The DSP toxins are all heat-stable polyether and lipophilic compounds isolated from various species of shellfish and dinoflagellates (Draisci et al., 1996a) (see Figure 3.1 and 3.2). Although diarrhoea is the most characteristic symptom of intoxication, several other effects may be of relevance and some of the toxins in the DSP complex (PTXs and YTXs) do not yield diarrhoea at all (Van Egmond et al., 1993). Re-evaluation of their toxicity will probably lead to these toxins being removed from their classification as DSP toxins (Quilliam, 1998a). The different chemical types of toxins associated with the DSP syndrome comprise:
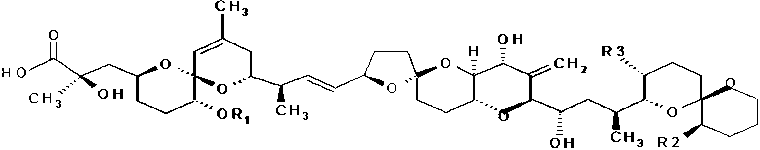
a) The first group, acidic toxins, includes okadaic acid (OA) and its derivatives named dinophysistoxins (DTXs). OA and its derivatives (DTX1, DTX2 and DTX3) are lipophilic and accumulate in the fatty tissue of shellfish. These compounds are potent phosphatase inhibitors and this property is linked to inflammation of the intestinal tract and diarrhoea in humans (Van Apeldoorn et al., 1998; Hallegraeff et al., 1995). OA and DTX1 are also tumour promoters in animal test systems (Draisci et al., 1996a; Van Egmond et al., 1993). DTX1 was first detected in Dinophysis fortii in Japan; DTX2 was identified in shellfish in Ireland during a DSP episode (Van Egmond et al., 1993). DTX2 was isolated also from a marine phytoplankton biomass mainly consisting of Dinophysis acuta (James et al., 1999). A new isomer of DTX2, named DTX2B, was isolated and identified in Irish mussel extracts (James et al., 1997). DTX3 originally described a group of DSP toxin derivatives in which saturated or unsaturated fatty acyl groups are attached to the 7-OH group of DTX1. More recently it has been shown that any of the parent toxins, OA, DTX1 and DTX2, can be acylated with a range of saturated and unsaturated fatty acids from C14 to C18 (Hallegraeff et al., 1995; Wright, 1995). In a report of an EU meeting it was stated that chain length of the fatty acid can vary from C14 to C22 and that the number of unsaturation is varying from 0 to 6. The most predominantly fatty acid in DTX3 was palmitoyl acid (EU/SANCO, 2001). These acylated compounds also possess toxic activity. Since these compounds have only been detected in the digestive gland of contaminated shellfish, it has been suggested that they are probably metabolic products and not de novo products of toxin producing micro algae (Wright, 1995). Suzuki et al. (1999) demonstrated the transformation of DTX1 to 7-O-acyl-DTX1 (DTX3) in the scallop Patinopecten yessoensis. The ester bond in the acylated compounds can be hydrolyzed by heating in 0.5 M NaOH/90 percent methanol solution at 75 °C for 40 minutes. The ester bond in DTX3 was also easily hydrolyzed by lipase and cholesterol esterase (EU/SANCO, 2001).
Two naturally occurring ester derivatives called diol esters were isolated from some Prorocentrum species. These diol esters did not inhibit phosphatase in vitro. However, it should be noted that these allylic diol esters may be somewhat labile and could be hydrolysed to yield the active parent DSP toxin (Hallegraeff et al., 1995). Draisci et al (1998) reported the detection of another OA isomer and called it DTX2C. The structure of DTX2C is not yet elucidated. The compound was isolated from D. acuta collected in Irish waters.
