In November 1995, at least eight people in the Netherlands became ill after eating mussels (Mytilus edulis) cultivated at Killary Harbour, Ireland. Although the symptoms resembled those of diarrhoeic shellfish poisoning (DSP), concentrations of the major DSP toxins were very low (McMahon and Silke, 1996; Satake et al., 1998a). The known organisms producing DSP toxins were not observed in water samples collected at that time. In addition, a slowly progressing paralysis was observed in the mouse assay using the mussel extracts. These neurotoxic symptoms were quite different from typical DSP toxicity (Satake et al., 1998a). It was then that azaspiracid (formerly called Killary Toxin-3 or KT3) was identified and the new toxic syndrome was called azaspiracid poisoning (AZP).
Satake et al. (1998b) elucidated the structure of azaspiracid after human intoxication due to the consumption of contaminated Irish mussels. Azaspiracid was extracted from contaminated whole mussel meat and appeared to be a colourless amorphous solid with no UV absorption maxima above 210 nm. In addition to azaspiracid (AZA), four analogues, AZA 2 to AZA5, were isolated and their chemical structures were elucidated (see Figure 6.1). Ofuji et al. (1999a) identified azaspiracid-2 (AZA-2) and azaspiracid-3 (AZA-3) and demonstrated that these compounds were 8-methylazaspiracid and 22-demethylazaspiracid, respectively. Ofuji et al. (2001) determined the structure of two further analogues of azaspiracid found in mussels namely azaspiracid-4 (AZA-4) and azaspiracid-5 (AZA-5) and showed that these compounds were 3-hydroxy-22-demethylazaspiracid and 23-hydroxy-22-demethylazaspiracid, respectively (thus hydroxylated analogues of AZA-3). No experimental data exist at present as to how and whether the toxins undergo structural modification in shellfish. By analogy with pectenotoxins and yessotoxins, that undergo structural modification by hydroxylation in mussels, it may be assumed that AZA-4 and AZA-5 are oxidized metabolites of AZA-3. Hence, AZA, AZA-2 and to AZA-3 are likely to be the genuine products of a causative marine organism. Azaspiracid was considered as the major causative agent (Satake et al, 1998b).
Azaspiracids differ from any of the previously known nitrogen-containing toxins found in shellfish or dinoflagellates (e.g. prorocentrolide, pinnatoxin, gymnodimine and the spirolides). Azaspiracids (AZAs) have unique spiro ring assemblies, a cyclic amine instead of a cyclic imine group and a carbocyclic or lactone ring is absent (Satake et al., 1998b).
Dounay and Forsyth (2001) performed synthetic studies toward the C5 - C20 domain of the azaspiracids to identify these sub-fragments of the azaspiracid.
Satake et al. (1998b) reported that mussel extracts did not show a significant decrease of toxicity when the extract was heated at 5 oC for 150 minutes in 1.0 N acetic acid/methanol or 1.0 N ammonium hydroxide solution and no significant change in toxicity occurred in solution during storage. Therefore AZAs are assumed to be relatively stable compounds.
Figure 6.1 Chemical structures of azaspiracids
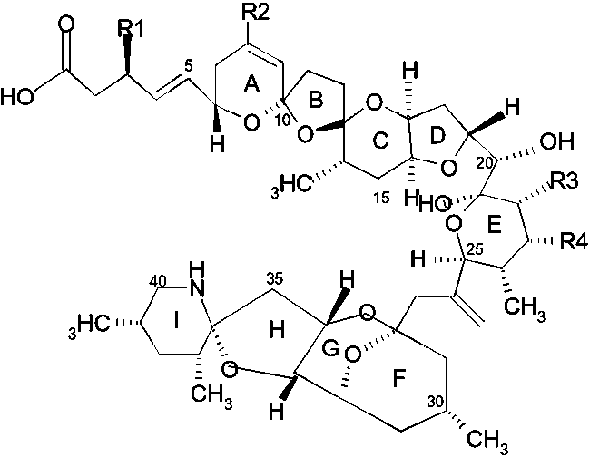
|
|
R1 |
R2 |
R3 |
R4 |
|
azaspiracid (AZA) |
H |
H |
CH3 |
H |
|
azaspiracid-2 (AZA2) |
H |
CH3 |
CH3 |
H |
|
azaspiracid-3 (AZA3) |
H |
H |
H |
H |
|
azaspiracid-4 (AZA4) |
OH |
H |
H |
H |
|
azaspiracid-5 (AZA5) |
H |
H |
H |
OH |
6.2.1 In general
Attention should be paid to the possible co-occurrence in mussels of OAs, PTXs and YTXs. Coexistence of these toxins with AZA was noticed in mussels collected in Norway (Yasumoto and Aune, unpublished data in EU/SANCO, 2001).
