The hatchery culture of bivalves is as much an art as it is a science and the old adage applies that "there are many ways to skin a cat". In a similar vein, the success of a hatchery is related more to the skill and intuitive "feel" for the work of the manager and technicians than it is to the location, the scale and quality of the physical structure and the sophistication of the available equipment. Every hatchery is different in the way it is managed and in the nuances of the manner in which the various aspects of culture are approached and the work done. There is no standard methodology as such but there are common denominators that relate to the need to fulfill the biological requirements of the different bivalve species through their early developmental stages.
This section of the manual synthesizes the various approaches and the methods used in the culture of larvae from the fertilized egg to settlement with emphasis on some of the more commonly cultured species.
Fertilized eggs are permitted to develop to the fully-shelled "D" veliger stage in tanks of the type shown in Figures 50 and 52. This early veliger stage is known as the D-larva stage because of the characteristic "D" shape of the shell valves (Figure 51). D-larvae of the various, commercially cultured bivalves are similar in appearance.
A wide range of circular or semisquare (square with rounded
corners) tanks can be used for embryo development and also for larval rearing
(Figure 52). They should be constructed from preferably either "virgin" (new,
non-recycled) polyethylene or fibreglass (alternatively known as GRP - glass
reinforced plastic or fibreglass). Previously unused vessels should be filled
with seawater and allowed to soak with weekly changes of water for 2 to 4 months
before use. Soaking removes toxic substances that leach from the surface of new
plastics which may be harmful to larvae.
Steam curing of fibreglass tanks
substantially reduces the period the tanks need to be seawater soaked.

Figure 50: Fertilized eggs can be incubated in various types of tanks in filtered seawater for a period of 2 to 3 days, depending on species and temperature.
Flat-bottomed or steeply tapering conical tanks (i.e. almost flat bottomed) are most commonly used for embryo development (Figure 52). Shallowly tapering conical tanks (shaped like an ice cream cone) are less satisfactory because the early embryos are immobile and will tend to aggregate together at the bottom of the cone.
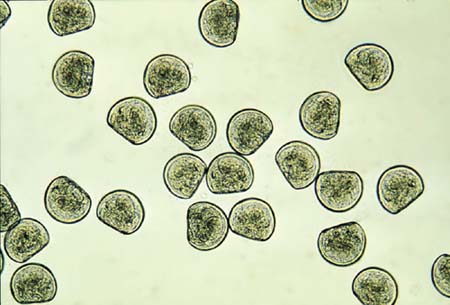
Figure 51: Photomicrograph of Crassostrea gigas D-larvae (48-h after fertilization). Mean size is 75 µm shell length.
Surface area of the tank base rather than water depth is more important. Aeration during this early stage is not recommended. The mechanical effects of the disturbance it creates can lead to abnormal development.
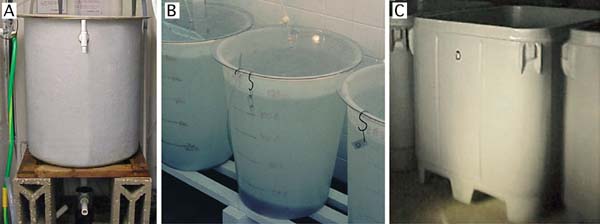
Figure 52: Suitable rearing vessels for embryo (and larval) development. A - 200 l steeply tapering conical fibreglass tank with bottom drain; B - 125 l polyethylene flat-bottomed tank; C - 1 000 l insulated polyethylene, square tank with rounded corners.
Culture tanks are filled with seawater filtered to 1 to 2 µm particle size (Figure 53A) and heated to the required temperature (usually 18 to 24ºC; cooler for cold water species). Some hatcheries disinfect the water following fine filtration by passing it through an ultra-violet light (UV) unit (Figure 53B), the value of which is questionable unless it is used properly and with discretion.
UV units should be maintained according to manufacturer’s recommendations and a record kept of hours of lamp usage. Lamps must be replaced as they reach the specified hours of use at which time the quartz silica sheath that separates the lamp from the water flow needs to be cleaned with a soft cloth soaked in alcohol. Moreover, these units are designed to disinfect freshwater and are not as efficient in killing or immobilizing marine bacteria and other micro-organisms.
As a rule of thumb - if UV disinfection is considered necessary - it is best to pass water through two or three similar units, connected in series, at half the flow-rate recommended for a single unit (Figure 53). It should be remembered that limiting the diversity of bacteria in a culture of embryos or larvae may reduce competition and thereby permit potentially harmful bacteria to predominate. Modern thinking is that the probiotic approach is the better option. This approach entails controlling the density of larvae carefully, feeding them properly with only the best cultured algae available and, paying attention to the hygienic operation of both the cultures and equipment.
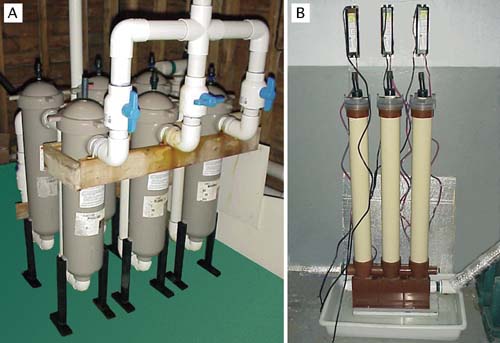
Figure 53: Examples of suitable equipment for water treatment. The multiple bag filtration unit (A) is arranged for fine water filtration. One bank of 3 filters is in use while the second bank is serviced and readied for use. These filtration units contain bags that progressively remove particulate matter from 10 µm down to 2 µin three stages. The uv disinfection unit (B) consists of lamp units arranged in series and is designed to treat a continuous flow of previously filtered seawater. This is the recommended arrangement in seawater treatment rather than relying on the water being treated by a single lamp unit.
It is sometimes beneficial to filter the water and to fill the culture tanks 24 hours before they are needed. This is more applicable in hatcheries located adjacent to estuaries contaminated by industrial or domestic wastes, or by the leachings from richly metaliferous geological strata (and mine workings) in the catchment area, which may contain elevated quantities of heavy metals. The water is then treated by adding 1 mg per l of EDTA (sodium salt - as used in the preparation of algal culture medium) and 20 mg per of sodium metasilicate and is vigorously aerated for 24 hours. Pre-treatment helps to complex heavy metals and render them non-toxic to the particularly vulnerable early stages in development of bivalve larvae. The water does not need to be re-filtered after pre-treatment but aeration is switched-off during embryo development.
Embryos are stocked in the culture tanks about 2 hours after fertilization and at the appropriate density. Fully developed D-larvae are recovered 24 to 48 hours later, depending on species and water temperature (Figure 54). Either no or very low aeration is used during embryo development.
Embryo stocking densities for many of the commonly cultured oviparous oysters and clams can be as high as 50 000 to 80 000 per l of culture, although 20 000 per l is more generally considered to be the safe upper limit (Table 10). In contrast, similarly high initial embryo densities of many of the scallop species leads to abnormal development and numbers are usually restricted to 10 000 to 15 000 fertilized eggs per l of culture tank volume in warmer water species. Egg densities are more commonly based on the surface area of the tanks rather than on tank volume in cold water scallop species, where maximum density should not exceed 1 000 per cm2 (Table 10).
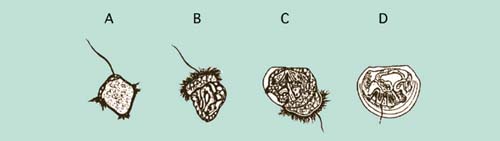
Figure 54: Development of embryos from the early trochophore (A) to the fully shelled D-larva stage (D). The ciliated swimming feeding organ (velum) can be seen in B and early shell valve formation in C. Fertilized eggs will develop to fully formed D-larvae in less than 2 days in many warm water species but the entire developmental process can take 4 or more days in cold water species.
