


D. Geesing, M. Al-Khawlani and M.L. Abba
Dieter Geesing is Research Associate in the Department of Plant Science, Technical University of Munich, Germany.
Mohamed Al-Khawlani is Assistant Professor in the Department of Agriculture, Sana’a University, Yemen.
Maman Laouali Abba is Forestry Engineer in the Department of Environment, Niamey, the Niger.
Case studies in the Niger and Yemen suggest that introduced Prosopis species can be invasive but that exploiting the resources for fuelwood, fodder and food can counterbalance the damage.
In recent decades Prosopis has quickly become one of the most important tree genera in many tropical and subtropical regions of the world as a result of intentional or unintentional introductions. Prosopis trees or shrubs are woody perennials belonging to the family Leguminosae. The genus consists of 44 recognized species, of which 40 are native to the Americas, distributed within a wide ecological range. Only one species, Prosopis africana, is native to Africa, occurring in the Sahelian zone from Senegal to the Sudan, Uganda and Ethiopia. The other three Old World species, P. cineraria, P. farcta and P. koelziana, are native to the Near East and Pakistan, with the range extending into India (P. cineraria) and into Cyprus and subtropical North Africa (P. farcta). The species have some common features (such as leaf, flower and fruiting characters) and wide inter and intraspecific variability.
Prosopis species are highly appreciated in their native range. Fuelwood from Prosopis spp. is of a high quality and makes excellent charcoal. “Mesquite” (Prosopis glandulosa) charcoal, for example, is popular in North America for the distinctive taste that it imparts to food. Prosopis timber is hard and resistant to decay, and finds uses in fence posts, small carpentry items, furniture, railway ties and parquet (Simpson, 1977). Its use as sawn timber is limited, however, by the low availability of long, straight, defect-free logs from most species.
The pods of some Prosopis species have been a staple food for many indigenous peoples, for example in the deserts of Mexico and in the southwestern United States (Simpson, 1977). The pods, containing 9 to 17 percent protein and 15 to 37 percent sugar (Oduol et al., 1986), are also important as livestock feed, especially in the dry season when other forage is sparse. The leaves of some species (especially the Afro-Asian species) are also valued as a source of animal feed, although the leaves of many American species are fairly unpalatable to livestock despite their high protein and mineral content and relative digestibility. The flowers of Prosopis species are regarded as a valuable source of bee forage, and honey has become the most widely derived food product from Prosopis. There is also local potential for many other products, such as gum from the resin or seeds, although methods have not yet been perfected for obtaining gum of sufficiently high quality to be competitive on the world market (FAO, 1995).
Prosopis spp. are extraordinarily drought resistant and therefore have been distributed widely for the regreening of arid lands both within and beyond their natural range. Prosopis plantations have been established for dune stabilization, (e.g. in the Niger and Mauritania; see Jensen and Hajej, 2001), restoration of degraded land (e.g. in Cape Verde), remediation of saline land (e.g. in India) and as shelterbelts, with animal fodder and other uses as co-products. Prosopis plantations have also been established primarily for fuelwood production, yet with the belief that such plantings would also benefit the environment.
In recent decades, however, and with evolution in perceptions and scientific knowledge about the practice of introducing exotic species, significant drawbacks have become apparent and the presence of introduced Prosopis spp. has become controversial. In a number of sites, under certain environmental conditions, some Prosopis species, in particular P. juliflora, P. glandulosa, P. pallida, P. chilensis, P. flexuosa and P. ruscifolia, invade valuable farm- and rangeland and sometimes grow into impenetrable thickets, causing enormous ecological and economic damage as a result of competition with the native vegetation and with agricultural crops.
Notwithstanding the unquestionable ecological changes produced by Prosopis invasion, where the species have been introduced it is necessary to make the best of a situation that is hardly reversible. Therefore, rather than seeking to investigate case by case the negative and/or positive impacts of introduced Prosopis species on their new environment, this article seeks to show how the benefits might be maximized, as illustrated by projects in the Niger and Yemen.