Therefore it is recommended to test for these toxins by LC or LC/MS. During AZP outbreaks the occurrence of unknown toxin(s) was noted in mussels, although at low levels (about 12 percent of total toxicity). Mice injected with this unknown toxin showed immediately after injection agitation, paralysis and PSP-like convulsions before death. Besides these symptoms in mice, the unknown toxin was distinct from AZP toxins in chromatographic properties (probably the toxin has no acidic moiety). Mice injected with this toxin died within 30 minutes. In surviving mice recovery was quick. No data are available at present as to whether the unknown toxin(s) reach to a level high enough to interfere with the results of the mouse bioassay (EU/SANCO, 2001).
6.2.2 Bioassays
in vivo studies
mouse bioassay
Mussel extracts are injected intraperitoneally in mice like for the DSP mouse bioassay is done. The results suggest that azaspiracids can be extracted with acetone from raw meat due to increased solubility by the presence of water and lipids in the meat (EU/SANCO, 2001). The azaspiracid response is characterized by hopping, scratching and progressing paralysis which is atypical for DSP (Flanagan et al., 2000; Satake et al., 1998a). The shortest time for mouse death was 35 minutes (at six times of the lethal dose) and the longest was 30 hours and 46 minutes (EU/SANCO, 2001). Usually, polyether toxins are concentrated in the digestive glands of shellfish, but this is not always the situation with azaspiracids. Azaspiracid and its analogues, AZA2 and AZA3, are distributed throughout shellfish tissues. Using conventional DSP mouse bioassay protocols (in which only the hepatopancreas is used for testing), only zero to 40 percent of the total azaspiracid content of the shellfish is measured, which can directly account for false-negative results (James et al., 2002a).
rat bioassay
This assay is based on diarrhoea induction in rats. The (starved) animals are fed with suspect shellfish tissue (mixed into the diet) and observed during 16 hours for signs of diarrhoea, consistency of the faeces and food refusal. The method is at best semi-quantitative (Hallegraeff et al., 1995 and Van Egmond et al., 1993). The test is still used routinely in the Netherlands and is an officially allowed procedure in EU legislation.
in general
The European Commission has recognised the needs of the analytical community to develop methods alternative to animal testing. A relevant call for proposals in the Commission’s Sixth Framework Programme in Area 5: “Food Quality and Safety” is expected (EC, 2003), in which one of the objectives is to develop cost-effective tools for analysis and detection of hazards associated with seafood from coastal waters including also Azaspiracid Shellfish Poisons. If granted, this will mean that progress can be expected in the coming years.
in vitro studies
mammalian cell culture assay
The development of alternative diagnostic strategies for the detection of phycotoxin contamination in shellfish is driven by scientific, ethical and financial concerns. To address this, an assay has been developed based upon the cytopathological responses of cultured mammalian cells to phycotoxins. The primary response of these cells to any okadaic acid family of toxins is to “round-up” and lose their distinctive morphology, within three hours, yet they remain about 90 percent viable for up to 48 hours. Azaspiracid positive samples, when applied to this system do not cause the “rounding up” effect on cultured cells. Instead the cellular viability, as measured by an MTT assay, drops to less than 10 percent of the viability of control cells after 18 to 24 hours. Combination of cell morphology observation at three hours with 24-hour viability measurement enables the detection of both okadaic acid type toxins and azaspiracid in shellfish (Flanagan et al., 2000; 2001).
