It is impossible to estimate on a global basis the percentage of all infants who receive one of the products under consideration. This is due, on the one hand to the variable rates of breastfeeding in different populations and, on the other hand to the availability of the products in different parts of the world.
Exclusive breastfeeding rates differ from one country to the next. In Scandinavian countries, for example, 95% of babies are breastfed shortly after birth with almost 75% of those still being breastfed at 6 months of age. In other European countries, initial breastfeeding rates are below 30%, decreasing to almost no exclusive breastfeeding at 6 months. Available data on rates and exclusivity of breastfeeding from Australia in 1995 (Donath and Amir, 2000) and from Germany in 1997/98 (Kersting and Dulon, 2002) allow an estimate of the percentage of infants at different ages exposed to infant formula (Table 1).
Infant formula may be a direct source, an indirect source (contributing to a reservoir of E. sakazakii in the environment), and/or a vehicle for E. sakazakii-induced illness; it may also be neither the source nor the vehicle for E. sakazakii-induced illness. The meeting considered, on the basis of the information available, that in between 50 and 80% of cases, powdered infant formula is both the vehicle and the source (direct or indirect) of E. sakazakii-induced illness. To estimate a range of the proportion of cases due to powdered infant formula versus some other source, the meeting considered data from the United States in 2003 (C. Braden, personal communication, 2004). For sporadic cases of E. sakazakii sepsis and meningitis, six out of seven cases had exposure to powdered infant formula; the exposure was unclear in the remaining case. Thus, for at least 85% of these cases, powdered infant formula was a potential source. A review of 48 E. sakazakii cases in English language literature since 1961 revealed that at least 25 cases (52%) were directly linked to powdered infant formula.
Table 1. Estimated percentage of (healthy term) infants exposed to powdered infant formula or follow-up formula in Australia and Germany.
|
Age |
Australia, 1995 |
Germany, 1997/98 |
|
1 month |
29% |
- |
|
2 months |
- |
42% |
|
3 months |
40% |
- |
|
4 months |
- |
51% |
|
6 months |
57% |
61% |
Reconstituted powdered infant formula is probably a common vehicle in transmitting Salmonella to infants, given its major role in the infant diet, but contamination of formula is more likely to occur from the preparer or preparation environment than from the manufacturing process. Infrequent occurrence of intrinsic contamination of powdered infant formula does occur and has resulted in outbreaks of illness, but this appears to be rare. Thus, the meeting considered that most cases of salmonellosis amongst infants were probably not caused by intrinsic contamination of powdered infant formula. Disease caused by contamination of powdered infant formula by rare serotypes is more likely to be detected. As stated above (section 2.1.2), it would be difficult to detect outbreaks or specific sources of salmonellosis due to common serotypes within the higher incidence of background illness.
There is a dearth of information on contamination of powdered infant formula sold in developing countries, and there has also been no surveillance on the disease burden resulting from consumption of contaminated powdered infant formula in developing countries. However, even if there have been no studies on whether the product used in developing countries is contaminated, the potential risks of contamination cannot be ruled out given that reports from different developed countries have shown that some batches of powdered infant formula are contaminated. Many developing countries import powdered infant formula from processing plants in a few countries, for example Bangladesh. The incidence and levels of E. sakazakii are likely to be the same as in products evaluated in exporting countries of origin and reported in published surveys. The levels should remain stable during transport and distribution.
In many developing countries, the proportion of special subpopulations consisting of low-birth-weight infants and infants of HIV-infected mothers is higher than in developed countries; therefore, the use of powdered infant formula in these circumstances may be increasing. The basis of the higher demand for powdered infant formula is the recommendation for infants of HIV-positive mothers that - where replacement feeding is acceptable, feasible, affordable, sustainable and safe - all breastfeeding be avoided. (WHO, 2001). Human milk fortifiers are required to compensatethe nutritional needs of very low-birth-weight infants. In circumstances when the mother cannot breastfeed or chooses not to breastfeed for any reason, special powdered infant formula may be required for feeding of low-birth-weight infants. Therefore, well-controlled studies need to be conducted to assess the extent of risk associated with contaminated powdered infant formula for infants in developing countries.
According to industry experts from the United States and Europe, powdered infant formula can be manufactured in different ways. A flow chart of the production and use of powdered infant formula highlights a number of points at which this product may be subject to microbial contamination (Figure 2).