Prosopis tree... |
 |
D. GEESING |
...flowers and leaves... |
 |
D. GEESING |
...pods and seeds |
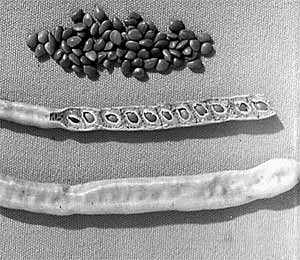 |
FAO |
The mean rainfall where Prosopis species grow, including areas where they have are native and where they have been introduced, varies from <70 mm for Prosopis tamarugo to over 1 000 mm for P. africana. Other species such as P. juliflora and P. pallida perform well in high rainfall zones but also grow in areas receiving <250 mm. Often, Prosopis species do not depend entirely on rainfall for their water needs and tap groundwater supplies with deep root systems or absorb foliar water as mechanisms for coping with drought. Some Prosopis species endure extremely high temperatures, but only a few can survive freezing. Prosopis species can thrive on nutrient-poor or degraded soils, and many species are tolerant of salinity and alkaline soils (Burkart, 1976). In trials in Cape Verde, P. juliflora had a greater survival and growth rate than any other tree species tested there, including a small number of other Prosopis species such as Prosopis cineraria and P. tamarugo and other known drought-tolerant species (e.g. Acacia species, Balanites aegyptica, Ziziphus spp., Azadirachta indica, Boscia spp., etc.), even under heavy browsing (Pasiecznik, Vera Cruz and Harris, 1995).
Invasive species are species that are non-native to a particular ecosystem and whose introduction causes, or is likely to cause, economic or environmental harm. Invasive species are characterized by rapid growth rates, extensive dispersal capabilities, large and rapid reproductive output and broad environmental tolerance. Forest invasive species can negatively affect forest ecosystems or damage specific forest products. Prosopis species, like any invasive species, are invasive only under conditions that are favourable to their spread.
Prosopis species usually require the presence of animals or flooding and drying cycles to germinate. One important reason for their invasive behaviour is certainly their outstanding viability under extreme conditions. Perhaps more important is the widespread propagation of Prosopis trees and shrubs (often from poor genetic material) by humans without measures for preventing further spread (discussed below). Many Prosopis species are also naturally protected from overgrazing by thorns and unpalatable leaves. Finally, Prosopis spp. were often introduced into areas of predominantly agrosilvipastoral land use, and animals became the major agent of Prosopis seed dispersal over long distances.
Prosopis spp. are often considered invasive from an economic viewpoint because they are in conflict with other human land use. Just as the effect of new Prosopis stands on native biological diversity depends on the ecosystems to which they spread, the economic damage or benefit depends on the socio-economic environment of the invaded land and its potential alternative uses. In some areas, for example in Australia, South Africa or the southwestern United States, invasion of rangeland has caused several million dollars of damage either because land has to be cleared before use or has become useless as pasture. (Note that calculations of the economic damage are theoretical and are often tangled with effects of other invasive species and environmental changes.) In other cases, for example in Cape Verde, some parts of Mauritania or the Niger, Prosopis spp. constitute the only viable woody vegetation cover and are important as a source as fuelwood and fodder.
Today, several options are available for eradicating Prosopis stands depending on the size and age of the trees and the density and habitat of the stands. Tall, dense infestations may require uprooting and root ploughing, which must remove the bud zone of the root system (about 30 cm below the surface) to prevent reshooting. This mechanical control may in some cases need to be followed by fire and foliar spraying of seedlings with herbicides (triclopyr and picloram). Isolated multiple-stemmed plants may require foliar sprays and are generally more difficult to treat. Isolated single-stemmed plants can be treated by carefully spraying herbicides completely around the base of the plant to a height of about 30 cm, or by cutting stems off horizontally as close to the ground as possible and immediately swabbing the cut surface with the above-mentioned herbicides (Csurhes, 1996). The high cost of herbicides and associated labour often impedes control, and all treatments require follow-up measures.
It is not only for economic reasons that eradication of Prosopis may be inexpedient. It is conceivable that the short-term benefits of successful eradication could create additional problems that are worse over the long term. Reports of the effects of Prosopis spp. on native flora or fauna are still primarily anecdotal. Some Prosopis species appear to colonize degraded dry-zone areas and to occupy in some ways an ecological niche previously occupied by other woody plants, e.g. native Acacia spp. Both negative and positive changes in the number and composition of plant species have been reported, but usually the effects of environmental degradation (climate change, human activity, overgrazing) cannot be separated from the effects of invasion of land by Prosopis spp.