Table 10: Summary data of typical embryo densities (thousands per l), initial D-larva size (shell length, µm), densities of D-larvae (thousands per ml) and culture conditions in terms of suitable temperature (+ 2ºC) and salinity (+ 5 PSU) for the culture of embryos and early larvae of a number of bivalves. Notes: N/A - not applicable: embryo development takes place within the mantle cavity in Ostrea edulis. * Embryo densities in cold water scallops are calculated as embryos per unit area of the base of tanks rather than per unit volume. Maximum density should not exceed 1 000 fertilized eggs/embryos per cm2.
|
Group/Species |
Embryo density |
D-larva |
D-larva density |
Temp |
Salinity |
|
Oysters: |
|||||
|
C. gigas |
15 - 20 |
75 |
10 - 20 |
25 |
28 |
|
C. virginica |
15 - 20 |
65 |
10 - 20 |
25 |
28 |
|
C. rhizophorae |
15 - 20 |
60 |
10 - 20 |
25 |
35 |
|
O. edulis |
N/A |
175 |
5 - 10 |
22 |
30 |
|
Clams: |
|||||
|
T. philippinarum |
20 - 40 |
95 |
10 - 20 |
25 |
30 |
|
M. mercenaria |
15 - 25 |
95 |
10 - 20 |
25 |
28 |
|
M. arenaria |
15 - 25 |
95 |
10 - 20 |
19 |
30 |
|
Scallops: |
|||||
|
P. yessoensis |
* |
105 |
1 - 2 |
15 |
30 |
|
P. magellanicus |
* |
90 |
1 - 2 |
15 |
30 |
|
P. maximus |
* |
95 |
1 - 2 |
14 |
30 |
|
P. ziczac |
10 - 15 |
95 |
2 - 5 |
25 |
32 |
|
A. gibbus |
10 - 15 |
95 |
5 - 10 |
24 |
30 |
|
A. irradians |
10 - 15 |
95 |
5 - 10 |
23 |
30 |
|
Mussels: |
|||||
|
M. edulis |
15 - 25 |
95 |
10 - 20 |
16 |
30 |
A recovery of from 30% to 85% perfectly formed D-larvae from the initial number of embryos stocked is normal in large-scale rearing. Imperfectly formed D-larvae - those with incomplete or misshapen shells - rarely develop further.
Fully shelled D-larvae have a mean shell length of 90 to 100 µm in most species of clams, scallops and mussels, and 55 to 75 µm in oviparous oysters of the genus Crassostrea (Table 10). Crassostrea gigas has larger D-larvae than either Crassostrea virginica or Crassostrea rhizophorae.
A special case is the larviparous oysters of the genera Ostrea and Tiostrea, which have considerably larger eggs. They brood larvae until they are released into the surrounding water at 170 to 200 µm shell length (retained by a 90 µm mesh screen) in the case of Ostrea edulis and an average length of 490 µm in Tiostrea species (see part 4.2.3). Tiostrea larvae are liberated at the pediveliger stage (the pre-settlement and metamorphosis stage) and are ready to set almost instantaneously (<1 hour after release).
Shell length is best measured with a monocular microscope (x100 magnification) fitted with an eye-piece graticule calibrated against a micrometer slide (Figure 55).

Figure 55: Measuring larvae: each larva is oriented and lined-up with the calibrated eye-piece graticule, as shown, and the number of small sub-divisions it spans on the scale, equivalent to shell length, is recorded. In this case, at x100 overall magnification (x10 eye-piece and x10 objective), each small division is 10 µm. Thus, the D-larva shown measures approximately 105 µm.
Normal D-larvae are retained by a 45 µm nylon mesh-based sieve (35 µm in the case of D-larvae of Crassostrea gigas, or 25 µm for C. rhizophorae and C. virginica) and the number recovered is estimated as described later in section 5.1.2.3.
Recovering D-larvae
Tanks containing newly developed D-larvae are drained 2 days after fertilization. There is merit in adding a small quantity of food to the tank on the day prior to draining, i.e. 24-h to 36-h after fertilization. While embryos develop to the D-larvae stage utilizing maternally derived reserves, fully developed D-stage larvae are able to ingest algal cells of the smaller food species and benefit from uptake of dissolved nutrients.

Figure 56: The arrangement of sieves to capture D-larvae from a tank where a smaller diameter, 60 µm mesh sieve is suspended over a larger diameter 40 µm sieve which is partially immersed in a shallow tray containing the discharge seawater. This arrangement permits grading of larvae by size at the point of collection and ensures larvae are not allowed to dry out.
The method employed to catch and retain larvae while tanks are being drained is illustrated in Figure 56. When the tank is full, the drain valve is opened a fraction to allow a slow flow of water into a sieve, or series of sieves, contained in a shallow tray. This arrangement ensures the mesh of the bottom sieve is always immersed in seawater, which minimizes damage to the shells of the fragile D-larvae during draining. As the tank drains down, the valve can be opened further making certain that the flow is not sufficiently violent so as to cause excessive turbulence. Any larvae retained in the tank once it is empty are flushed out with a flow of filtered seawater. Tanks without drains can be emptied by siphoning the water through a similar arrangement of sieves using a length of flexible hose.
D-larvae can be graded during tank emptying by suspending a slightly larger aperture sieve over one with a smaller mesh aperture, as in Figure 56. This is often of benefit and can help separate the better, larger D-larvae from those that are imperfectly formed and abnormal (Figure 57). Once the tank has been completely emptied, filtered seawater is gently sprayed over larvae retained in the upper sieve to wash any smaller individuals into the lower sieve. The contents of larvae in both sieves are washed into separate, graduated containers and are then estimated for numbers and examined as described below. At the same time small sub-samples are often taken to be subsequently measured for shell length.
Figure 57: The appearance of almost 5 million calico scallop, Argopecten gibbus, larvae concentrated in a 20 cm diameter sieve (A) and after transferring to a 4 l graduated jug, preparatory to estimation (B).
Estimating Egg, Embryo and Larval Numbers
Care needs to be taken in handling eggs and larvae. When transferring eggs, embryos or larvae from one container to another through a mesh-based sieve, always ensure that the mesh of the sieve is submerged below the surface of the receiving container. All equipment used in transfers of this kind and in the estimation of numbers should be thoroughly cleaned beforehand and rinsed with filtered seawater.
(i) equipment required
Much of the equipment used for the purpose of estimating larval numbers needs to be specially made. For example, sieves are prepared from PVC pipe or high-impact, rigid styrene garden plant pots or horticultural containers. (Proprietary screens/sieves constructed of metal should be avoided).
To make suitable sieves, the bases of plastic containers are removed and nylon monofilament mesh is fixed tautly in place at the cut end with suitable solvent cement. Alternatively, 15 cm sections of suitable diameter PVC pipe (20 to 30 cm diameter is convenient) are cut and nylon mono-filament mesh fitted to one end in the same way. Sieves should be indelibly marked with mesh size for easy identification.
It is useful to make a number of sieves for each of a wide range of mesh sizes ranging from 20 µm to 250 µm for the various purposes of embryo, larvae and early juvenile culture. Useful sieve sets for clam and scallop larvae are 40, 60, 80, 120 and150 µm mesh aperture (Table 11). The range needs to be extended upwards for larvae of the various commonly cultured oyster species.
Perforated plunger agitators and counting slides can be made in the workshop from plexiglass or transparent PVC pipe, sheet, and rod. Proprietary sedgewick rafter slides, available from scientific and aquaculture suppliers are useful for counting purposes (Figure 58).
Table 11: The relationship between the mesh aperture of sieves (screens) and the minimum size of larvae they will retain. This information is for guidance only and differs from species to species according to the shape of larvae. Experienced hatchery technicians can estimate the mean size of larvae from a culture by their distribution and retention on a range of mesh sizes at grading.
|
Mesh aperture (µm) |
Minimum size of larvae retained shell length (µm) |
|
45 |
75 |
|
80 |
120 |
|
120 |
145 |
|
150 |
170 |
|
160 |
210 |
|
180 |
255 |
|
200 |
280 |
|
220 |
300 |

Figure 58: Equipment used in estimating numbers of larvae. A - perforated plungers for evenly suspending larvae in containers from which known volume sub-samples are taken in the estimation of larval numbers. B - a sedgewick rafter microscope slide, which has a chamber designed to take a 1 ml sample. The chamber has a grid marked on the base for easy tracking while viewing and counting larvae or spat in the sample. Similar slides can be made from transparent plastic.
Specific equipment that needs to be purchased includes adjustable volume automatic pipettes (0.1 to 1.0 ml and 1.0 to 5.0 ml ranges are useful); various measuring cylinders from 25 ml to 2 l volume and wash bottles.
Where budget permits, an electronic particle counter performs the same task as described below and saves time. It is also extremely useful for counting cell density in algal cultures and in determining food cell consumption at all stages in the hatchery process (refer back to Figure 21).
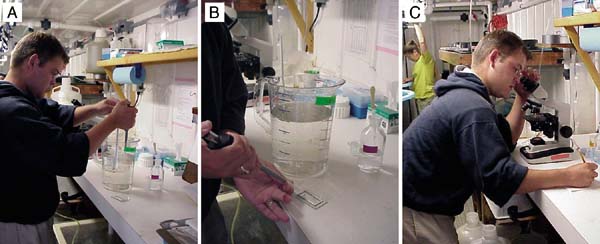

Figure 59: The steps in taking sub-samples of larvae for counting in the estimation of total numbers. A - taking a sub-sample with an automatic pipette while agitating the contents of the jug containing the pool of larvae; B - transferring the sub-sample to a sedgewick rafter slide; C - counting and recording the number of larvae in the sub-sample. The lower photographs (D and E) show a similar technique where larvae, concentrated in a 2 l graduated cylinder are sampled by automatic pipette during agitation.