6.2.3 Chemical assays
mass spectrometry
The first LC-MS quantitative determination method reported for azaspiracids was based on selected ion monitoring (SIM) detection (Ofuji et al., 1999b), with one ion per compound and external calibration. Linearity was checked over a relatively wide concentration range (50 pg to 100 ng). The recovery data seemed correct, but it remained unclear how many different samples formed the basis for the recovery experiment(s). As only one ion per compound was monitored, no check on specificity was possible with ion intensity ratio. In short, the analytical basis of the method is not strong, which makes questionable its applicability in practice.
The start for application of LC-MSn methods for AZAs - as published later on - was presented by James et al. (2001). A micro liquid chromatography-tandem mass spectrometry method (micro-LC-MS-MS) was developed for the determination of azaspiracids (Draisci et al., 2000). The method reported focused on the identification of azaspiracids, so in fact it had a qualitative accent. Eventually the aim was formulated as “...to investigate the suitability of LC-MS and LC-MS-MS in order to unambiguously detect azaspiracid in shellfish.” By applying selected ion monitoring (SIM) on the ions corresponding to the protonated molecules only, the most sensitive form of detection was obtained (maximum intensities). Good sensitivity (defined as low detection limit) was also obtained by applying micro-LC (1.0 mm I.D. column), which is effective because Electrospray-MS is a concentration dependent detector. In this study a triple quadrupole MS (tripleQ) was used. In the area of marine biotoxins this instrument is more commonly used than the ion trap MS. Structural information was obtained by using the CID-MS-MS capabilities of the tripleQ, first in full scan mode to find appropriate daughter ions which could afterwards be used in SRM-mode. The optimized SRM mode resulted also in quantitative data. Good linearity (r2>0.995) was observed for a small concentration range (0.1-1 µg/ml), while the detection limit was approximately 20 ng of azaspiracid per gram of whole mussel. In conclusion, the developed method provided very selective and specific data. However, as stated by the authors, a “full validation was hampered by the lack of availability of the azaspiracid standard necessary for recovery experiments”.
Lehane et al. (2002) reported the development of an LC-ESI-MSn method for the determination of the three most prevalent AZA toxins (AZA1-3), as well as the isometric hydroxylated analogues (AZA4-5). They demonstrated that LC-multiple tandem MS resulted in more sensitive analysis than LC-single-MS, which suggests “.. that the reduction in background noise in MSn is more dramatic than the decline in analyte signal.” Notable is their use of WideBand activation, which allows them to reduce total elution time aiming at the determination of the five azaspiracids. Although the authors state to have developed a method that requires minimal sample preparation steps, total sample preparation will most probably require the major part of total analysis time.
Next to the article just mentioned, the same research group reported a comparison of solid-phase extraction methods for the determination of azaspiracids in shellfish by the LC-ESI-MSn method of Lehane (Moroney et al., 2002). Good recovery and reproducibility data were obtained for one diol SPE cartridge and two C18 SPE cartridge types. As they state: “...the efficient SPE methods presented here for sample preparation should prove more useful in the development of alternative analytical methods for AZP toxins in shellfish.” This fits well to their earlier statement: “Sample preparation for the determination of phycotoxins in shellfish can be problematic due, in part, to an extensive variation in the toxic content.”
The same group reported the same method development in a different journal (Furey et al., 2002) “with the primary objective to produce a protocol that could be used for the regulatory control of azaspiracids in shellfish”. Especially their extensive linearity studies for the determination in shellfish extracts are worth mentioning: rather good results were obtained for a concentration range over two decades. The data look convincing that regulatory control can be conducted with the methods reported. An application based on the just mentioned method was reported by the same group (James et al., 2002a; 2002b). The report shows LC-MS3 spectra of AZA1-3 both as standards and as analytes in mussel extracts.
