F. Mbithi[33], [34], H.Wesonga[35], F. Thiaucourt[36] and E.L.N. Taracha[37]
Introduction
Contagious bovine pleuropneumonia (CBPP) is one of the most important transboundary animal diseases in Africa. In recent years, CBPP has been found in countries like Botswana from where it was previously eradicated (Amanfu et al., 1998). There is growing evidence to indicate that the incidence of the disease is increasing in endemic areas. These recent increases can be attributed to uncontrolled movement of cattle, poor disease control strategies and application of sub-standard vaccines (FAO, 2000; Masiga, 1996). There is sufficient consensus that efficacious vaccines could contribute substantially to an integrated control program for CBPP (Anonymous, 2000; Tuslane et al., 1996). This would involve improvement of existing and development of a new generation of vaccines. This study was aimed at investigating the immunogenicity and efficacy of two different vaccines against CBPP.
Materials and Methods
Vaccines
The T1 44 vaccine strain and a saponin formulated inactivated virulent strain of Mycoplasma mycoides subspecies mycoides small colony (Mmm SC), strain 237, were used. Strain 237 was isolated from an outbreak of CBPP in Kenya and confirmed to be Mmm SC by the polymerase chain reaction (PCR) assay. Briefly, the preparation of the saponinadjuvanted vaccine involved centrifugation of a 500 ml culture of strain 237 at 11000 g, washing the pellet twice in phosphate buffered saline (PBS) and finally resuspending in 10 ml of PBS. Inactivation of the cultured organisms was achieved at 56°C for 30 minutes. A protein estimation using the Bicinchoninic Acid method was done and the preparation stored at -20°C. On the day of vaccination, 1 ml of saponin obtained from the Kenya Vaccine Production Institute (KEVAPI) at a concentration of 1 mg/ml was added to 1 ml of the inactivated preparation at 2 mg/ml. The vaccine was used at 2 ml per inoculation. The T1 44 vaccine was administered at a dosage of 107.3 per ml viable mycoplasmas.
Cattle and vaccination
Bos indicus cattle were obtained from a CBPP free area in Kenya. The cattle were screened for the presence of anti-Mmm SC antibodies using the complement fixation test (CFT) and the competitive enzyme linked immunosorbent assay (cELISA) and found to be negative by both tests. The experimental animals were divided into 3 groups of 9 each:
Group 1: Vaccinated twice with the T1 44 at two months interval.
Group 2: Vaccinated once with the T1 44.
Group 3: Vaccinated once with the inactivated saponin-adjuvanted vaccine.
The vaccine was administered subcutaneously on the right shoulder and in the case of group 1 animals, the booster inoculation was performed on the left shoulder.
Preparation of antigens for the proliferative assay
Four antigen preparations were derived from strain 237 including precipitate form, supernatant form, a whole viable mycoplasma and a heat-killed whole mycoplasma. Precipitate and supernatant fractions were obtained after 3 cycles of freeze-thawing mycoplasmal organisms, sonication and centrifugation. The heat-killed antigen preparation was obtained by boiling mycoplasma organisms for 1 hour in a water bath. Protein estimations using the Bicinchoninic Acid method were done to determine concentration of the antigen preparations.
Sampling and T cell assays
Sampling was done twice a week. Blood was collected for the isolation of peripheral
blood mononuclear cells (PBMC) and preparation of sera. PBMC at a cell density
of 5 × 105 cells/ml were dispensed in aliquots of 100 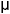 l per
well of 96-well, round-bottomed microtiter plates. Antigen preparations were
added at a concentration of 10
l per
well of 96-well, round-bottomed microtiter plates. Antigen preparations were
added at a concentration of 10 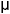 g/ml and cultures maintained at 37°C in
a humidified carbon dioxide incubator for 4 days. Tritiated thymidine was added
at 0.5
g/ml and cultures maintained at 37°C in
a humidified carbon dioxide incubator for 4 days. Tritiated thymidine was added
at 0.5 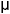 Ci per well during the last 20 hours. Cells cultured with the mitogen
Concanavalin A (Sigma) served as positive controls, while those cultured in
medium alone (RPMI 1640 with 20% fetal calf serum) as negative controls. Results
were presented as stimulation indices, which were calculated as counts obtained
with cells cultured in the presence of antigen/counts obtained with cells cultured
in medium alone. For each time point, the mean stimulation index (SI) of the
9 animals in each group was calculated. Flowcytometric analysis of ex-vivo PBMC
and T cell cultures were carried out at selected timepoints.