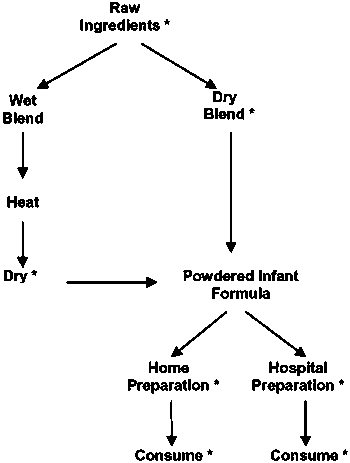
Figure 2. Flow chart for the production and use of powdered infant formula. The heat step during wet blending is assumed to effectively eliminate Enterobacteriaceae.
* = potential sites for environmental contamination.
Dry infant formula is manufactured according to three process types:
a. Wet-mix process: all ingredients are handled in a liquid phase and heat-treated (critical control point [CCP]), e.g. pasteurized or sterilized, and then dried.
b. Dry-mix process: individual ingredients are prepared, heat-treated as appropriate, dried and then dry-blended.
c. Combined process: part of the ingredients are processed according to (a), in order to produce a base powder to which the rest of the ingredients are added according to (b).
The main microbiological issues of current public health concern associated with powdered infant formula are related to the presence of Salmonella and other Enterobacteriaceae (coliforms) including E. sakazakii. The presence of these microorganisms may occur as a result of:
contamination through ingredients not submitted to a heat treatment during the powdered infant formula manufacturing process (this applies for dry-mix and combined processes).
contamination from the processing environment during the dry steps of the process, i.e. contamination post-thermal processing, presumably acquired from the processing environment during drying or packing (this applies for dry, wet and combined processes).
It must be emphasized that dry-mix ingredients are not “raw”; they are processed by the suppliers to fulfil the same requirements as the finished powdered infant formula. The presence of Enterobacteriaceae is due to post-heat-treatment recontamination. The results of an unpublished industry survey of ingredients are summarized in Table 2 (J.L. Cordier, personal communication, 2004). In order to ensure that ingredients are microbiologically suitable, a number of factors need to be considered:
The likelihood of occurrence in ingredients - some are considered to have a high risk of containing Enterobacteriaceae (e.g. starch) while others have a low risk (e.g. oils). The rating may depend on the local situation (Table 2).
Selection of the supplier according to stringent criteria (e.g. appropriate control measures, good hygienic practices [GHPs], verification and release procedures in place).
Testing of the ingredients to verify effectiveness of the above measures (not to ensure safety).
Powdered infant formula is produced from ingredients that may include milk, milk derivatives, soy protein isolates, carbohydrates, fats, minerals, vitamins and some food additives. These ingredients, in either liquid or powdered form, are typically mixed with water to form a liquid mix, which is then dried to a powder (aw < 0.3) in large spray dryers. Prior to drying, the liquid mix is heated (pasteurized at 71.6°C for 15 seconds or 74.4°C for 25 seconds [for products containing starches or thickeners] or at higher temperatures [e.g. 105°-125°C for at least 5 seconds]), homogenized, in some cases evaporated and sometimes stored in large, chilled holding tanks. Vitamins are added just prior to drying. During the drying process, the liquid mix is heated to approximately 82°C and is pumped under high pressure to spray nozzles or an atomizer mounted in a large drying chamber through which flows filtered, high-temperature air. Inlet air temperature ranges from 135° to 204°C, and the exhaust temperature ranges from 45° to 80°C. The liquid mix is dried nearly instantaneously in the hot air and the resultant powder falls to the bottom of the dryer for collection. Alternatively, it is collected from the exhaust stream in cyclone collectors or bag houses. The powder then passes from the drying chamber to a fluidized cooling bed where it is quickly cooled to below 38°C using cool, high efficiency particulate air (HEPA) > EU 10.[12] Next, the powder is sifted and pneumatically or mechanically transported to storage silos, tote bins or big bins, or directly to filling operations.