Since 1996, several AZP incidents have been identified in Ireland. In November 1997, cases of contamination recurred in the Avianmore Island region of Donegal, Northwest Ireland and caused human intoxication repeatedly (McMahon and Silke, 1998), also in other European countries (mainly by mussels cultivated in Ireland). The ultimate origin of azaspiracids is probably a dinoflagellate because of the highly oxygenated polyether structure and seasonal occurrence. However, none of the known toxic phytoplankton species was observed in water samples collected at the time of the intoxication (James et al., 2000b; Satake et al., 1998b). Recent information (Peperzak et al., 2002) suggests that Protoceratum crassipes is the AZP producing dinoflagellate. McMahon (2000) reported that an organism belonging to the genus Protoperidinium has been suggested as the source organism.
6.4.1 Uptake and elimination of AZP toxins in aquatic organisms
Typically, polyether toxins are concentrated in the digestive glands of shellfish but this is not always the situation with azaspiracids. Azaspiracid and its methyl- and demethyl-analogues, AZA2 and AZA3, respectively, are not confined to the hepatopancreas but are also distributed throughout shellfish tissues. The toxin profiles differed significantly in various mussel tissues with AZA as the predominant toxin in the digestive glands and AZA3 and an isomer of AZA predominant in the remaining tissues. Mussel digestive glands initially contained most of the azaspiracids due to grazing on toxic dinoflagellates. However, the transportation of these toxins to other shellfish tissues is unpredictable but, if this occurs, a prolonged period of shellfish intoxication is likely due to a low rate of natural depuration. Azaspiracids show an unusual solvent distribution during the extraction process of these toxins. This leads to the speculation that the polar amino acid and the non-polar polyether regions of azaspiracid impart detergent properties to this molecule. The ease with which azaspiracids can move through different polarities probably plays a significant role in the increased penetration of these toxins in shellfish and mammalian tissues. There can be a significant variation in the total level of AZAs in mussels from different sites in the same cultivation region. See Table 6.1 (James et al., 2002a).
Table 6.1 Distribution of AZP toxins through mussel tissues
|
Site No. |
Meata (total AZAs) µg/100g |
HP (total AZAs) µg/100g |
Total AZAsµg/100g |
AZAs distribution (% meat/HP)b |
|
1 |
14 |
0 |
12 |
100/0 |
|
2 |
14 |
34 |
17 |
67/33 |
|
3 |
6 |
10 |
7 |
75/25 |
|
4 |
7 |
18 |
9 |
67/33 |
|
5 |
84 |
12 |
72 |
96/4 |
|
6 |
37 |
100 |
48 |
64/36 |
|
7 |
48 |
33 |
45 |
88/12 |
a mussel meat without hepatopancreas
b average weight is 4.8 g; HP was 15-18% of total mussel tissue
6.4.2 Shellfish containing AZP toxins
Mussels and oysters were found to contain AZP toxins (James et al., 2000b).
6.5.1 Mechanism of toxicity
No data
6.5.2 Pharmacokinetics
No data
6.5.3 Toxicity to laboratory animals
acute toxicity
oral studies
Acute oral studies with azaspiracid were performed in mice. Azaspiracid was extracted from mussels collected in Killary Harbour, Ireland in February 1996. During the course of toxin purification, the major toxin was concentrated in a lipid fraction coded KT3 (Ito et al., 2000). By oral administration (by gavage) of 60 µl of this KT3 fraction mice did not show any clinical changes during 24 hours. At autopsy after four hours, active secretion of fluid from the ileum and debris of necrotizing epithelial cells from upper portion of the villi were observed in the lumen (SEM) and after eight hours, erosion of the villi from the top resulted in the shortened villi, and prominent accumulation of fluid was observed accompanying edema in the lamina propria. Then after 24 hours, these changes were not observed but epithelial cells of adjacent villi were fused to each other (Ito et al., 1998).