(ii) estimating procedure (Figure 59)
a) After sieving and rinsing eggs, newly fertilized embryos or larvae, transfer them to a graduated measuring cylinder (1 or 2 l volume) or, if numbers are anticipated to exceed 5 to 10 million, to a bucket or similar larger volume container graduated in l, pints or gallons.
Note: For greater accuracy when using larger volume containers, use an accurately calibrated measuring cylinder or jug to fill the container to about 8 cm below the rim. Note the volume added and mark a calibration line on the inside of the container at the waterline with an indelible marker.
b) Add filtered seawater to the container to the graduation mark. (The total volume needs to be known).
c) With an automatic pipette set at 0.5 ml (volume may vary - see later) take 3 replicate sub-samples of the contents while agitating the contents of the measuring cylinder, or other container, with a suitable diameter perforated plunger. Make sure that the eggs or larvae are evenly distributed in the water column during sub-sampling (Figure 59A).
Note: The diameter of the plunger should be slightly, but not considerably, smaller than the diameter of the container to be sampled. Agitation should be sufficient to lift eggs or larvae off the bottom of the container and evenly suspend them, but not vigorous enough to cause excessive turbulence. A slow, rhythmical up and down motion at about 1 complete cycle per 4 seconds is recommended.
d) Transfer the sub-samples to the compartments of the counting slide (Figure 59B). The compartments should be etched with a suitable grid, as illustrated in.
Note: Take smaller volume sub-samples when numbers of larvae are anticipated to be large, or use a larger volume measuring cylinder/graduated container, or a combination of the two. In the case of eggs, which are very delicate, it may be easier to transfer the egg suspension to a large container, for example, a 10 l polyethylene bucket. Make up the volume to the calibration line and withdraw sub-samples while gently agitating the bucket contents with a large diameter, perforated plastic plunger.
e) Count the eggs or larvae in each sub-sample using a microscope (x40 magnification - Figure 59C).
Note: In the case of eggs and newly fertilized embryos, separate counts can be made of the total number per sub-sample and the number that have not rounded-off and appear abnormal. The same procedure applies to D-larvae where calculation can be made, based on the counts, of the percentage that have developed normally. Similarly, mortality rate estimation can be made on later larvae as part of the counting procedure by counting the live and dead or moribund larvae separately.
f) Calculate the total number as in the following example:
|
Example: Larvae counts in the three sub-samples = 414; 389; 402. Mean = 414 + 389 + 402/3 = 402 Volume of sub-sample = 0.5 ml. Total volume of cylinder = 2 000 ml. Total number of larvae = 2 000/0.5 x 402 = 1 608 000 |
Eggs and larvae can also be counted using an electronic particle counter (e.g. Coulter Counter) fitted with a suitable aperture, sampling head. While this method is quick and convenient, it is impossible to distinguish between normally and abnormally developing larvae or between dead and live larvae. There is no substitute for visual examination and discrimination of the quality of a culture by an experienced hatchery technician.
D-larvae recovered are counted as described above. They are now at the stage where they need feeding with unicellular, cultured algae. Species of good nutritional value include the diatoms,
Chaetoceros calcitrans,
Chaetoceros muelleri,
Thalassiosira pseudonana (3h)
and the flagellates,
Isochrysis galbana (or the ‘T-Iso’ clone),
Pavlova lutherii, and one of the
Tetraselmis species (but only for larvae >120 µm length).
Details of diets, rations and how to calculate them are described in part 5.2
Larvae can be grown in the same flat-bottomed tanks used for embryo development or in conical-based fibreglass tanks fitted with bottom drains (see Figure 52). Tanks may be of relatively small volume (200 to 1 000 l) for experimental purposes and smallvolume production or much greater in both size and volume in high volume output commercial hatcheries. They may be operated as static systems or on flow-through. Water is changed in static systems on a periodic basis, whereas in flow-through culture, it is continuously introduced, a fixed volume being exchanged and replaced daily. This topic is discussed in depth in section 5.1.4.2.
D-larvae of hardier species (including Crassostrea and Tapes) can be grown at densities of 15 000 to 20 000 per l, but growth and survival are generally improved at lower densities (Table 10). Reduced densities are recommended for scallop species of the genera Pecten, Patinopecten, Placopecten and species of Chlamys and Argopecten, where between 5 000 and 10 000 early-stage larvae per l is appropriate. The larviparous flat oyster, Ostrea edulis is generally grown at 2 000 to 5 000 larvae per l because of the large size of the intial D-larvae. Some species can be successfully grown more intensively than above using high-density culture techniques (see section 5.1.4.1).
Rearing tanks are aerated - most commonly by a single, central air outlet located just off the tank bottom - at flow rates ranging from a slow bubble rate for D-larvae increasing to 200 l per hour for later stage larvae. The source of pressurized air needs to be free of carbon and oil. Low pressure, high volume, regenerative air blowers are ideal for the purpose. The air is filtered at source to 0.22 or 0.45.m particle size by a series of cartridge filters of decreasing porosity. This is to reduce air-borne contaminants which may include harmful micro-organisms. It is also advisable in humid conditions to dry the air before it enters the tanks by passing it through a sealed unit, such as filtercartridge housings, containing either anhydrous calcium chloride or silica gel. These drying agents need to be replaced as they become saturated to be effective.
Larval culture tanks and all equipment to be used must be thoroughly cleaned and then rinsed with either freshwater or filtered seawater. Mild liquid detergents added to hot water, or suitably diluted sterilizing/disinfecting agents such as bleach (sodium hypochlorite solution) at 20 mg per l free-chlorine can be used for cleaning purposes. The process of starting a new culture is as follows:
a) Fill the required number of clean larval rearing tanks with seawater filtered to 1 or 2 µm at the required temperature and salinity.
Note: It may be beneficial to reduce near oceanic salinities when rearing euryhaline species such as the American eastern oyster, C. virginica, by adding finely filtered freshwater from a clean, unpolluted source. A salinity of between 20 and 25 psu is recommended for this and other Crassostrea species).
b) If problems have been experienced with abnormal mortality of larvae in the recent past, bacteria may have been responsible. In this situation UV-light treatment of the water before filling the tanks is used by some hatcheries and may help. As a last resort if mortalities persist, a broad-spectrum antibiotic such as chloramphenicol at 2-5 mg per l of seawater may be used experimentally under veterinary prescription.
c) Add D-larvae to the vessel at the appropriate density.
d) Calculate, following the procedure given in part 5.2.3.2, The volumes of harvested algae to add to the vessels to provide the required food ration.
e) Turn on the flow of air so that there is a good turnover of water to suspend and evenly mix both the larvae and food.
f) The culture is then left for 24 hours before further husbandry is necessary.
Cultures of larvae require daily maintenance. They are operated most commonly as static water systems, i.e. without a continuous exchange of water, although some hatcheries operate flow-through culture systems (see section 5.1.4.2). Food cell concentrations need to be maintained at levels conducive to efficient feeding activity.
To prevent the accumulation of potentially harmful, metabolites, tanks require complete water changes at regular intervals throughout larval development from the D-stage to the onset of metamorphosis. The frequency with which this is done depends on the number and mean size of larvae being cultured. Water is changed either at 48-hour intervals or 3 times each week:
- at higher densities of early-stage larvae (15 000 to 20 000 per l at <120 µm),
- at low densities of late-stage larvae (<5 000 per litre at 150 to 200 µm) or,
- at about 2 000 per litre at 250 to 300 µm).Note: The values given are a rough guide to applicable densities related to mean shell length. More accurately, if a culture requires feeding less than 200 cells per µl Isochrysis equivalents per day then it is regarded as low density - see part 5.2.3.1). Water changes need to be daily at higher daily additions of food or alternatively, the tanks operated on the flow-through principle.
(i) Husbandry on non water change day
Husbandry on days between water changes consists of restoring the food cell concentration to compensate for cells eaten in the previous 24-h period by adding sufficient freshly harvested algae. A sample of water is taken from each vessel and the remaining algae cells (residual algae) per unit volume are counted, either by using a microscope with a haemocytometer slide at x100 magnification, or, more easily, by a coulter counter or similar particle counter. Where it is impractical to determine residual algae, either a full or partial ration of food can be added on intermediate days between water changes based on the previous day’s ration fed.
Daily records should be kept of culture temperatures, residual algae and any additions of food to restore optimum food cell concentrations. An example of such a record form is shown in Figure 60. Volumes of additional algae are calculated as described in part 5.2.3.2.
(ii) Husbandry on water change days
The procedure is similar to that described and illustrated in the previous section on embryo development (Figure 56). The tank is emptied by means of a siphon or from a bottom drain, delivering the discharge flow into a sieve which is of sufficient aperture to retain large debris but not the larvae - a 250 µm sieve is ideal (Figure 61). The larvae are retained on the mesh of a lower sieve of suitable mesh aperture.

Figure 60: An example of a daily record sheet and the type of information that is useful to record in order to follow the progress of a batch or a tank of larvae. The steps in calculating the mean shell length of larvae from size/frequency plots are also shown.