Table 2. Industry survey for the presence of Enterobacteriaceae and E. sakazakii in ingredients used in dry mixing operations for all types of powdered formula (up to 3 years).
|
Ingredients |
n (10 g) |
Coliform or Enterobacteriaceae positives |
E. sakazakii positives |
|
Vitamins |
793 |
8 |
0 |
|
Skimmed milk powder |
835 |
1 |
1 |
|
Dem. whey powder |
23 |
3 |
0 |
|
Sucrose |
1 691 |
28 |
0 |
|
Lactose |
2 219 |
70 |
2 |
|
Banana powder/flakes |
105 |
3 |
1 |
|
Orange powder/flakes |
61 |
1 |
1 |
|
Lecithin |
136 |
1 |
1 |
|
Starch |
1 389 |
155 |
40 |
In some cases, manufacturers produce infant formula by first drying a wet mixture of the major ingredients (protein, fat and carbohydrate). This is typically called infant formula base powder. Then, in large mixers or blenders, the dry minor ingredients, such as vitamins, minerals and additional carbohydrates, are blended into the base powder to produce the final product formulation. This option allows for longer drying campaigns and reduces the frequency of changeovers between different product formulations. Another option is to blend all of the pre-dried ingredients together to make a finished infant formula powder. This process is more efficient from an energy standpoint and provides more flexibility in formulation modifications. In the dry-blending process, it is essential that the dry ingredients meet the same microbiological standards as the final product because they receive no additional heat treatment. Because incoming “raw” material testing alone does not guarantee conformance to the high quality standards required by the industry, manufacturers employing these processes maintain close relationships with their “raw” material suppliers and require strict adherence to good manufacturing practices (GMPs) and Hazard Analysis Critical Control Point (HACCP) principles.
On completion of the drying or blending steps, the final product is conveyed from the storage silos or blenders to filling machinery where it is filled into cans or flexible containers. The containers are flushed with inert gas, sealed, coded, labelled and packed into shipping cartons. The finished product is typically held until it undergoes final testing, including nutrient content, uniformity and microbiological analysis.
5.3.3.1 Heat treatment
It has been suggested that the high thermal resistance of E. sakazakii strains in comparison to other members of the Enterobacteriaceae can possibly explain their high prevalence in powdered and prepared formula milk (Nazarowec-White and Farber, 1997a). However, recent studies suggest that the osmotolerance of the organism may be more important in this latter regard (Breeuwer et al., 2003). The ability to be osmotolerant may increase the risk of the organism becoming more dominant in the environment, thus increasing the risk of post-processing contamination of powdered infant formula. Previous work done by Nazarowec-White and Farber (1997b) and others (Nazarowec-White, McKellar, and Piyasena, 1999; Iversen, Lane, and Forsythe, 2004) showed that standard pasteurization practices are effective for the inactivation of E. sakazakii. Edelson-Mammel and Buchanan (2004) showed that a greater than 4-log reduction can be obtained by rehydrating dried infant formula with water pre-equilibrated to > 70°C. This implies that preparing reconstituted formula using the latter approach (using 70°C for rehydration) is likely to result in a high probability that a serving would not contain this organism. Interestingly, there appeared to be two distinct phenotypes of E. sakazakii, and heat resistance varied as much as twentyfold (Edelson-Mammel and Buchanan, 2004). Figure 3 illustrates the difference in heat resistance and provides a comparison with other Enterobacteriaceae (Edelson-Mammel and Buchanan, 2004). A complete listing of D- and z- values can be seen in Table 3. In summary:
There appears to be substantial diversity in thermal resistance among strains.
Inactivation of the organism can occur very quickly at temperatures above 70°C.
This suggests that the use of relatively mild thermal treatments is a potential risk reduction strategy that can be directed towards reducing or eliminating E. sakazakii in reconstituted powdered infant formula.
The difficulty which has to be considered in the evaluation of potential treatments for inactivating microbial pathogens in powdered infant formula is the behaviour of vegetative cells in dry products, i.e. very frequently there is increased heat resistance. Based on currently available knowledge, sterilization of the final product in its dry form in a processing environment in cans or sachets seems only possible using irradiation. However, with the doses that are likely to be required to inactivate E. sakazakii in the dry state, the technology does not appear to be feasible due to organoleptic deterioration of the product.
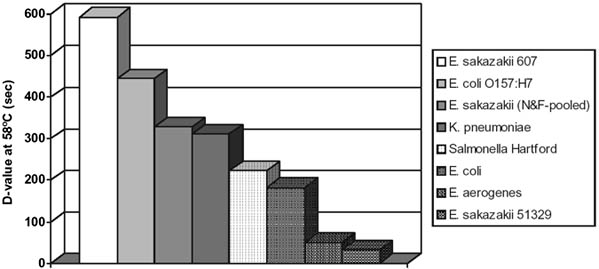
Figure 3. Heat resistance of different strains of E. sakazakii and other Enterobacteriaceae (Buchanan, 2003).