Male ICR mice receiving orally by gavage a single dose of 500, 600 or 700 mg purified AZA/kg bw did not show any behavioural changes within four hours. The number of survivors after 24 hours were 0/2, 3/6 and 1/2 at 500 mg/kg bw (eight weeks old), 600 mg/kg bw (five weeks old) and 700 mg/kg bw (five weeks old) respectively. At 600 mg/kg bw and 700 mg/kg bw, diarrhoea and body weight decrease were observed within 24 hours. At single oral doses of 300 to 700 mg/kg bw, AZA caused dose-dependent changes in small intestines (necrotic atrophy in the lamina propria of the villi) and in lymphoid tissues such as thymus, spleen and Peyer’s patches. In the spleen the number of non-granulocytes was reduced and damage to both T and B lymphocytes occurred. Additionally liver weight increased, the colour of the liver changed from dark red to pinkish red and fatty changes in the liver were observed. AZA did not cause prominent changes in the stomach mucosa but the appearance of many degenerating cells was observed in the large intestine. The pancreas appeared to loose zymogen granules locally, but cells were not injured. Histopathological damage to other organs (kidney, heart, and lung) was not observed. The acute morphological changes in the mouse, induced by AZA, were distinctly different from those of okadaic acid (Ito et al., 2000).
In the latest experiments from Ito et al. (2002), a total of 18 four-week old mice, five six-week old mice and two five-month old mice were used to produce severe injuries and then to observe recovery. Four dose levels (250, 300, 350 and 450 µg AZA (more purified extract from blue mussels at Killary Harbour and Arranmore Island in Ireland)/kg bw (dissolved in 50 percent ethanol) were given orally to five groups. Ten mice that survived the initial treatment received a second treatment on day three. Nine mice that survived the second treatment were killed between day seven and 90 after treatment. Thirteen control mice were used. The highest dose of 450 µg/kg bw caused death in 11/16 treated (four-week old) mice. Two out of two six-week old mice and another two out of two five-month old mice, receiving 300 and 250 µg/kg bw, respectively, also died. Of ten mice that survived the first treatment, one died after the second treatment with 350 µg/kg bw. Slow recoveries were revealed after oral administration of 300, 350 and 450 mg/kg bw. Erosions and shortened villi in the stomach and the small intestine persisted for more than three months, edema, bleeding, and infiltration of cells in the alveolar wall of the lung for 56 days, fatty changes in the liver for 20 days and necrosis of lymphocytes in the thymus and spleen for 10 days. Thus, the lowest oral dose of 250 µg AZA/kg bw appeared to be lethal to mice in this study.
It has to be noted that the partially purified KT3 toxin caused much more severe intestinal fluid accumulation and histological damage to the pancreas (Ito et al., 1998) than the more purified toxin used in the studies of Ito et al. (2002). May be several unknown analogues of azaspiracid are present in the crude fraction. It should also be mentioned that the difference between the mouse lethality by oral and intraperitoneal administration was much less significant with azaspiracid than with other phycotoxins (Ito et al., 2000).
intraperitoneal studies
Mice exposed to AZA by intraperitoneal injection react differently than those exposed to other shellfish toxins. After i.p. dosing of the partially purified KT3 to male ddY mice, the animals became sluggish, sat still in the corners and showed progressive paralysis and laboured breathing. No diarrhoea was observed. At low doses the animals died two to three days after dosing. The minimal lethal dose was reported to be 150 µg/kg bw (Satake et al., 1998a). Ito et al. (1998) injected 10 µl of the partially purified KT3 i.p. to 10 male ICR mice (age three-weeks). All animals showed inactivity and general weakness and died within 24 hours. Morphological changes caused by KT3 were distinctly different from those induced by DSP, PSP or ASP toxins. The main target organs of KT3 were liver, spleen, pancreas, thymus and digestive tract. In contrast, those of DSP toxins are the digestive tract, of PSP toxins the central nervous system and of ASP toxins the brain. The target site of KT3 was the small intestine, where villi degenerated from the top. At the histopathological level, parenchym cells of the pancreas and hepatocytes, which contain numerous rough endoplasmic reticula, are preferentially affected and it is probable that KT3 inhibits protein synthesis.