b) The second group, neutral toxins, consists of polyether-lactones of the pectenotoxin group (PTXs). Ten PTXs have been isolated until now and six out of these have been chemically identified; PTX1, -2, -3, -4, -6 and -7. Since PTX2 (PTX2,CH3) is found in phytoplankton only (D. fortii in Japan and Europe) and never in shellfish, it is suggested that an oxidation occurs in the hepatopancreas of shellfish producing other PTXs (PTX1, CH2OH; PTX3, CHO; PTX6, COOH) (Draisci et al., 1996a; Yasumoto et al., 2001 and cited from Van Apeldoorn et al., 1998). Suzuki et al. (1998) demonstrated oxidation of PTX2 to PTX6 in scallops (Patinopecten yessoensis). Sasaki et al. (1998) identified PTX4 and PTX7 as spiroketal isomers of PTX1 and PTX6, namely epi-PTX1 and epi-PTX6, respectively. Two new artefacts, PTX8 and PTX9, were also isolated but their structures are not yet elucidated. Daiguji et al. (1998) isolated two new pectenotoxins from the greenshell mussel Perna canalicus from New Zealand and from D. acuta from Ireland and elucidated the structures as pectenotoxin-2-seco acid (PTX2SA) and 7-epi-pectenotoxin-2 seco acid (7-epi-PTX2SA), respectively. Suzuki et al. (2001) demonstrated that PTX2SA and 7-epi-PTX2SA (the most important PTX homologues in the New Zealand mussel Perna canaliculus) are converted from PTX2 by these mussels. This transformation is also expected to occur in the blue mussel Mytilus galloprovincialis as PTX2SA is also the predominant PTX homologue in this mussel species.
c) The third group includes a sulphated compound called yessotoxin (YTX), a brevetoxin-type polyether, and its derivative 45-hydroxyyessotoxin (45-OH-YTX) (Draisci et al., 1996a; Van Egmond et al., 1993). Yessotoxin was first isolated from the digestive organs from scallops (Patinopecten yessoensis) in Japan (Ciminiello et al., 1999) and is believed to be produced by microalgae. The yessotoxins do not cause diarrhoea. Yessotoxin attacks the cardiac muscle in mice after i.p. injection, while desulphated yessotoxin damages the liver (Van Egmond et al., 1993). In the digestive gland of Adriatic mussels (Mytilus galloprovincialis) besides yessotoxin, two new analogues of yessotoxin, homoyessotoxin and 45-hydroxyhomoyessotoxin were identified by Ciminiello et al. (1997; 1999). Tubaro et al. (1998) also detected homoyessotoxin in M. galloprovincialis from the Adriatic sea during a bloom of Gonyaulax polyhedra (=Lingulodinium polyedrum). Satake et al. (1997) and Satake et al. (1999) isolated YTX and 45,46,47-trinoryessotoxin from cultured cells of the marine dinoflagellate Protoceratium reticulatum. The production of yessotoxins by P. reticulatum differed from strain to strain. Ciminiello et al. (1998) detected again a new analogue of YTX, adriatoxin (ATX), in the digestive glands of DSP infested Adriatic mussels collected in 1997 along the Italian coast (Emilia Romagna). In addition, four further analogues of yessotoxin, carboxyyessotoxin (COOH group on C44 of YTX instead of double bond), carboxyhomoyessotoxin (COOH group on C44 of homoYTX instead of double bond) (Ciminiello et al., 2000a; 2000b), 42,43,44,45,46,47,55-heptanor-41-oxo YTX and 42,43,44,45,46,47,55-heptanor-41-oxohomo YTX (Ciminiello et al., 2001; 2002) in Adriatic mussels (M. galloprovincialis) were identified.
d) Unexplained human intoxication, with DSP symptoms, following the consumption of mussels from Killary, Ireland in 1995 was resolved by the isolation of a new toxin (C47H71NO12), tentatively named Killary Toxin-3 or KT3 (Satake et al., 1998a). This toxin was later called azaspiracid (see Chapter 6).