In Bundala National Park, the only wetland in Sri Lanka listed under the Ramsar Convention, P. juliflora introduced to improve saline soils has now become invasive and a threat to native flora and fauna (Algama and Seneviratne, 2000). Elsewhere, however, in areas where Prosopis trees and shrubs restore degraded land and offer food and shelter to animals, it can be hypothesized that they generally have a beneficial effect on wildlife. The impact on soil biodiversity and fertility may also be assumed to be generally positive, particularly in comparison with bare land, since vegetation cover reduces erosion by wind and water, stabilizes dunes and increases soil fertility through nitrogen fixation and litter fall. On the other hand, Prosopis invasion could theoretically impair the water supply.
In many countries where Prosopis species have been introduced to fight desertification, they are not particularly recognized for their economic value. Even when fuelwood becomes scarce, many people still prefer to go far to exploit traditional sources rather than take advantage of the now ubiquitous Prosopis stands, perhaps because the plants are thorny or because the odour of the smoke is considered disagreeable by some cultures. Since it is typically the women who collect fuelwood and prepare food, exploitation of Prosopis species is also a gender issue.
Since it is typically women who collect fuelwood, exploitation of Prosopis species has become a gender issue |
 |
FAO |
The ability of Prosopis trees to establish over a large area from a single introduction is confirmed by the encroachment of Prosopis trees on arable land around Lake Chad. It is assumed that the plants date originally from a dune stabilization programme carried out on only 10 ha by the Niger’s national forestry service in 1977, although animal transhumance may have contributed to the establishment by introducing pods from outside the area. The trees were probably disseminated in the wild by livestock (goat, sheep, cattle, camels), as pastoralism is the most important source of livelihood in the area. Today, this recently established forest extends over more than 300 000 ha. It has caused serious problems not only for farmers, but also for fisherfolk, who can no longer move in the shallow waters of the lake because the Prosopis trees and roots impede the movement of boats.
The most common method of control has been the cutting and burning of the trees, without any attempt to use the wood economically. The socio-economic and environmental conditions of the area preclude chemical spraying or large-scale mechanized clearing. The release of insects to feed on the pods as a biocontrol method is unlikely to succeed in reducing the spread of Prosopis trees, since the pods are already heavily infested by bruchid beetles. Other biocontrol methods such as the use of leaf-tying moths (Evippe spp.) to cause defoliation or the use of a sap-sucking psyllid (Prosopidopsylla flava) that causes dieback are, at present, even less developed and are unlikely to have much impact. Furthermore, considering the spreading desertification observed in many other parts of the Sahel, it is debatable whether the eradication of Prosopis trees is even advisable.
Recognizing a threat to the arable land and to the already precarious food situation, but also bearing in mind that Prosopis provides food for human consumption in other parts of the world (notably in South America and Mexico), the Government of the Niger requested assistance from FAO to develop a strategy for improved management and exploitation of the Prosopis forest in the Lake Chad district of N’Guigmi. The technical assistance, provided in close cooperation with national, regional and local authorities, started in December 2000 and ended 18 months later.
The project commissioned several in-depth studies, carried out by national institutions and non-governmental organizations (NGOs), on the extent of the Prosopis resource, the chemical composition of the pods, the socio-economics and market potential of Prosopis products and their palatability to people and animals.
The Prosopis wood resource on the Niger side of Lake Chad was estimated to be 2.2 million cubic metres and the average yearly increment to be around 75 000 m³. Boureima, Mayaki and Issa (2001) predicted that the yearly sustainable gross return could be around €2.5 million per year if this resource were traded on rural wood markets supplying major nearby communities; its exploitation would not only help contain the forest, but would also cover the costs of clearing fields and even create additional income.
Salissou and Nourou (2001) reported that most pastoralists feed Prosopis pods to their animals, but only a small fraction of them crush the pods first. Crushing makes the protein in the seeds more available and at the same time destroys the seeds, preventing germination of new plants and thus contributing to containment of the Prosopis invasion. Many livestock breeders observed that the exclusive and prolonged consumption of Prosopis pods had adverse effects on animal health and anti-nutritional effects. Therefore, Kangar, a local NGO, is currently testing the effects of Prosopis feed on small ruminants to find the most appropriate ways of using the pods as fodder to increase productivity and avoid adverse effects on animal health.