Figure 61: Draining static larval tanks on water change days.
The procedure is as follows:
a) Wash any remaining larvae from the vessel into the sieve.
b) Clean the vessel with a sponge and hot detergent or bleach solution and rinse well.
c) Refill the vessel with appropriately treated seawater at the required temperature and salinity.
d) Grade the larvae by washing them through a stack of sieves of descending aperture size with filtered seawater. A guide to suitable mesh sizes for larvae of different shell lengths is given in Table 11.
e) Take small samples from each sieve upon which larvae have been retained and observe the appearance and activity of the larvae with the aid of a microscope. Discard any sieve fraction containing predominantly dead or slow-growing larvae.
Note: Sieve fractions containing mainly empty shells and larvae with decomposing tissues need to be discarded. The tissues of healthy larvae are of golden-brown colouration with a welldefined and darkly coloured digestive gland. Moribund larvae tend to be more darkly and uniformly granular in appearance.
f) Wash fractions containing healthy larvae into a measuring cylinder.
g) Take sub-samples as described before and determine the total number surviving. Measure a sample of 50 to 100 and calculate mean shell length.
Note: The addition of a few drops of formalin (10% formaldehyde solution, neutralized over calcium carbonate in the form of limestone or marble chips) will immobilize the larvae. Discard the counted sample(s).
h) Return the larvae to the culture tank and restore the aeration.
i) Repeat this procedure at 48-hour intervals.
Mention has been made earlier in this section of methods that can be employed to improve the efficiency of larval culture, either by operating the culture tanks on a flowthrough of seawater or by rearing larvae at higher densities in static water tanks. In fact, the two methodologies can be combined to good effect in increasing production where space is limited with the added benefit of reducing the labour component in husbandry.
Although some hatcheries are beginning to turn to flow-through, the practice is not yet widespread. There is, however, ample scope to improve productivity by raising the density at which larvae are reared either by using existing equipment more efficiently or by investing in electronic devices to control feeding. The usual larval densities can be doubled or trebled by feeding to the number of larvae in a tank and their size, rather than by adding food to the water volume to a certain food cell density per unit volume, irrespective of the number and size of larvae. But if larval densities are further increased then the rate at which the feed is supplied becomes critical and needs to be continuously monitored. This approach is more suitable for the hardier species. If mortalities do occur for one reason or another, the effects in terms of lost productivity can be drastic. Most hatcheries prefer to opt for the more cautious approach.
The rate at which food cells are ingested by larvae of different sizes (or weights) of the bivalve species being cultured needs to be known. This information is shown later in Table 12 (part 5.2.3.2) For three commonly cultured species when grown at 24+1ºC. Where such information is lacking it will need to be determined experimentally or by the "trial and error" principle.
Knowing the relationship between larval size and food cell ingestion rate, it is a simple matter to calculate how much food needs to be added to the tank during the next 24-h period for a given number of larvae in culture of a particular mean shell length. Details of the calculation with worked examples and an explanation are given in the next section (part 5.2.3.2). At higher densities than the norm it will be necessary to provide part of the ration as a bulk feed at the beginning of the day; the remainder being dosed at a constant rate by drip feed or peristaltic pump over the next 24 hours.
At more than 20 000 larvae per l, particularly as they approach metamorphosis, feeding rate becomes more critical. It is more detrimental to over-feed larvae than it is to underfeed them. The amount of faecal waste and metabolites will accumulate in the culture water and can give rise to great increases in bacterial numbers. This can reduce feeding rate and result in more food being added to the tank than can be filtered by the larvae when added at a constant fixed rate. The solution has been tackled experimentally by the use of electronic sensors and control equipment to continuously monitor the food cell density in the culture tank (Figure 62). [A full explanation is given in Higgins et al. (1987) - see suggested reading list].
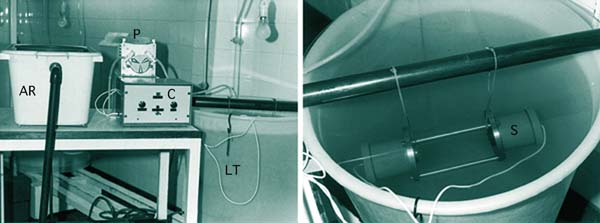
Figure 62: Experimental automatic control of food cell density in high-density cultures of bivalve larvae. AR - chilled, aerated algal reservoir containing the daily food ration; P - peristaltic pump which delivers the required amount of algae upon demand; C - control equipment containing a relay that switches the pump on when the sensor (S) detects a decrease in food cell concentration in the larvae tank (LT) below a certain pre-set threshold. This device utilizes an infra-red transmitter and receiver and could be greatly improved with modern electronics.
A summary of comparative results is given in Table 12, which includes data from trials with European flat oyster and Pacific oyster larvae.
Table 12. The average number of larvae stocked initially (No) and surviving immediately prior to settlement (Np) in 5 comparisons of high and normal density rearing with the European flat oyster, O. edulis, and 3 comparisons with the Pacific oyster, C. gigas. The number of days to the onset of settlement and information on average spat yields (both as % of the initial number of larvae and as spat per l of water used during culture) are also shown.
|
|
Larvae per l |
Days to set |
Yield |
||
|
|
No |
Np |
|
% set |
spat per l |
|
O. edulis |
|||||
|
High density |
9 954 |
5 942 |
9.8 |
40.5 |
512 |
|
Normal density |
1 440 |
1 083 |
10.0 |
40.3 |
161 |
|
C. gigas |
|||||
|
High density |
56 667 |
24 900 |
20.7* |
21.6 |
735 |
|
Normal density |
5 333 |
2 766 |
19.0* |
25.0 |
202 |
* Days to set from the D-larva stage allowing a 4-day settlement period from the time settlement began.
The impetus to develop flow-through methods of larval culture stem from a number of objectives. Larvae of some species are less tolerant than others to generally used methods of culture in hatcheries. Those of the various pectinid species are a good case in point. They usually exhibit higher mortality rates and are not as amenable to highdensity culture in static systems.
Other hatcheries are testing the potential of flow-through technology to make better and more efficient use of available resources. There may be the need for greater production within physical space constraints or to reduce labour costs and time spent in larval husbandry. Flow-through culture does offer these benefits. Time can be saved in raising the density of larvae without the need to drain statically operated tanks 3 or 4 times each week. The method may be wasteful in the use of cultured algae in that water is continuously being exchanged, albeit at a slow rate, and is run to waste. But the food is relatively inexpensive to produce at the required volumes at this stage in the production cycle.
Tank design is important when considering flow-through. Larvae need to be retained within the tank and the volume large enough to ensure that added food has a sufficient residence time to be eaten. The exchange rate needs to be sufficient to prevent metabolic wastes and debris from accumulating, yet there may still be the need to flush out the tank periodically after cleaning the interior surfaces. The general approach is commonly used in culturing the early pre-feeding stages of marine fin-fish, e.g. Halibut, where purpose made tanks are available and can be adapted with minimal alteration. Rather than the flat-bottomed or steeply tapering conical tanks generally used in bivalve larval culture, these conical tanks have shallowly tapering, long cones (Figure 63).
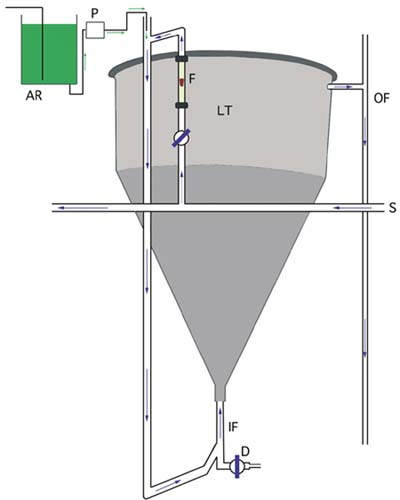
Figure 63: A typical arrangement for flow-through larval culture. See text for a description. Arrows show the direction of flow of both algae and seawater.
The rate of flow of suitably treated seawater (from the delivery pipe, S) is controlled and adjusted by a diaphragm valve and flowmeter (F). Depending on the density of larvae, the flow is adjusted such that the total daily throughput (IF - in-flow, OF - outflow) is the same or greater than the total tank (LT - flow-through larval tank) volume that the number of larvae would require when cultured at normal densities. If, for example, larvae are normally cultured at 5 000 per l in a 500 l tank, then 20 000 larvae per l in a flow-through tank of the same volume will require a minimum throughput of 2 000 l per day. Larvae are retained in the tank by a large diameter "banjo" filter fitted with a suitable aperture mesh screen (see Figure 64 for details).