Note: E. sakazakii (N&F pooled) = pool of 10 strains as reported in Nazarowec-White and Farber (1997a).
A number of other technologies, such as ultra-high pressure and magnetic fields, may be potential candidates. These new technologies are at an early stage of development and currently none are suitable for dried foods. It is recommended that research be done in this field, bearing in mind the needs for quantitative validation of the killing effect.
In the United States and Europe, for many years now, infant formula manufacturers have recognized that GHP and HACCP play a primary role in the control of microbiological, chemical and physical hazards as well as allergens. Although there is currently no worldwide regulatory requirement for infant formula manufacturers to have HACCP plans, most (if not all) incorporate HACCP principles into their control programmes as well as GHP. Quality of raw materials, air and liquid filters, sifter screens, magnets/metal detectors, pasteurization and storage temperatures are important control points and must be addressed specifically.
Table 3. Decimal reduction time (D-value)a and z-valueb for E. sakazakii in powdered infant formula.
|
D-value (min.) |
z-value (°C) |
Reference |
||||||||
|
52°C |
53°C |
54°C |
56°C |
58°C |
60°C |
62°C |
65°C |
70°C |
|
|
|
(Temperature at which D-values were determined) |
|
|
||||||||
|
54.8 |
|
23.7 |
10.3 |
4.2 |
2.5 |
|
|
|
5.8 |
Nazarowec-White and Farber, 1997ac |
| |
8.3, |
6.4, |
1.1, |
0.27, |
|
|
|
|
3.1, |
Breeuwer et al., 2003d |
| |
|
|
|
0.50 |
|
|
|
|
|
Breeuwer et al., 2003 |
| |
|
|
21.1 |
9.9 ± 0.8 |
4.4 |
|
0.6 |
0.07 |
5.6 |
Edelson-Mammel and Buchanan, 2004e |
| |
|
16.4 |
5.1 |
2.6 |
1.1 |
0.3 |
|
|
5.8 |
Iversen, Druggan, and Forsythe, 2004f |
| |
|
11.7 |
3.9 |
3.8 |
1.8 |
0.2 |
|
|
5.7 |
Iversen, Druggan, and Forsythe, 2004g |
a D-value is the time required for a 10-fold reduction in viable numbers of organisms at a given temperature.
b z-value is the temperature change required to change the D-value by a factor of 10.
c D-values of a pool of 10 strains (5 clinical isolates and 5 food isolates) of E. sakazakii.
d These D-values for 4 different strains of E. sakazakii were determined in phosphate buffer. The authors report that heat treatment in reconstituted powdered infant formula did not influence the D-value.
e D-value for E. sakazakii strain 607 described as the most heat resistant of a range of E. sakazakii strains that were examined in the study.
f Data for E. sakazakii type strain.
g Data for E. sakazakii capsulated strain.
The heat treatments (CCP) applied are theoretically sufficient to ensure the destruction of eight or more log units of Enterobacteriaceae, including Salmonella and E. sakazakii, as well as other vegetative microorganisms, such as L. monocytogenes or S. aureus. Spore-formers, such as B. cereus and C. botulinum, are inactivated in part - to what extent depends on the processing conditions. Other heating steps are typically applied in a wet-mix process, but they are not considered CCPs; they include:
1. thermization or pasteurization of, for example, raw materials (e.g. incoming raw milk or raw whey);
2. preheating of the liquid formula before spray-drying; and
3. actual spray-drying.
Although these steps may have some killing effect (in particular, steps 1 and 2), they are performed for technological reasons and are not considered CCPs.
5.3.6.1 Methods for detection
Different genera and species of Enterobacteriaceae have been isolated from reconstituted powdered infant formula after enrichment (Muytjens, Roelofs-Willemse, and Jasper, 1988; Iversen and Forsythe, 2004), including E. sakazakii, E. cloacae, C. koseri, C. freundii, Pantoea agglomerans and Escherichia vulneris (the latter two formerly known as E. agglomerans). Specific detection methods are required to isolate and distinguish between closely related members of the Enterobacteriaceae.