Satake et al. (1998b) reported an i.p. lethal dose of purified AZA to mice of 200 mg/kg bw. Intraperitoneal lethal doses for AZA-2 and -3 to mice were 110 and 140 µg/kg bw, respectively (Ofuji et al., 1999a) and for AZA-4 and AZA-5 approximately 470 and less than 1000 mg/kg bw, respectively (Ofuji et al., 2001).
repeated dose toxicity
oral studies
Oral doses of 50, 20, 5 and 1 mg AZA/kg bw were given twice a week, up to 40 times, within 145 days, to four groups of 10, 10, 5 and 6 mice (four-weeks old), respectively. Nineteen control mice were used. Nine mice out of ten at 50 µg/kg bw and three out of ten at 20 µg/kg bw became so weak (inactivity and weight loss) that they were sacrificed before being treated 40 times (mainly after 30 treatments). Interstitial pneumonia and shortened small intestinal villi were observed. At 5 and 1 µg/kg bw no mortality was seen. The mice that survived 40 treatments were kept for up to three months after withdrawal. No fatty changes in the liver, previously seen at acute or lethal oral doses, were observed. At 50 mg/kg bw, a lung tumour was seen in 1/10 mice dosed 32 times. At 20 mg/kg bw a lung tumour was observed in 1/10 mice dosed 36 times and in two additional mice after withdrawal. In addition, hyperplasia of epithelial cells in the stomach was seen in 6/10 mice at 20 mg/kg bw. At 5 µg/kg all 5 mice showed erosion of small intestine (possibly attributed to unhealed injuries rather than late effects developed during withdrawal period). At 1 mg/kg, one out of six mice developed hyperplastic nodules in the liver and two mice out of six showed mitosis in liver (Ito et al., 2002).
reproduction teratogenicity
No data
mutagenicity
No data
in vitro toxicity
Azaspiracids were cytotoxic to P388 cells but to KB cells the potency was much less prominent (EU/SANCO, 2001). AZA did not inhibit protein phosphatase 2A. It was noted that in vitro studies performed in human cells from healthy donors suggest that the threshold for azaspiracid analogues to modify cellular function would be 24 mg/kg for a 60 kg person.
6.5.4 Toxicity to humans
In November 1995, at least eight people in the Netherlands became ill after eating mussels (Mytilus edulis) cultivated at Killary Harbour, Ireland. Although human symptoms such as nausea, vomiting, severe diarrhoea, and stomach cramps were similar of those of diarrhoeic shellfish poisoning (DSP), contaminations with the major DSP toxins okadaic acid (OA) and dinophysistoxins (DTXs) were very low. These observations prompted the investigators to explore the causative toxin in the mussels for structural studies. After chemical analytical research, the investigators identified and quantified AZA (Satake et al., 1998a; 1998b). Based on these results, the toxicity of the mussels was estimated to be 0.15 mouse units (MU)/g (equivalent to 0.6 mg AZA/g) (EU/SANCO, 2001). A higher toxin content of 1.4 mg AZAs/g of meat (0.4 MU/g of meat) was reported by Ofuji et al. (1999b). Human toxicity was seen between 6.7 (5 percent) and 24.8 (95 percent) mg/person with a mean value of 15 mg/person. However, new data on the heat stability of azaspiracid suggest that it is not appropriate to take into account a reduction in AZAs concentration due to heating. Therefore the recalculated range of the LOEL is 23 to 86 mg per person with a mean value of 51.7 mg/person (EU/SANCO, 2001).