The tank is fitted with a drain valve (D), which also serves as the input port for a "saltplug" of saturated brine solution. When the in-flow is switched off, this "salt plug" of 2 or 3 l volume is gravity-fed into the drain and the valve closed. Living larvae will swim to the water surface and thereby avoid the dense salt solution, which traps the dead and moribund. After a few minutes, the drain is opened a fraction to eliminate the "salt plug" and the dead larvae, much in the same way as dead marine fish eggs are accumulated and eliminated from incubators.
Larvae are supplied with the required food ration by peristaltic pump (P) from a cooled and aerated algal reservoir (AR). The quantity and rate at which the ration is supplied depends on the number of larvae, the size they are and the flow rate through the tank (refer to parts 5.1.4.1 and 5.2.3.2). Mean shell length can be determined by taking daily samples, but survival is more problematic. An estimate of mortality can be obtained by sub-sampling the volume of saturated brine collected after a "salt plug" and counting the dead larvae it contains. This can be done at 2 to 3 days intervals to keep track of survival.
Internal surfaces of flow-through tanks need to be cleaned with a soft-bristle brush, attached to a pole of suitable length, at least once during the culture period of a batch of larvae. The flow rate should be increased during cleaning to flush out the dislodged debris.
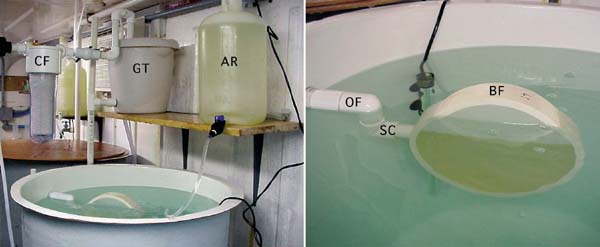
Figure 64: Detail of the top of an experimental flow-through tank showing the "banjo" filter (BF) attached to the out-flow pipe (OF). In this example, seawater filtered to 1 µm by a filter cartridge (CF) is supplied to a gravity tank (GT) from which it flows at a controlled, constant rate into the base of the larvae tank. Food is drip-fed into the tank from an algal reservoir (AR). The "banjo" filter is made from a 20 cm diameter section of PVC pipe and is fitted both sides with 60 µm nylon mesh screen, solvent cemented to the cut faces of the cylinder. A short length of suitable diameter PVC pipe is welded into a hole drilled through the plastic to connect with the out-flow pipe. Large diameter "banjos" are recommended to reduce the per unit area force generated by the out-flow. They should be totally, or almost totally, submerged and need to be cleaned daily. For this purpose, the "banjo" is a push-fit into a swivel coupling (SC) made from a pair of 90o PVC elbows. This coupling can be swivelled upwards to raise the filter above the water level for removal and cleaning, or replacement.
Rearing larvae in flow-through tanks does have disadvantages but they are potentially more than outweighed by the benefits. Since larvae are not graded on a regular basis as in static culture, considerable variability in size will develop over time. Also, larvae will need to be transferred to settling tanks when they reach the pediveliger stage. This is standard practice in many hatcheries but not in others, where larvae are allowed to set on the sides and bases of the larval rearing tanks from which they are later removed.
Removing attached pediveligers and spat from the internal surfaces of shallowly tapering cones will be extremely difficult. Settlement tanks will be needed, particularly for late-stage larvae of the various oyster species that cement themselves to surfaces (see 5.4.3).
The growth and stages in development of larvae of the Pacific oyster, Crassostrea gigas, and the sand scallop, Pecten ziczac, from the D-stage to metamorphosis are shown in Figure 65.
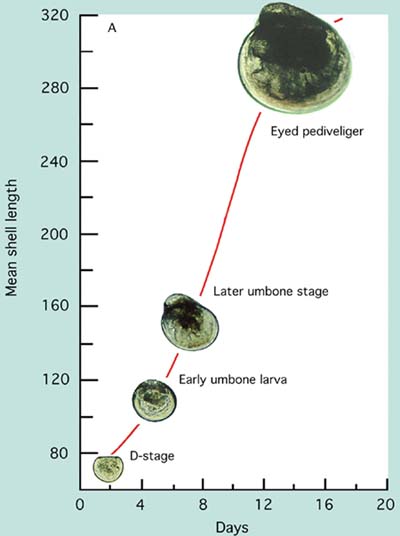
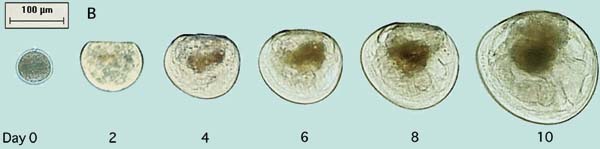
Figure 65: Photomicrographs of the growth and development of Pacific oyster, Crassostrea gigas, (A) and sand scallop, Pecten ziczac, (B) larvae.
Larvae of the different groups of bivalves grow at different rates. Those of scallops and clams have initially larger D-larvae and reach settlement size and metamorphosis at a considerable smaller shell length (210 to 230 µm) than do larvae of the oviparous oysters (320 to 340 µm). Comparative growth rate of a number of species cultured at 24+2ºC is shown in Figure 66. Some species, including the calico scallop, are slower in reaching an exponential rate of growth. They tend to have a lag phase before rapid growth commences. Others like the Manila clam and Pacific oyster grow rapidly from the initial D-stage. Growth rate slows in all species as they approach the settlement stage.
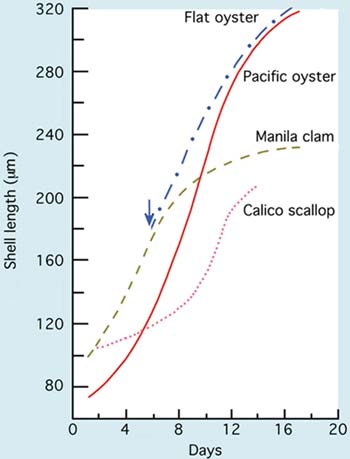
Figure 66: Comparative growth of larvae of some warmer water bivalve species (European flat oyster, Ostrea edulis, Pacific oyster, Crassostrea gigas, Manila clam, Tapes philippinarum and the calico scallop, Argopecten gibbus) from the D-larva stage to metamorphosis when cultured at 24+2ºC. Day 0 denotes the day that eggs were fertilized. The blue arrow shows the day that flat oyster larvae were liberated by brooding adults.
Similarly, survival of larvae from the D-stage to metamorphosis is variable among different species. It may be as high as 50 to 70% on average in some of the oyster and clam species and as low as 15 to 30% in scallops. Much depends on culture protocols and the extent of the culling (removal) of slower growing larvae during the rearing process. A considerable part of losses in larval numbers through the culture period is often associated more with culling and discarding of slower growing individuals than it is to the death of larvae. The proportion of larvae that reach metamorphosis is also related to culture conditions including diet and ration, temperature and salinity and to relatively uncontrollable factors such as seawater quality and disease (see 5.3).
Feeding commences as soon as larvae become fully shelled and their organs including the digestive system have developed. Prior to that time, energy for respiration and development is derived from reserves laid down during egg development (oogenesis) by the maturing females (see 5.3.4). It is also likely that developing embryos are able to absorb organic nutrients from the surrounding seawater. Indeed, there is often benefit in adding a little cultured algal food to tanks containing embryos 12 hours before they have reached the D-larva stage and are capable of ingesting particulate food. It may not be the algal cells themselves that are important but rather organic nutrients in solution in the algal cultures. In this respect, the addition of small quantities of diatoms (e.g. Chaetoceros muelleri at 10 to 20 cells per µl) from cultures nearing the stationary phase appears to be most effective.
Once the velum is developed at the D-larva stage and the fully shelled larvae are swimming, the beat of the velar cilia direct food particles towards the mouth as well as providing the motive force for swimming activity (Figure 67). At this point - Day 0 as it is usually referred to - the quality (diet composition) and quantity (ration) of food added to culture tanks becomes important.

Figure 67: Larvae feed as they swim. Beating of the cilia of the swimming organ, the velum, also directs food particles towards the mouth. The three Day 8 scallop larvae shown are swimming on a collision course. Their darkly coloured digestive glands are clearly visible.
Mixed algal diets are beneficial. A combination of two or three high nutritional value species including a suitably sized diatom and a flagellate invariably provide improved rates of larval growth and development than do single species diets (Figure 68). They also improve spat yields and influence the subsequent performance of spat in terms of both growth and survival.
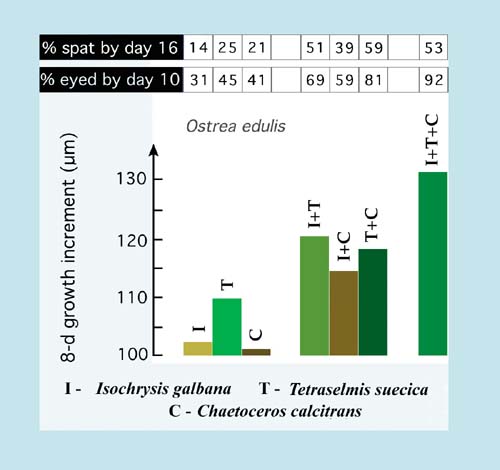
Figure 68: Growth (in an 8-day period), development (% eyed larvae by Day 10) and settlement (percentage spat of the initial larval number at Day 0) of Ostrea edulis larvae fed various single and mixed diets of the three algal species indicated. Values are the means of a large number of trials.