Another study (Geesing, 2002) found that the production of easily storable food from sweet Prosopis pods (about 25 percent of all pods in the area of intervention) amounted to about 1.3 kg per day per inhabitant (approximately 38 000 at present). Tasting panels found that replacing up to 10 percent of the traditional flour (millet, maize or sorghum) with Prosopis flour did not negatively affect the taste of traditional dishes or even made the taste agreeable (Kaka and Seydou, 2001).
Assisted by a Brazilian expert on pod processing, several mills were locally manufactured and adapted to local needs to produce flour from Prosopis pods. Several millers and technicians were trained to produce the different flour fractions for human and animal consumption and to keep the mills from becoming clogged as a result of the high sugar content in the pods. At the same time, a committee of local women trained by a Peruvian expert promoted the use of Prosopis flour in human food (including as a coffee substitute). The techniques were also demonstrated at local markets and by NGOs in the area. Today, more than 500 women have been trained in the use of the flour in local dishes, and more than 500 pastoralists, farmers and technical staff were taught improved techniques of exploiting the new resource. The results were presented in two workshops to a local, national and international audience. The project produced extension material in the form of booklets and a video which was shown on the Niger’s national television.
Today’s visitor to the area will not find the ingredients of traditional dishes replaced by Prosopis pod flour, but the authorities and policy-makers have become aware that eradication is not feasible and that the resource is underexploited. The Prosopis forest, which was before considered threatening weeds, is today considered a resource whose exploitation can contribute to containing its uncontrolled spread and can also help mitigate, rather than aggravate, the precarious food situation, especially in times of severe drought and food shortage.
Prosopis encroaches on Lake Chad and the surrounding fertile lands |
 |
D. GEESING |
Women demonstrating the preparation of biscuits using Prosopis pod flour in the Niger |
 |
D. GEESING |
The details of the first introduction of non-native Prosopis species in Yemen are not known, but it is likely that the trees were introduced through animal trading in the nineteenth and early twentieth centuries, when the presence of these species was already documented in countries such as Egypt, India, Oman and the Sudan. In 1974, the Tihama Development Authority introduced and tested several non-native drought-resistant species from different genera. The American Prosopis species showed the highest survival rate and the highest biomass productivity. The seedlings were planted in shelterbelts around cities and villages and were further spread by sheep and goats.
The presence of native and introduced Prosopis species in Yemen is limited to coastal plains and low altitudes where there is no frost. Where these species have been planted to stabilize sand dunes their presence generally does not yet constitute a major concern. In the former Acacia ehrenbergiana woodlands and Ziziphus spina-christi and Dobera glabra lands, however, which are today widely used as farmland and rangeland, introduced Prosopis spp. have spread into adjacent fields, wadis and fallow land. A particular problem is the establishment of Prosopis spp. (together with other invasive species such as Tamarix spp.) in irrigation systems where they impede the flow of water. Thus in the 1990s, a growing number of voices were raised against the Prosopis invasion of farmland. Complaints came in particular from large landowners growing irrigated cash crops (cotton, onions, watermelons, wheat and various vegetables), even though the offending species had often been planted by the farmers themselves.
The situation was perceived to be threatening food security; hence it became of primary importance to explore alternative ways to improve food and fodder availability. Information about the plants, their ecology, management and presence in Yemen was insufficient and not well organized. Farmers had no experience with the management and use of introduced Prosopis species, and many considered the plants dangerous weeds. Scientists were worried about ecological changes that might occur in the natural rangelands and risks for the maintenance and conservation of native flora and fauna.
Thus, at the request of the Yemen Government, in 2002 and 2003 FAO implemented a project to manage, use and control Prosopis better. Research and development were conducted in three major agricultural centres: on the west coast at the Tihama Agriculture Development Authority in Al Hodedah, along the south coast at the El Kod Research Station, and in the Hadramaut Governorate of the northeastern interior region at the Seiyun Research Station. Additional research on the human use of Prosopis flour was conducted at the Food Research and Post Harvest Technology Center in Aden. Unfortunately the use of Prosopis pods in human food had to be excluded because the pods that grew in Yemen proved to be almost exclusively bitter.
With the help of international specialists, local technicians and scientists were acquainted with techniques employed in other countries for Prosopis management and use, i.e. silviculture, rhizobiology, pod harvesting and processing, utilization of fuelwood, timber production and honey harvesting.