Primary isolation: Both E. sakazakii and S. enterica are isolated using pre-enrichment, enrichment and selective-differential agar. For Salmonella, multiple 25 g volumes (n = 60) are tested (CAC, 1979; ICMSF, 1986), whereas for E. sakazakii a most probable number approach is used with multiple 100 g, 10 g and 1 g volumes (Figure 4) (Muytjens, Roelofs-Willemse, and Jasper, 1988; Nazarowec-White and Farber 1997b; USFDA, 2002). For both organisms, presumptive isolates are confirmed using biochemical or gene-based tests (Anon., 1996; Kandhai, Reij, and Gorris, 2004). For Salmonella, methods have been validated by international organizations, but this is not the case for E. sakazakii. A characteristic of most E. sakazakii strains is the production of a yellow non-diffusible pigment below 37°C. However, this is not a unique trait and it is commonly found in the closely-related genus Pantoea which has also been isolated from reconstituted powdered infant formula (Muytjens, Roelofs-Willemse, and Jasper, 1988; Iversen and Forsythe, 2004). In addition, there are white E. sakazakii strains (Block et al., 2002). A second common trait used in presumptive identification is the production of a-glucosidase (Muytjens, van der Rose-van de Repe, and van Druten, 1984; Iversen, Druggan, and Forsythe, 2004). Internationally-validated methods exist for the specified pathogens, B. cereus, C. perfringens and S. aureus, and the indicator organisms coliforms and Enterobacteriaceae (USFDA Bacteriological Analytical Manual; Health Canada Compendium of Methods, ISO, Geneva). No internationally validated methods exist for specific Enterobacteriaceae such as E. sakazakii, E. cloacae and C. koseri. Biochemical profiles are frequently used following primary isolation (Figure 4), but contradictions in identification may occur in different biochemical kits for the same strain (Iversen, Druggan, and Forsythe, 2004). Further research is required into the genetic diversity and distinguishing traits of E. sakazakii and related organisms.
Typing: Salmonella isolates are subject to established typing methods, including serotyping, phage typing and antibiograms. Central alert centres exist (PulseNet and Salm-Net) to detect multinational Salmonella outbreaks due to a common food source (Swaminathan et al., 2001; Rowe et al., 2004). Methods used to fingerprint E. sakazakii isolates include plasmid typing, ribotyping, pulsed field gel electrophoresis (PFGE) and random amplified polymorphic DNA (RAPD) typing (Biering et al., 1989; Clark et al., 1990; Nazarowec-White and Farber, 1999). These methods enable the tracing of specific strains in powdered infant formula production and are very useful during epidemiological investigations, to observe if clinical and food isolates are indistinguishable (Smeets et al., 1998).
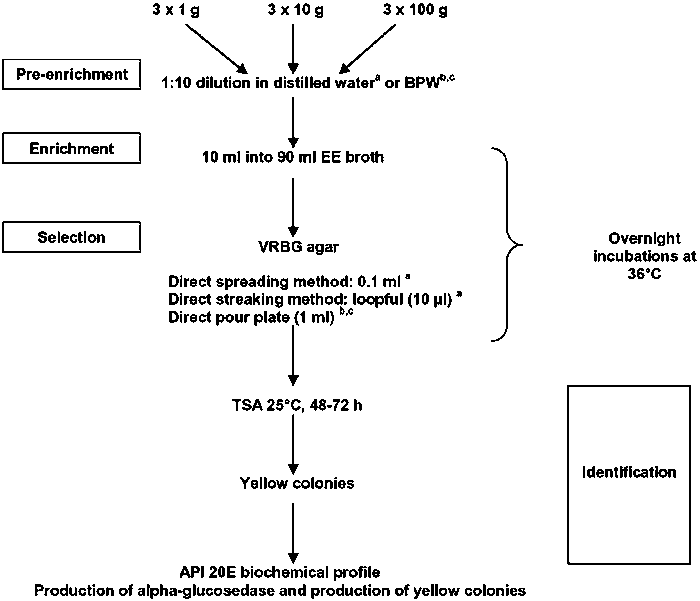
Figure 4. Quantitative E. sakazakii isolation procedure.
a USFDA (2002); b Muytjens, Roelofs-Willemse, and Jasper (1988); c Nazarowec-White and Farber (1997b) from Iversen and Forsythe (2003).
BPW, Buffered peptone water; EE Broth, Enterobacteriaceae enrichment broth; VRBG, Violet red bile glucose agar.
E. sakazakii isolates from powdered infant formula available in Canada and Canadian clinical isolates were characterized by phenotypic (biotype and antibiograms) and genotypic (ribotyping, RAPD and PFGE) methods (Nazarowec-White & Farber, 1999). There is currently at least one large food company that is using ribotyping of E. sakazakii to track the spread or trace the source of the organism in powdered milk plants. Molecular typing methods, such as ribotyping and PFGE, are very suitable tools for studying environmental contamination in plant processing environments, in trouble-shooting, as well as in tracing sources of contamination, and should, wherever feasible, be encouraged.