Not all of the suitably sized and readily cultured algal species available from culture collections are of good food value to larvae. But generally those that are valuable foods for the larvae of one species will be of similar value to others. There are exceptions to this rule as will be explained later. The food value of a particular alga is determined not only by its biochemical composition but also its "ingestability" and digestibility. For example, diatoms with long, silicaceous spines may be difficult to ingest and be an irritant to be expelled by larvae closing their shell valves. Some varieties of Phaeodactylum are a good case in point. Other species, such as Chlamydomonas coccoides have thick cell walls that render them almost indigestible. Yet others, including Dunaliella tertiolecta, lack certain essential highly unsaturated fatty acids (HUFAs) required for larval development and while digestible, have little or no nutritional value.
A comparison of the HUFA profiles of a number of algal species that are either good or poor foods for larvae is given in Figure 69. Also shown are values of total lipid content as a percentage of ash-free dry weight.
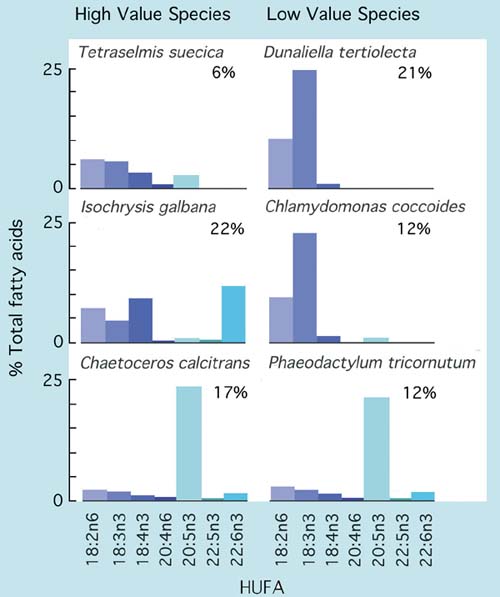
Figure 69: Comparison of total lipid as a percentage of ash-free dry weight and the relative abundance of various highly unsaturated fatty acids (HUFAs) in a number of algal species of both high and low nutritional value to bivalve larvae.
High food value species tend to have relatively high proportions of either 20:5n3 (EPA - eicosapentaenoic acid) or 22:6n3 (DHA - docosahexaenoic acid) compared with many of the poor value species. If these components are lacking in the diet, it appears that the larvae of most bivalves have either no or only a limited ability to synthesise them from less highly unsaturated precursors. It is the case for many of the diet fastidious species that feeding a combination of species rich in either EPA or DHA (or both) will provide the best results. Larvae of clams tend to be less dependent in this respect than those of oysters or scallops.
The relative proportions of HUFAs and the overall lipid content of species of algae useful for hatchery production vary according to phase of the culture cycle and also culture conditions, which differ from hatchery to hatchery. However, species that are of good nutritional value in one hatchery situation will always be of similar value elsewhere given reasonably good attention to details of the culture conditions.
The various diatom species commonly grown in hatcheries have very similar HUFA profiles, all being rich in EPA. Total quantities of particular fatty acids in the different diatom species are somewhat variable. They tend to be higher in cultures entering the stationary phase than during exponential growth.
Among the small cell-size brown flagellates, Pavlova lutherii has a similar HUFA profile to Isochrysis galbana (Figure 69) but tends to have more DHA. In contrast, the T-ISO clone of Isochrysis has only 50 to 70% of the DHA of Isochrysis galbana when grown in side-by-side culture in the same conditions of light and nutrients. T-ISO tends to be grown in more hatcheries than its near relatives because it is easier to culture year round and is tolerant to higher temperatures. Useful substitutes for Tetraselmis are species of Pyramimonas (e.g. P. obovata and P. virginica). They have HUFA profiles intermediate between Tetraselmis and Isochrysis but can be difficult to culture at certain times of year.
A suitable starter diet for D- and early-stage larvae (<125 µm shell length) of most commonly cultured bivalves is a mixture of:
One of the following diatoms:
Chaetoceros calcitrans or Thalassiosira pseudonana (for larvae >55 µm) or Chaetoceros muelleri (for larvae >90 µm),combined with:
One of the following flagellates:
Isochrysis galbana or ‘T-Iso’ or Pavlova lutheri, in equal proportion by cell numbers.
When the mean size of larvae exceeds 120 µm shell length, the larger cell-size flagellate, Tetraselmis spp. (T. chuii, T. suecica, T. tetrahele, etc.), can usefully be added to the diet.
Food rations are usually quoted as the total number of algal cells per microlitre (cells per µl) or per millilitre (cells per ml) of the culture tank volume. Note that 100 cells per µl are equivalent to 100 000 cells per ml.
Account needs to be taken of the fact that the cells of the different algal species vary widely in mean size and, thereby, in volume and mass (see Table 1, Part 3.1). In calculating a ration for a diet incorporating two or three species, the representation of each in the ration is calculated on a cell volume equivalency basis, where (in approximate terms):
1.0 cell of Isochrysis galbana, T-Iso or Pavlova lutherii =
0.1 cells of Tetraselmis sp., or
1.0 cells of Thalassiosira pseudonana, or
2.25 cells of Chaetoceros calcitrans, or
0.75 cells of Chaetoceros muelleri
Thus, a suitable food ration for early-stage Crassostrea or Tapes larvae (and for most other species), where the target food cell density is equivalent to 100 cells Isochrysis per µl, can be satisfied by the following dietary combinations:
125 cells per µl C. calcitrans + 50 cells per µl I. galbana, or
37.5 cells per µl C. muelleri + 50 cells per µl P. lutherii, or
50 cells per µl T. pseudonana + 50 cells per µl P. lutherii
Any of these mixed-species diets are excellent for larvae of bivalves most commonly cultured in hatcheries, although the ration as cells per µl will vary both with species and density of larvae in the culture. Cell densities quoted above are ideal for larvae of the various Crassostrea sp., Ostrea edulis, the clams Tapes philippinarum, Tapes decussatus, Mercenaria mercenaria, Mya arenaria (and many others), and the mussels Mytilus edulis and Perna perna at previously quoted larval densities (refer to Table 10, Part 5.1.2.3). In contrast, larvae of many scallop species show better overall performance when fed the same diets but at lower rations. For example, larvae of the scallops, Pecten ziczac and Argopecten gibbus, express maximum growth rates at a total ration of between 5 cells per µl at the D-larva stage, increasing to 18 cells per µl prior to the pediveliger stage. Other hatcheries use rations two to three times greater with larvae of different scallops, but rarely do they use rations as high as for oysters, clams and mussels, over a similar range of larval sizes.
It will be noted in the example of dietary combinations given above that T-Iso has been omitted. While T-Iso is a perfectly good species to feed to larvae of clams, mussels and scallops, there are reservations to its use in diets for the early larvae of Crassostrea species (Figure 70). Compared with Isochrysis galbana and, more so, Pavlova lutherii, levels of the important highly unsaturated fatty acid (HUFA) DHA are considerably lower. When T-Iso is fed as a single species diet to larvae of the various Crassostrea sp., both larval growth and development are severely retarded beyond a shell length of 110 µm. For this reason, it is recommended that hatcheries should concentrate their small flagellate, algal culture on well proven strains of Isochrysis galbana and Pavlova lutherii.
Larvae can be grown from D-larvae to metamorphosis on two-species combination diets, such as those shown above. However, once mean larval shell length exceeds 120 µm, it is advantageous to add a third species in the form of one of the smaller Tetraselmis sp. Evidence shows that growth rate and the proportion of larvae that successfully complete metamorphosis improves when Tetraselmis is included in the diet (refer to Figure 68).
Either Tetraselmis can be used as a direct substitute for Isochrysis or Pavlova in the diet or, better still, it can be used as an additional species in formulating a three-species diet. It should not, however, be substituted for the diatom in the diet. Each of the three recommended diatoms, mentioned above, contains another important HUFA (EPA) of known nutritional and developmental significance.
When substituted for Isochrysis or Pavlova in a two-species diet, Tetraselmis is fed at 10% of the cell density appropriate to the smaller flagellates, thus:
37.5 cells per µ l C. muelleri + 50 cells per µl P. lutherii
becomes:-
37.5 cells per µ l C. muelleri + 0.5 cells per µl T. suecica
When used as an additional species to make a three-species combination, each of the component species is provided at 33.3% of the target cell density, which may be 100 cells per µ l Isochrysis equivalents. Thus:
Two-species combination:
37.5 cells per µ l C. muelleri + 50 cells per µl P. lutherii =
100 cells per µl Isochrysis equivalents;Three-species combination: 25 cells per µl C. muelleri + 33.3 cells per µl
P. lutherii + 3.33 cells per µl T. suecica =
100 cells per µ l Isochrysis equivalents
The overall amount of algae fed in terms of cell volume/mass is approximately the same for these two example diets, both of which are suitable for larvae >120 µm shell length.