Ci per well during the last 20 hours. Cells cultured with the mitogen
Concanavalin A (Sigma) served as positive controls, while those cultured in
medium alone (RPMI 1640 with 20% fetal calf serum) as negative controls. Results
were presented as stimulation indices, which were calculated as counts obtained
with cells cultured in the presence of antigen/counts obtained with cells cultured
in medium alone. For each time point, the mean stimulation index (SI) of the
9 animals in each group was calculated. Flowcytometric analysis of ex-vivo PBMC
and T cell cultures were carried out at selected timepoints.
Results
Lymphoproliferative responses
Assays performed before vaccination demonstrated a marginal response in some animals and no response in others. Following vaccination, responses were detected in all vaccinates suggesting that this was a consequence of immunisation. Lymphoproliferative activity was detected to all antigen preparations albeit to varying magnitude. Stimulation indices ranged between 4 and 27. In general, the reactivity detected with the precipitate form of antigen was comparable to responses obtained with viable and heat-killed whole mycoplasma cells. This response was 2-fold more than that obtained with the supernate form of antigen. The proliferative responses were detectable over a period of 19 weeks post vaccination (group 1) and the analysis is on-going up to the time of challenge. Data showing the reactivity of PBMC obtained from the 3 groups of animals to the precipitate form of antigen is depicted in Figures 1, 2 & 3.
Figure 1. Lymphoproliferative responses to the precipitate form antigen in cattle vaccinated twice with T1 44
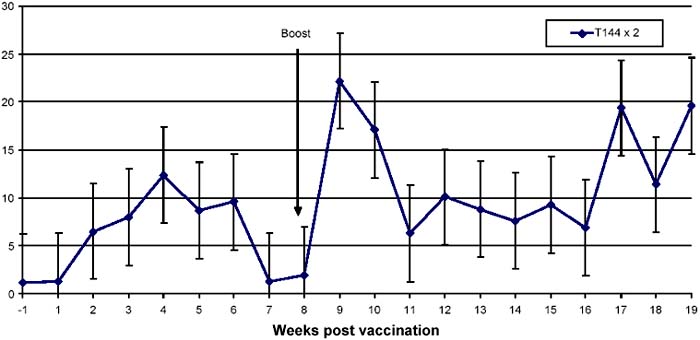
Figure 2. Lymphoproliferative responses to the precipitate form of antigen in cattle vaccinated once with the T1 44 vaccine
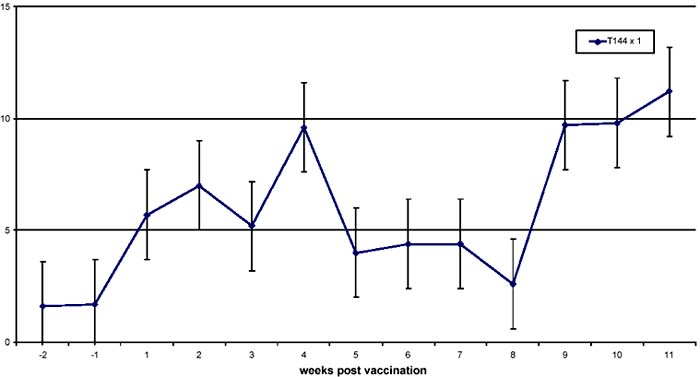
Figure 3: Lymphoproliferative responses to the precipitate form of antigen in cattle vaccinated with saponin - adjuvanted vaccine
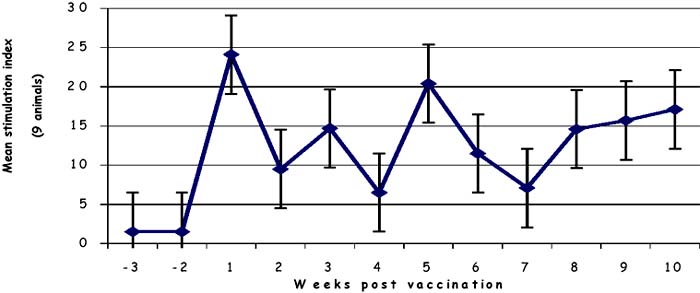
As shown in Figures 1 and 2, cattle immunised with T1 44 developed a response that peaked at week 4 (mean SI of 10). This response generally reduced in magnitude over the next 4 weeks, even though it was detected at near-peak levels in group 2 animals after this period of time. Significantly, as shown in Figure 1, the responses doubled (mean SI of >20) during the first week following a booster vaccination, and declined gradually to pre-boost levels after 3 weeks. By contrast, responses in cattle that received saponin-formulated vaccine (group3) were detected at high levels (mean SI of 25) by week 1 of vaccination. These responses declined to levels that ranged between mean SI values of 6 and 20 from week 2 to week 11. It is evident that the magnitude of the response observed after boost with T1 44 is comparable to that detected following immunisation with the saponinadjuvanted vaccine.