5.3.6.2 Monitoring and testing by industry
Sampling and testing regimes in processing facilities are integrated sampling plans which are implemented to demonstrate the efficiency of the control measures taken to eliminate or minimize the presence of Salmonella and other Enterobacteriaceae (including E. sakazakii) in finished products, as well as other specified pathogens such as B. cereus and S. aureus. Such sampling plans are not necessarily identical to the ones applied by official control laboratories - they may be as or more stringent, but with a different focus and different types of samples. Such integrated sampling plans are flexible and may be adapted to the findings. In particular, indications for deviations in the processing environment and line (indication for an increased risk of contamination) could lead to increased sampling (number and size of samples) and testing and investigation of the deviation.
Such sampling plans may vary among manufacturers and the parameters included (pathogens, indicators, visual inspections etc.) are adapted to the particular processing line. They integrate the following types of sample:
dry-mix ingredients;
finished products;
food contact surfaces at critical processing steps; and
environmental samples from the processing environment, in particular critical ones.
For a detailed consideration of the above, see ICMSF (2002).
The current Codex microbiological specifications relating to mesophilic aerobic bacteria, coliforms and Salmonella for powdered infant formula (CAC/RCP 21-1979) are outlined in Table 4. These criteria were established many years ago and need to be reviewed in light of new developments and knowledge.
Table 4. Current Codex advisory microbiological specifications for dried and instant products.a
| |
Case |
Class plan |
n |
c |
Limit per g b |
|
|
m |
M |
|||||
|
Mesophilic aerobic bacteria |
6 |
3 |
5 |
2 |
103 |
104 |
|
Coliforms |
6 |
3 |
5 |
1 |
<3 c |
20 |
|
Salmonellae d |
12 |
2 |
60 |
0 |
0 |
- |
a Including products intended for consumption after the addition of liquid, dried infant formula, instant infant cereals etc.
b The microbial limits apply to the dry product (CAC/RCP 21-1979).
c <3 means no positive tube in the standard 3-tube MPN (most probable number) method (ICMSF, 1978).
d For Salmonellae, 25 g samples should be used.
The current microbiological specifications for Salmonella were considered by the meeting to be adequate and are near the limit of practical microbiological testing. However, there is currently no requirement to test for E. sakazakii and the meeting concluded that the current specifications should be reviewed based on the information presented to the meeting. A revision of the Codex specifications should include consideration of the following:
microorganisms and reasons for concern;
analytical methods to be used;
sampling plan and size of analytical units;
microbiological limits; and
numbers of units to be in conformity.
Using current dry-mix technology, it does not seem to be possible to ensure that powdered infant formula is free from Enterobacteriaceae such as E. sakazakii. Even a more stringent microbiological specification may not be reliably effective at detecting very low numbers of organisms. Given the large quantity of the product consumed and the fact that even one contaminating bacteria is capable of growing to large numbers, a combination of risk reduction measures may be required for the effective management of the risk.
5.3.8.1 Storage of open packs of formula
Edelson-Mammel and Buchanan (R. Buchanan, personal communication, 2004) studied the long-term survival of E. sakazakii in powdered infant formula by preparing a quantity of powdered formula to contain approximately 106 cfu/ml E. sakazakii when reconstituted according to the manufacturer’s instructions. Over the course of approximately 1.5 years, the spiked dry infant formula was stored at room temperature in a closed screw-cap bottle. Periodically, samples of the formula were taken, hydrated, and the level of viable cells determined by plating in duplicate on tryptic soy agar plates. During the initial 5 months of storage, the level of viable E. sakazakii declined approximately 2.5 log cycles (6.0 log cfu/ml to 3.5 log cfu/ml) at a rate of approximately 0.5 log cycles per month. Over the course of the subsequent year, the level of viable E. sakazakii declined an additional 0.5 log cycles to approximately 3.0 log cfu/ml (Figure 5). These results clearly demonstrate that E. sakazakii can survive for extended periods in powdered infant formula.