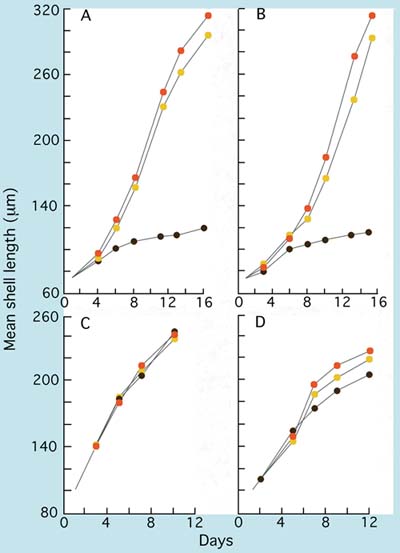
Figure 70: The growth of (A) Crassostrea gigas, (B) Crassostrea rhizophorae, (C) Mercenaria mercenaria and (D) Tapes philippinarum larvae fed T-Iso (brown circles), Chaetoceros calcitrans (yellow circles) and a two species mixture of these two algae (orange circles).
Attention so far in this section has focussed on general guidelines for diets and rations for larvae. In many small owner operator hatcheries run on the "wet thumb" principle - and often on a very tight budget - little or no regard is paid to counting the density of algae at harvest, or in formulating a combination of species to feed with any great degree of precision. An experienced operator rearing one or more of the hardy and tolerant bivalve species will judge which algae cultures look to be the best on a particular day and will add enough of each to the larvae tanks until the water colour looks about right.
At the other end of the spectrum - in large hatcheries supplying seed at industrialscale either for their own growout, or in support of private growers within a region - responsibility and the scale of financial investment dictate that husbandry is maintained under closer control. Here, the priority is to maximize cost-efficient output of the seed products and make a profit. How this can be achieved by making the most effective use of cultured algae in feeding larvae is discussed next.
There are two basic strategies used in hatcheries to ensure that a sufficient food ration is provided to larvae. The first is to add algae to the seawater volume contained in the larval culture tanks with the objective of raising food cell density to a concentration that supports maximum larval growth rate. The second strategy involves feeding the everincreasing biomass of larvae, as development proceeds, according to known food-cell ingestion rates of larvae of different mean shell lengths. This latter approach has already been briefly mentioned in discussion of high density larval culture in Part 5.1.4.1.
Strategy 1 is the easier option, since the volume of water in a tank is unlikely to be varied during the culture period. It is the appropriate strategy where lower densities of larvae are maintained. In effect, the food ration is delivered once per day. Over the course of the next 24-hour period the food will be grazed to a low level. It is only for a brief period in the 24-hour period that food cell concentration is optimal. However, the strategy can be modified, and often is, by feeding an additional 50% (or more) ration 8 to 12 hours after the main ration. The intention is to maintain food cell density nearer the optimum for the greater part of the day. At low mortality rates, the numbers of larvae may need to be reduced as development proceeds by division between two or more tanks so that the ration is not grazed too quickly.
Strategy 2 requires knowledge of the rate at which food cells are depleted from the water by a known number of larvae at all shell lengths (or weights) for all stages in development from the D-larva stage to metamorphosis. Having determined mean size and the number of larvae surviving at successive tank water changes the operator can calculate how much feed needs to be added to the tank to support maximum growth rate for the biomass of larvae at that time. In this way, higher densities of larvae can successfully be maintained in a given tank volume.
However, over-feeding is equally if not more damaging to the performance of larvae than is under feeding. As mentioned previously, at higher larval densities it may be necessary to feed twice each day as two separate rations at the recommended optimum cell density for the larvae of most species, i.e. at close to 100 cells per µl Isochrysis equivalents. Providing a double ration as one bulk feed per day to a culture of larvae will exceed the density and volume of food cells at which feeding activity of larvae is at its most efficient. Overfeeding may lead to bacterially related disease in situations where larvae are already stressed. In this case, the required ration is divided into two equal parts. The first part is added directly to the tank and the remaining half is dosed or drip fed over the following 24-h period.
A logical development in ensuring proper feeding of larvae is the use of modern, sophisticated opto-electronics. Some progress has been made with more primitive devices that shine an infra-red light beam through a culture to a detector, comparing the turbidity caused by the presence of the optimal food cell density in the culture volume with a reference signal. When food cells are grazed by the larvae, turbidity of the water decreases. At a certain preset value, a relay is triggered which activates a small peristaltic pump that adds more algae to the tank from an aerated reservoir until the desired turbidity is restored. The reader is referred to Part 5.1.4 for further information.
Feeding Strategy 1: Volumes of the algal species necessary for addition to larval rearing vessels to achieve the required cell densities are calculated from the following equation:

where V = volume of the larvae culture tank in litres.
|
Example: Basic Information: Diet and cell density to be fed:
Cell densities of harvested algae:
Volume of larvae culture = 800 l Calculation: Volume of C. muelleri required = 37.5x800/4 800 =
6.25 l |
Feeding Strategy 2: Calculation involves determining the number of food cells additional to an initial daily ration equivalent to 75 cells per µl Isochrysis required during the following 24 hours to maintain the algal cell density constant. Steps in the calculation are detailed in the following example that applies to Crassostrea gigas larvae:
|
Example: |
|
|
|
|
|
|
|
Basic Information: |
|
|
|
|
|
|
|
Larvae culture tank volume |
- 1 000 l |
|
|
Number of C. gigas larvae |
- 22.5 million |
|
|
Mean shell length of larvae |
- 170 µm |
|
|
Algal diet provided |
- equal mixture by cell volume of: P. lutherii, C. muelleri, T. suecica |
|
|
Harvest densities of algae |
- P. lutherii = 15 000 cells per µl |
|
|
|
C. muelleri = 7 400 cells per µl |
|
|
|
T. suecica = 1 200 cells per µl |
|
|
Calculation: a) Provide an initial ration of 25 cells per µl P. lutherii, 18.75 cells per µl C. muelleri and 2.5 cells per µl T. suecica = 1.67, 2.53 and 2.08 l respectively at the harvest cell densities given above (see Feeding Strategy 1 for the method of calculation). b) Read the number of cells consumed by a 170 µm Pacific oyster larvae in 24 hours from Table 13 = 30 100 c) Divide 30 100 by the number of algae species in the diet = 10 033 cells per µl Pavlova (1 003 cells in the case of Tetraselmis and 7 525 cells in the case of C. muelleri to account for cell volume differences). d) Calculate the volume of harvested algae of each species required to maintain the optimum food cell density in the 1 000 l tank stocked with 22.5 million larvae:
|
||
|
Similarly, the volume of C. muelleri required is: |
7 525x22.5/7 400 = 22.88 l |
|
|
and for T. suecica it is: |
1 003x22.5/1 200 = 18.81 l |
|
|
e) Add the volumes calculated in (a) above directly to the larval tank. The remainder (15.04 minus 1.67 l for Pavlova, etc.) is mixed in a cooled, aerated reservoir of sufficient volume. Dose this volume at a constant rate over the 24-hour period. From the practical point of view, it is advisable to make up the algae contained in the reservoir with filtered seawater to the volume pumped in 24 hours by the peristaltic pump. Note: Data given in Table 13 applies to larvae grown at 24+1°C. At a fixed rearing temperature, the growth of larvae is generally predictable so that daily measurements of shell length are not essential. Measurements should, however, be made at 48-hour intervals and can be estimated on the intermediate days based on experience. |
||
Table 13: The number of algal cells ingested per larva per day by three commonly cultured bivalves relative to the mean shell length of the larvae. Values are shown as cells equivalent in size to Isochrysis galbana.
|
|
Cells (Isochrysis equiv.) ingested per larva per day |
||
|
Mean Shell Length (mm) |
C. gigas |
O. edulis |
T. philippinarum |
|
|
|
|
|
|
100 |
2 800 |
|
4 400 |
|
110 |
6 700 |
|
6 000 |
|
120 |
10 600 |
|
8 000 |
|
130 |
14 500 |
|
10 200 |
|
140 |
18 400 |
|
12 800 |
|
150 |
22 300 |
|
15 700 |
|
160 |
26 200 |
|
18 900 |
|
170 |
30 100 |
19 200 |
22 300 |
|
180 |
34 000 |
28 200 |
26 000 |
|
190 |
37 900 |
37 300 |
29 900 |
|
200 |
41 900 |
46 300 |
29 100 |
|
210 |
45 800 |
55 400 |
21 900 |
|
220 |
49 700 |
64 500 |
14 900 |
|
230 |
53 600 |
73 500 |
|
|
240 |
57 500 |
82 600 |
|
|
250 |
61 400 |
91 600 |
|
|
260 |
65 300 |
100 600 |
|
|
270 |
69 200 |
109 800 |
|
|
280 |
73 100 |
118 800 |
|
Growth rates of larvae are not significantly different whether they are grown at low density using Feeding Strategy 1 or at high density using Feeding Strategy 2. The advantage of the latter strategy is in operational cost efficiency, both in terms of labour and the better use of hatchery space. When using opto-electronic control of feeding (as in Part 5.1.4.1) estimated volumes of the required food species are calculated as in Feeding Strategy 2.