Like many other countries in the dry areas of the world, Yemen is facing a rapid decline of its fuelwood supply. If available, native species (mostly Acacia trees) are generally preferred to Prosopis spp. for fuelwood and charcoal production. (They are thought to have a higher energy yield and the smell emitted by burning Prosopis wood does not appeal to local tastes.) Thus, while native species fail to regenerate because of overuse, desertification and grazing, the underexploited resource of introduced Prosopis species ironically grows larger. Interestingly, Prosopis cineraria, the only native Prosopis species in Yemen, was overexploited to such an extent that in the 1980s FAO was engaged in in situ conservation activities
(Cossalter, 1985).
The project in Yemen suggested that by encouraging the use of the existing stands of introduced Prosopis spp. through extension, economic incentives or a legal framework, i.e through subsidies for the use of introduced Prosopis species (for example, allowing the use of machinery free of charge) or taxes on the use of native species, it should be possible to take pressure off the native vegetation and to contain at least partially the Prosopis spread.
A training manual was prepared for technicians and farmers on Prosopis management and processing. Local extension personnel were trained in the use of wood chippers to convert small thorny branches into easy-to-handle wood chips, and in the use of hammermills to grind pods for livestock. The use of long-handled pruning poles and locally purchased gloves and safety glasses makes the harvest of the thorny stems safer and more convenient.
The project also acquainted local personnel with state-of-the-art eradication methods. Experiments to identify the most appropriate methods for the socio-economic and environmental conditions in Yemen are still under way. However, trials clearly demonstrated that eradication is only cost effective in exceptional cases (for example, in irrigation channels) and that all methods will fail without follow-up treatments. It was also shown that preventive measures such as a routine control and eradication of established Prosopis seedlings on agricultural land two to three times a year, rather than large-scale eradication of established and dense Prosopis stands, are fundamental to containing further spread.
Perhaps the most important outcome of the project, however, was its catalytic role. Many of the activities were carried out in pilot villages and with pilot farmers. The activities are now being replicated and continued in all affected areas nationwide by trained local extension personnel with the support of involved policy-makers. Research has been initiated in several Yemeni research centres to optimize the use of Prosopis pods in animal fodder and to use Prosopis spp. as a component in agroforestry systems. It can be expected that the importance of pods as fodder supply will increase when natural conditions become harsher, seasonally and locally. The collection and sale of pods has already become a profitable enterprise for local people.
Prosopis flowers as bee forage in Yemen |
 |
D. GEESING |
In Yemen the collection of Prosopis pods has already become a profitable enterprise for local people, who collect them in the plains and transport them to feed animals in higher altitudes |
 |
D. GEESING |
Although they have provided benefits to drylands worldwide, a number of Prosopis species have escaped control in many locations where they have been introduced and have become weeds encroaching on valuable fertile farmlands and rangelands. In many cases, spontaneous, uncontrolled spread of Prosopis spp. has become more of a problem than the situation for which the plants were introduced. Such cases are conspicuous examples of human influence on biodiversity and of environmental risks brought about by introducing a new species into an ecosystem without adequate testing and subsequent management. But there are human needs to be respected as well as biology. Prosopis species have many favourable attributes under proper management, and can offer under certain conditions a singular solution to particular environmental and socio-economic problems. Particular situations must be judged case by case.
For example, the introduced P. velutina in South Africa and native P. ruscifolia in Argentina form dense, thorny, impenetrable and perhaps economically useless thickets and are unquestionably undesirable, at least from the human land-use viewpoint. The presence of P. juliflora introduced in dryland areas out of its native range has pros and cons; it can be weedy but also provides fuel, controls sand dunes and provides livestock food. Similarly, P. glandulosa in the state of Texas in the United States invades pastures but also provides wildlife habitat and commercial products such as charcoal, wood flooring and furniture. In other situations Prosopis species are unquestionably desirable: native P. pallida in Peru provides pods useful for human food and livestock feed; native P. alba in Argentina provides 100 000 tonnes of logs per year to the furniture industry and is heavily overexploited; P. cineraria in the Rajasthan desert of India is intercropped with cereals such as millet.