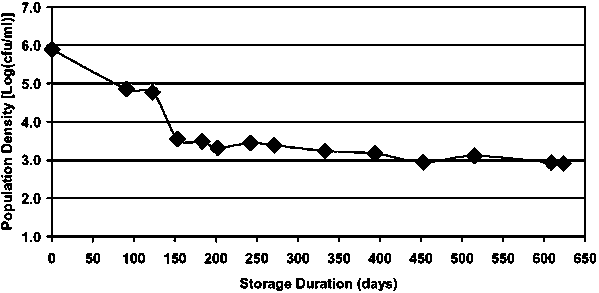
Figure 5. Long-term survival of Enterobacter sakazakii in powdered infant formula.
Little is known about the fate of intrinsically contaminated powdered infant formula once opened and then stored at high ambient temperature and humidity which is characteristic of tropical countries. Current information indicates that the moisture content of powdered infant formula in such a setting would not increase to the extent that it may support growth of intrinsic contaminants.
5.3.9.1 Labelling
Labelling of powdered infant formula is very comprehensive as a rule, with elements of information, advice and warning. The Codex standard for powdered infant formula requires: complete ingredient and nutrition labelling; advice on the feeding of infants (“breastfeeding is the best for your baby”); warning about inappropriate feeding of infants; and recommendations for the preparation, feeding and storage of the product as sold, opened and prepared for consumption. Depending on the law of the country, it may also contain information on particular properties of the product.
The recommendations for the preparation of infant formula, formula for special medical purposes intended for infants, and follow-up formula at home are detailed and often accompanied by illustrations. Presently, these recommendations include the following:
“Prepare each bottle freshly before feeding - boil water - put it into a clean bottle and cool it down to about 50°C - add measured amount of powder (number of scoops) - shake vigorously - cool to drinking temperature (skin test) - feed directly - discard residues in the bottle.”
The labelling of formula for special medical purposes (FSMP) has to contain, in addition, more specific information about the product: what makes it special? what makes it suitable for the indication it is presented for? for what disease, disorder, medical condition is it intended? are there interactions with drugs? A warning statement should be included that the product is not intended to be eaten by healthy persons and that it is only to be used under medical supervision.
5.3.9.2 Preparation
In the home, manufacturers recommend that formula be prepared before each feeding using boiled water. It is recommended to boil the water and then cool it to 50°C before the addition of the measured amounts of the powdered product. Manufacturers recommend that label directions as described above should be followed carefully.
The reasons for the recommendation of cooling the water appear to be threefold. First, there does appear to be some nutrient loss associated with particular formulas, particularly loss of vitamin C. Second, clumping of formula upon rehydration with hot water can occur with certain formula powders. Finally, there are concerns that use of water at elevated temperatures could lead to increased incidence of burns either to the infant or to the formula preparer (the latter being especially pertinent to the inappropriate heating of bottles in microwave ovens). The United States Food and Drug Administration (FDA) provided data (Buchanan, 2003) on nutrient losses associated with rehydrating infant formulas with boiling water. No data were available to the workshop on the effect of the use of hot water on clumping or on burn issues.
After mixing the powder and water by shaking the bottle and cooling it to drinking temperature under water (cheek test), the formula is to be fed to the infant immediately. While rewarming of the bottle cannot be excluded with slow-feeding infants, it should be discouraged. For practical reasons, parents might be tempted to prepare all the bottles needed for one day in advance and keep them in the refrigerator. In this case, rapid cooling of the prepared formula and storage at low temperature are important factors with regard to the microbiological safety of the reconstituted formula.
In hospitals, practices will vary according to local arrangements and availability of trained personnel and facilities.[13] A centralized preparation of ready-to-feed formula and on-ward preparation are possible and both have advantages and disadvantages. For both, the availability of safe (sterile) water and aseptic conditions for the preparation are required. The transport of ready-to-feed preparations to the wards under sustained refrigeration and refrigeration on the ward up to the feeding time are important factors to control.
Infants who can coordinate sucking, swallowing and breathing will receive formula from the bottle which has been quickly warmed immediately prior to feeding. Feeding times can be prolonged in sick and hypotonic infants and need to be controlled. Bottles should not be rewarmed. Formula remaining in the bottle should be discarded after a specified time limit.