6.5.5 Toxicity to aquatic organisms
No data
6.6.1 Depuration
In the winter when shellfish are free of contamination by DSP toxins, AZP toxins may occur in mussels. The long duration of toxicity periods, which often extend to nearly six months, is troublesome (Ofuji et al., 2001). During the initial stages of intoxication, mussel digestive glands contain most of the AZP toxins. Migration of AZP toxins to other mussel tissues can occur leading to persistent intoxication. This unusual distribution of AZP toxins within the shellfish tissue can lead to the slow rates of natural depuration. In addition the DSP mouse bioassay protocol in which only the hepatopancreas is used at extraction, may fail to detect AZP toxins in mussels (James et al., 2002a).
6.7.1 Europe
The presence of azaspiracids in European ICES countries is illustrated in Figure 6.2.
Ireland
In November 1995, at least eight people in the Netherlands became ill after eating mussels (Mytilus edulis) cultivated at Killary Harbour, Ireland (McMahon and Silke, 1996; Satake et al., 1998a). A toxin then called Killary Toxin-3 or KT3 was detected. Satake et al. (1998b) elucidated the structure of KT3 and called the toxin azaspiracid. Mussels collected in February 1996, showed a toxin content of 0.15 MU/g (=0.6 µg AZA/g) (EU/SANCO, 2001).
Since 1996 several AZP incidents have been identified in Ireland. Cases of contamination recurred in 1997 and repeatedly caused human intoxication in Ireland - in the Arranmore Island region of Donegal, Northwest Ireland (McMahon and Silke, 1998) - and other European countries. Although no known toxic phytoplankton were observed in cultivation areas after these intoxications, it is probable that AZP toxins were produced by marine dinoflagellates (James et al., 2002a).
Mussels collected at Killary Harbour on 23 April 1996 (five months after the incident) contained 1.14 µg AZA/g of meat, 0.23 µg AZA2/g of meat and 0.06 µg AZA3/g of meat (total AZAs 1.4 µg/g of meat). Mussels collected at Arranmore Island on 3 November 1997 (one to two months after the incident) contained 0.865 µg AZA/g of whole mussel meat (including hepatopancreas), 0.25 µg AZA2/g and 0.24 µg AZA3/g (total AZAs 1.36 µg/g). Results of the mouse bioassay revealed 0.4 MU/g of meat (Ofuji et al., 1999b). McMahon (2000) reported that the maximum AZA content in shellfish during the Arranmore Island incident was 10.7 µg/g of hepatopancreas. In November 1997, James and Furey (2000) detected 2.21 µg AZAs/g in raw whole meat of mussels.
After the initial intoxication in Arranmore Island and Killary Harbour, the toxin persisted for a further seven to eight months. Oysters seem to be just as susceptible as mussels to intoxication by AZP toxins as illustrated in Table 6.2 below (James et al., 2000).
Table 6.2 Levels of AZAs in mussels and oysters from Ireland
|
Location in Ireland |
Date |
Total AZAs |
Total AZAs |
|
County Cork |
Nov. 1998 |
70 |
70 |
|
County Cork |
Feb. 2000 |
10 |
20 |
|
Bruckless, Co. Donegal |
Nov. 1999 |
10 |
30 |
In Ireland during 1999, some 1800 samples were tested for DSP/AZP toxins using the mouse bioassay of Yasumoto et al. (1978). Approximately 5 percent of the samples were positive. Azaspiracid was detected in several production areas and harvesting of all bivalves has been prohibited in Bruckless Bay, Northwest Ireland since August 1999 due to detection of AZP toxins in samples tested weekly (EU-NRL, 2000).
Norway
Azaspiracids have been identified in mussels (James et al., 2000b).
Portugal
A strange toxicity in cockles (Cerastoderma edule) similar to AZP and not found in mussels was reported (EU-NRL, 1998).
United Kingdom
Azaspiracids have been identified in mussels (James et al., 2000b).