Thus, there area many case-specific appropriate ways to manage Prosopis species. Unfortunately, too many introductions still take place without proper taxonomic identification or documentation and are often based on narrow and/or poor genetic material. Potential weediness is still not a selection criterion. Little has been done to commercialize Prosopis products, and there have been relatively few attempts to industrialize processing technologies, especially outside the natural range of the species. As a consequence, there are still many underexploited Prosopis resources. In too many cases Prosopis species are chosen only for convenience, because other solutions are ignored, or because growth is immediate and often impressive. Research on the prevention (rather than remediation) of spread, and on the impact of Prosopis invasion on plant and animal diversity in different ecosystems, is still inadequate. Prosopis trees are a resource for the poorest communities in the world because they are a low-investment resource. Yet, no matter how little the investment may be, it exceeds the capacities (financial, legal and social) of many local populations. As a consequence, the decision to eradicate or to introduce Prosopis species is not usually made by the people concerned, but by the strongest lobby.
Finally, Prosopis trees and shrubs have become naturalized constituents of many natural and cultivated ecosystems; their total eradication is not only ecologically risky but in many areas technically and economically impossible.
Thus, future efforts must be concentrated on integrated management, i.e. far-sighted and sustainable control of the species, including prevention of spread, selective eradication and full exploitation of the resource, while its potential to fight desertification and to provide fuelwood, good-quality fodder and sometimes even human food is respected. Local people, policy-makers, scientists and technicians have to be aware of all aspects. The management of Prosopis spp. will also have to be transboundary, because animals and in particular livestock are the main factor in dissemination of the plants. International institutions and national and local policy-makers have a responsibility to ensure that sufficient training and research support are provided within the framework of national and international strategies related to the sustainable use of introduced species. In forestry there is a need to raise awareness of biosecurity – the management of biological risks, including the introduction of exotic trees – and a need to raise awareness that even trees can be “invaders”.
 Bibliography
Bibliography
Algama, A.M.N.S. & Seneviratne, G.I. 2000. Invasive nature of Prosopis juliflora in Sri Lanka. In H.P.M. Gunasena, ed. Invasive alien species in Sri Lanka: impact of ecosystems and management. Peradeniya, Sri Lanka, National Agricultural Society of Sri Lanka.
Boureima, M., Mayaki, A. & Issa, M. 2001. Études socio-économiques sur la commercialisation des produits et sous-produits de la forêt de Prosopis et sur la mise en place des marchés ruraux dans l’arrondissement de N’Guigmi. Niamey, the Niger, Institut national de recherches agronomiques du Niger (INRAN) and FAO.
Burkart, A. 1976. A monograph of the genus Prosopis (Leguminosae subfam. Mimosoideae). Journal of the Arnold Arboretum, 57: 219-525.
Cossalter, C. 1985. Propositions pour la conservation des ressources génétiques de Prosopis cineraria en République Démocratique et Populaire du Yémen. Rome, FAO. (Also in Arabic)
Csurhes, S. 1996. Mesquite (Prosopis spp.) in Queensland. Pest Status Review Series. Queensland, Australia, Department of Natural Resources and Mines, Land Protection Branch.
FAO. 1995. Gums, resins and latexes of plant origin, by J.J.W. Coppen. Non-Wood Forest Products No. 6. Rome.
Geesing, D. 2002. Rapport de mission du 24 février au 22 mars 2002. TCP/NER/0068. Rome, FAO.
Jensen, A.M. & Hajej, M.S. 2001. The Road of Hope: control of moving sand dunes in Mauritania. Unasylva, 207: 31-36. Also available on the Internet: www.fao.org/DOCREP/004/Y2795e/y2795e07.htm#h
Kaka, S. & Seydou, R. 2001. Tests d’utilisation du Prosopis en alimentation humain à N’Guigmi et Bosso. Niamey, the Niger, INRAN and FAO.
Oduol, P.A., Felker, P., McKinley, C.R. & Meier, C.E. 1986. Variation among selected Prosopis families for pod sugar and pod protein contents. Forest Ecology and Management, 16: 423-431.
Pasiecznik, N.M., Vera Cruz, M.T. & Harris, P.J.C. 1995. Prosopis juliflora withstands extreme aridity and goat browsing in the Republic of Cape Verde. Nitrogen Fixing Tree Research Reports, 13: 186-188.
Salissou, I. & Nourou, A. 2001. Valorisation du Prosopis en alimentation animale par les éleveurs dans la zone de N’Guigmi. Niamey, the Niger, INRAN and FAO.
Simpson, B.B. 1977. Mesquite: its biology in two desert ecosystems. Stroudsbourg, Pennsylvania, USA, Dowden, Hutchinson and Ross, Inc.