In the case of immature or sick infants without coordinated sucking/swallowing, feeding by naso- or orogastreal tube or gastrotomy tube is practised. Formula can be applied continuously using a pump or by giving boluses which are adapted in volume to the tolerance of the infant (gastric volume and gastrointestinal motility). Continuous infusion into the gastrointestinal tract by pump requires control of the time of administration of one selected syringe volume as well as observation of the homogeneity of the formula in the syringe. Pre-administrative warming can be omitted. Handling of the infusion system should observe the same precautions as for parental feeding systems. Flushing of the tube after each feeding with sterile solutions may reduce microbial contamination and the formation of biofilms within the feeding delivery systems. Gastric residues of feeds and removed tube systems should be regularly checked for the presence of pathogenic bacteria.
Farmer et al. (1980) examined 57 strains of E. sakazakii and reported growth of the organism at 25°, 36° and 45°C. Fifty of the tested strains grew at 47°C, but not at 4° or 50°C. Nazarowec-White and Farber (1997b) reported that minimum growth temperatures for E. sakazakii in Brain Heart Infusion (BHI) broth varied from 5.5° to 8°C; and strains actually began to die off slowly at 4°C. In addition, maximum growth temperatures for clinical and food isolates ranged from 41° to 45°C (see also Gavini, Lefebvre, and Leclerc, 1983). This has implications for enrichment broths which have a recommended incubation temperature of 45°C. Iversen, Lane, and Forsythe (2004) and Zwietering (personal communication, 2004) have measured the growth rate of E. sakazakii in powdered infant formula (Figure 6). Generation times for E. sakazakii in reconstituted infant formula varied at 10°C from 4.15 to 5.52 hours and at 22°C from 37 to 44 minutes. Lag times at 10° and 23°C ranged from 19 to 47 hours and 2 to 3 hours, respectively (Nazarowec-White and Farber, 1997b). Iversen, Lane, and Forsythe (2004) examined clinical and food strains and found that the generation times for E. sakazakii in reconstituted infant formula were 13.7 hours, 1.7 hours and 19-21 minutes at 6°, 21° and 37°C, respectively. The relationship between temperature and specific growth rate across the various studies is summarized in Figure 6. Therefore, it is evident that improper storage of contaminated reconstituted powdered infant formula can support rapid growth of E. sakazakii.
It is important to stress that the addition - in hospitals or at home - of ingredients such as starch or sugar to powdered infant formula may present a risk of contamination of the product. Such added ingredients need to comply with the same requirements as the powdered infant formula. However, the specific risk associated with the addition of such ingredients was not considered in this meeting.
Many consumers, including those directly involved in caring for infants, are not aware that powdered infant formula is not a sterile product and may be contaminated with pathogens that can cause serious illness, and they lack information on how handling, storage and preparation practices can influence the risk. Effective risk communication efforts for both the public and health professionals are needed. Information and education about basic hygiene practices in connection with food handling, storage and preparation at home also need to be emphasized.
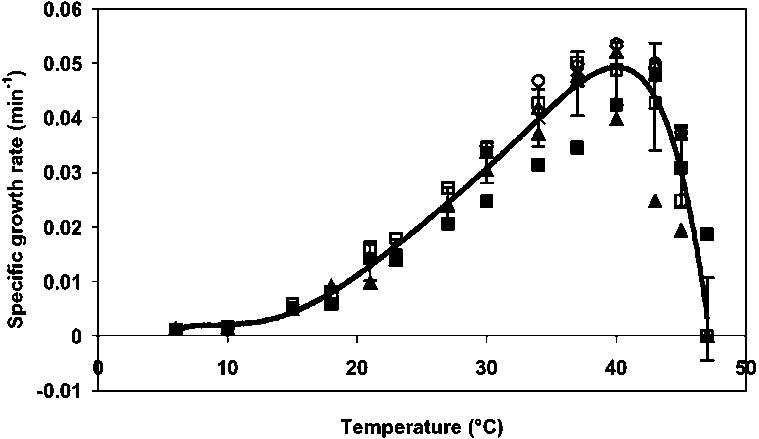
Figure 6. Growth rate of E. sakazakii (n=27) in reconstituted powdered infant formula according to temperature (Iversen, Lane and Forsythe, 2004; Zwietering personal communication, 2004).
|
[12] The Eurovent 4/4
standard has classified HEPA (high efficiency particle air) and ULPA (ultra low
particle air) filters in five different classes, EU 10 - EU 14, based on the
efficiency determined by using the Sodium Flame test. EU 10 exhibits 95-99.9%
efficiency, while EU 14 exhibits >99.999% efficiency. [13] See, for example: Infant feedings; Guidelines for the preparation of formula and breast milk in health care facilities. American Dietetics Association. 2004. |