Figure 6.2 Occurrence of AZP toxins in coastal waters of European ICES countries from 1991 to 2000
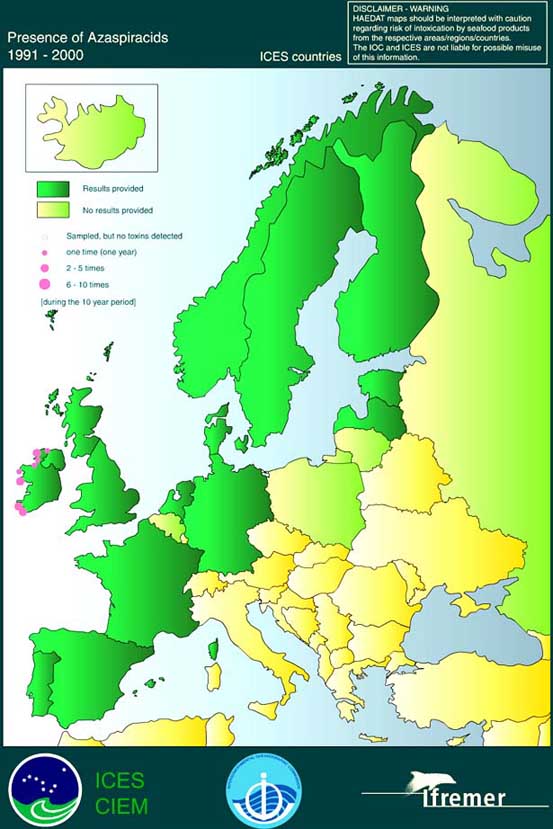
Source: http://www.ifremer.fr/envlit/documentation/dossiers/ciem/aindex.htm
It was the opinion of the Irish experts who carried out the risk assessment that, because of the lack of data on AZP toxins and the uncertainty outlined in the risk assessment, the prevailing tolerable limit of 8 mg/100 g of shellfish (see Chapter 8.5 Risk Assessment for Azaspiracid Shellfish Poisoning (AZP) and Chapter 9.1.5 Conclusions related to AZP) should be reviewed prior to being adopted into legislation.
Attention should be paid to the possible co-occurrence of okadaic acid, pectenotoxins and yessotoxins in the shellfish. Coexistence of these toxins with AZP toxins was noticed in mussels collected in Norway (Yasumoto and Aune in EU/SANCO, 2001).
6.8.1 Europe
In March 2002 the European Commission laid down the following rules (EU, 2002a):
Maximum levels of AZP toxins in bivalve molluscs, echinoderms, tunicates and marine gastropods (whole body or any part edible separately) shall be 160 µg/kg.
The mouse or the rat bioassay is the preferred methods of analysis. A series of analytical methods such as LC with fluorimetric detection, LC-MS and immunoassays can be used as alternative or complementary methods to the biological testing methods, provided that either alone or combined they can detect at least the following analogues, that they are not less effective than the biological methods and that their implementation provides an equivalent level of public health protection: AZA, AZA2 and AZA3
When results of analyses demonstrate discrepancies between the different methods, the mouse bioassay should be considered as the reference method.
Ireland
The Biotoxin Monitoring Programme in Ireland began in 1984 and was initially based on the screening of samples for the presence of DSP toxins by bioassays. In recent years, the detection of additional toxins, including DA and in particular the azaspiracids, has led to an increase in monitoring effort and the programme now includes weekly shellfish testing using DSP mouse bioassay, LC-MS (okadaic acid, DTX2, azaspiracids) and LC (DA) as well as phytoplankton analysis. Regular reports of the results of sample analysis are sent to the regulatory authorities, health officials as well as the shellfish producers and processors. A Web-based information system is being developed to increase access to information (McMahon et al., 2001).
McMahon (2000) reported that the Food Safety Authority Ireland proposed an interim threshold concentration of 0.1 µg AZA/g of whole mussel. It was proposed to review and, if necessary, to revise this value as new data become available.