Responding cell populations
Flow cytometric analysis of ex-vivo PBMC was performed
before and after vaccination to define responding cell populations. As shown in
Table 1, there was no significant increase of any cell population tested.
Preliminary results from analysis of cultured cells, indicate a high proportion
of CD4 and 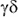 T
cells.
T
cells.
Table 1: Phenotypic analysis of PBMC from cattle before and after vaccination
|
Weeks PV |
Media |
CD4 |
CD8 |
|
B cells |
Monocytes |
|
-3 |
0.52 |
29.76 |
30.62 |
23.89 |
37.15 |
1.43 |
|
-1 |
0.83 |
29.52 |
25.80 |
30.36 |
26.22 |
2.11 |
|
1 |
0.26 |
30.01 |
23.65 |
22.10 |
26.73 |
0.68 |
|
2 |
0.20 |
32.55 |
18.85 |
15.08 |
23.98 |
0.99 |
|
3 |
0.48 |
35.73 |
28.68 |
17.47 |
|
0.66 |
Discussion
The results indicate a lymphoproliferative response as a consequence of vaccination. It is notable that animals vaccinated with the saponin adjuvanted vaccine gave a response comparable to that obtained with the T1 44 booster dose, which was about 2-fold higher than that observed after a single T1 44 vaccination. There were no marked changes in cell populations in the course of vaccination. Further analyses are being conducted to determine cytokine profiles in ex-vivo PBMC. It would be interesting to relate the immune responses observed with the outcome to challenge. This would provide an initial platform from which to develop correlates of immunity.
References
Amanfu, W., Masupu, K. V., Adom, E. K., Raborokgwe, M. V. and Bashiruddin, J. B. (1998) An outbreak of contagious bovine pleuropneumonia in Ngamiland district of northwestern Botswana. Veterinary Record 143, 46-48.
FAO (2000) CBPP status in Africa. In Report of the second meeting of the FAO/OIE/OAU/IAEA consultative group meeting on Contagious Bovine Pleuropneumonia (CBPP) Rome, Italy. 24-26 October.
Masiga, W. N., Domenench, J. and Windsor, R. S. (1996) Manifestation and epidemiology of contagious bovine pleuropneumonia in Africa. Revue Scientifique et technique Office International des Epizooties 15(4), 1283-1308.
Tuslane, J. J., Litamoi, J. K., Morein, B., Dedieu, L., Palya V. J., Yami M., Abusugura, I., Sylla, D. and Bensaid, A. (1996) Contagious bovine pleuropneumonia vaccines: the current situation and the need for improvement. Revue Scientifique et technique Office International des Epizooties 15(4), 1373-1396.
|
[33] Kenya Agricultural
Research Institute, Biotechnology laboratory, Nairobi, Kenya; [34] International Livestock Research Institute, Nairobi, Kenya [35] Kenya Agricultural Research Institute, Muguga, Kikuyu, Kenya; [36] 3CIRAD-EMVT, Montpelier, France; [37] International Livestock Research Institute, Nairobi, Kenya |