approve the revised DGD, explaining why this chemical has been removed, the Secretariat will circulate this information to all Parties.