Figure 3.1 Chemical structures of okadaic acid, dinophysistoxins and pectenotoxins
|
|
R1 |
R2 |
R3 |
|
okadaic acid (OA) |
H |
H |
CH 3 |
|
dinophysistoxin-1 (DTX1) |
H |
CH 3 |
CH 3 |
|
dinophysistoxin-2 (DTX2) |
H |
CH 3 |
H |
|
dinophysistoxin-3 (DTX3) |
acyl |
CH 3 |
CH 3 |
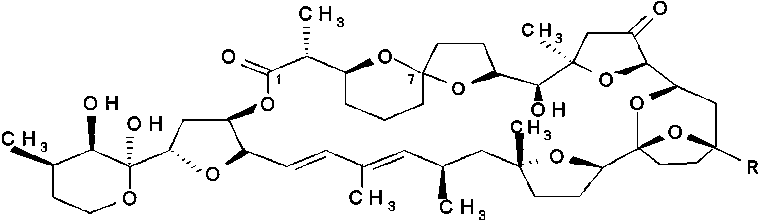
|
|
|
R |
C-7 |
|
pectenotoxin-1 |
(PTX1) |
CH 2OH |
R |
|
pectenotoxin-2 |
(PTX2) |
CH 3 |
R |
|
pectenotoxin-3 |
(PTX3) |
CHO |
R |
|
pectenotoxin-4 |
(PTX4) |
CH 2OH |
S |
|
pectenotoxin-6 |
(PTX6) |
COOH |
R |
|
pectenotoxin-7 |
(PTX7) |
COOH |
S |
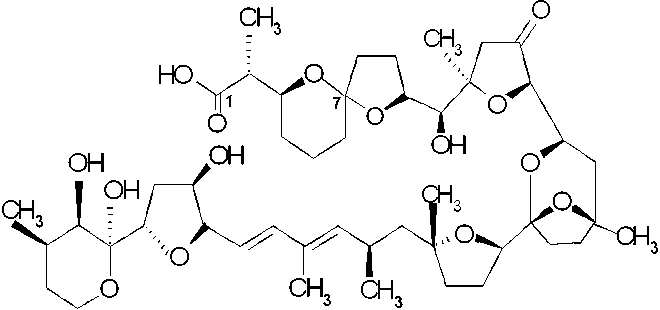
|
|
C-7 |
|
pectenotoxin-2 seco acid (PTX2SA) |
R |
|
7-epi-PTX2SA |
S |
Source: Yasumoto et al., 2001
Figure 3.2 Chemical structures of yessotoxins and adriatoxin
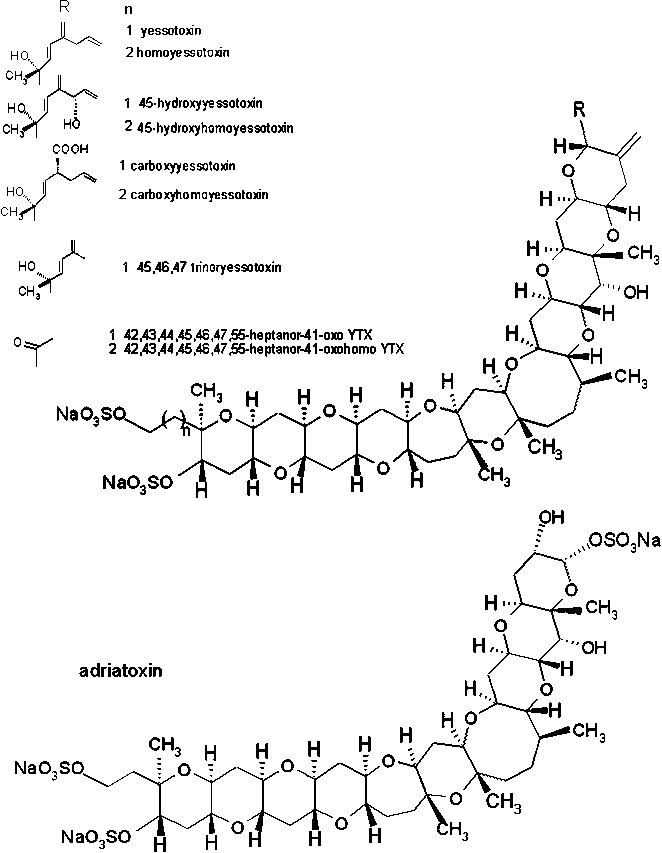
Source: Ciminiello et al., 1998; 2002 and Yasumoto et al., 2001