Effects of diet and food ration were specifically dealt with in the previous section. This section provides useful background information in discussing other aspects of culture conditions and how they influence the performance of both embryos and larvae. Topics include temperature and salinity, seawater quality, egg and larval quality and disease.
Much of the information included has not previously been published and in contrast to other sections of this manual references have been cited in the text to enable the reader to pursue topics of interest in greater depth.
Of all the factors that impinge on growth, development and survival of larvae in culture, temperature is one of the most important since metabolic rate is dictated by temperature of the water in which they swim. Larvae of many of the commonly cultured bivalves exhibit a wide tolerance to both temperature and salinity, often well beyond conditions they may be exposed to in their natural environment. It should not be assumed when one is dealing with a species normally inhabiting cooler, offshore habitats, that larvae will necessarily show optimum performance within the temperature range to which the wild stock is exposed. Often, larvae grow best at higher temperatures than they would experience in nature. Similarly, tolerance limits of larvae to salinity is often much wider than might be anticipated. For example, calico scallop, Argopecten gibbus, larvae from stock adapted to an almost invariable salinity of 36 PSU in Bermuda are able to grow and develop to settlement at 20 PSU. Growth and development are slower, but survival to settlement is little different than in cultures raised at the higher salinity.
The growth of larvae of the Japanese scallop, Patinopecten yessoensis, larvae at various salinities and temperatures is shown in Figure 71. Tolerance exhibited to temperature by this species is quite typical of growth rates that apply to other cooler water scallop species including Placopecten magellanicus and Pecten maximus, which are normally cultured at 14 to 16ºC. Growth, development and survival are adversely affected at higher temperatures. Offshore scallops such as Placopecten magellanicus and Pecten maximus have a higher salinity requirement (>30 PSU). In contrast, larvae of Argopecten species, e.g. the calico scallop (Argopecten gibbus) and the Bay scallop (Argopecten irradians concentricus) can be successfully cultured at temperatures as high as 26 to 28ºC.
Oysters of the genus Crassostrea are extremely tolerant to both temperature and salinity in the culture environment. Interactions of these two factors on growth are shown in Figure 72. In both the Mangrove oyster, Crassostrea rhizophorae, and the Pacific oyster, Crassostrea gigas - and the same is true for the American oyster, Crassostrea virginica - growth, development and survival are near optimal at 28ºC and at a salinity of 25 PSU. Larvae will also tolerate salinities as low as 10 PSU but survival suffers at 5 PSU. Survival exceeded 80% in all treatments over the duration of the trials with the two species.
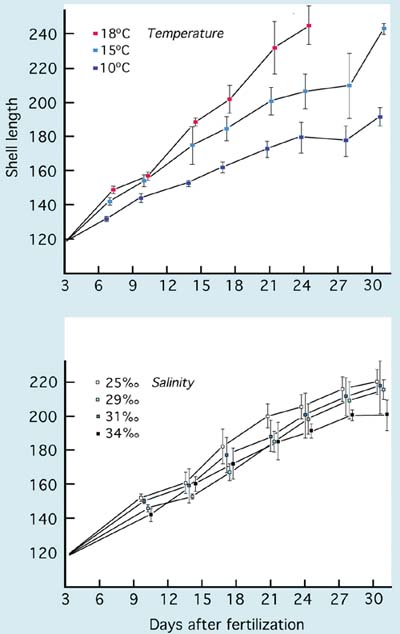
Figure 71: Effects of temperature and salinity on the growth of larvae of the Japanese scallop, Patinopecten yessoensis. Larvae were grown at a salinity of 29 PSU in the temperature trial and at 15ºC in the salinity trial. From Bourne et al. (1989)
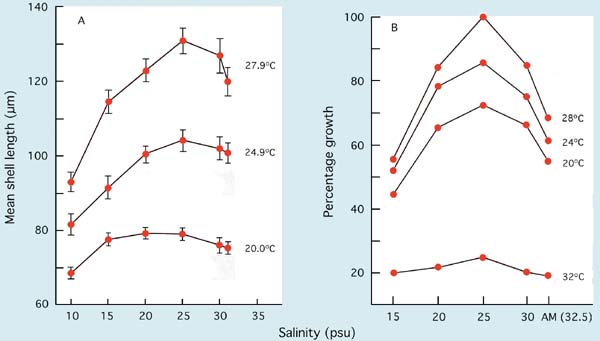
Figure 72: The growth of A, Mangrove oyster, Crassostrea rhizophorae, in a 7-day period from D-stage (initial mean length 65 µm) and B, Pacific oyster, Crassostrea gigas, larvae in a 10-day trial at various temperatures and salinities. Pacific oyster results are expressed as a percentage of the growth of larvae in the best treatment (28ºC at 25 PSU). AM denotes ambient salinity which was 32.5 PSU in B.
Larvae of the European flat oyster, Ostrea edulis, are as tolerant to temperature as the Crassostrea species, but they are not as widely tolerant to lower salinities. While they will survive brief exposure to 20 PSU, growth and development rates in culture are near optimal at 28 to 32 PSU.
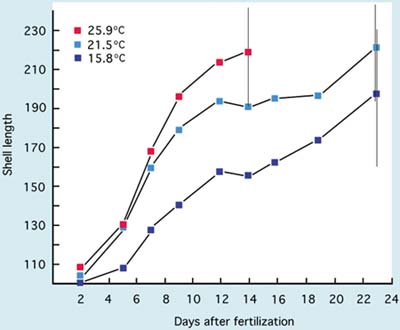
Figure 73: The growth of Manila clam, Tapes philippinarum, larvae from D-stage to metamorphosis at 3 temperatures. Vertical bars denote the range of larval shell lengths (µm) when pediveligers were first noted in cultures (A. Lovatelli, MSc thesis).
The growth of larvae of commercially cultivated, inshore and estuarine clams including the Manila clam, Tapes philippinarum, American hard shell clam, Mercenaria mercenaria, and the soft shell clam, Mya arenaria, also show tolerance to wide-ranging temperature and salinity conditions. Except for Mya arenaria, which is normally cultured at 18 to 20ºC, larvae are generally grown at 25+2ºC and at salinities ranging from 25 to 34 PSU. The effect of temperature on the growth of Manila clam larvae is shown in Figure 73.
It is generally the exception for a hatchery to operate at a constant rate of production throughout the year. Factors of a seasonal nature that cannot easily be controlled may bring about periods in the year when the performance of larvae in terms of growth rate and survival is significantly poorer than at others. In the absence of a technical explanation, such as filter failure or the corrosion of equipment - among other possibilities - or the use of poor quality algal cultures that may have become contaminated, or lapses in husbandry (human error), seawater quality may be responsible.
It has long been established that seawater varies seasonally in its ability to support the growth and survival of embryos and larvae. This may not happen globally, but adverse conditions do occur on both sides of the Atlantic Ocean, particularly when the sea begins to warm in spring and coincident with periods of intense phytoplankton blooming both in spring and in early autumn. The precise reasons for deterioration in seawater quality during these times are not completely understood and they may not recur annually. Some years are better than others in that respect.
By comparing the development of embryos or the growth of larvae week by week in normally treated hatchery seawater and in an artificial seawater control medium using standardized bioassay techniques it is possible to detect and quantify variations in seawater quality. The methodology for bivalve embryo bioassays is detailed in Utting and Helm (1985). Adaptation can be made at the beaker or bucket scale to determine variability as it effects larval growth and survival. Artificial seawater can be prepared according to various recipes from analytical grade chemicals or be purchased as proprietary brands from laboratory suppliers and from fish hobbyist stores. It must always be prepared in the same way as the constant quality, control medium.
An example of seawater quality variability as it influences development of Pacific oyster embryos in the hatchery is given in Figure 74. The development of fertilized eggs to perfectly formed D-stage larvae is expressed as net treatment mortality (NTM), where
A net treatment mortality value of 0 indicates as great a number of fertilized eggs surviving to the D-stage in both media and an NTM of 100 denotes a total failure in development in the normally treated seawater. Negative values indicate that the hatchery water was superior to the artificial seawater.
In the early part of the year in northern temperate latitudes of the Atlantic Ocean, when sea temperatures are cool and day length short, seawater quality is relatively stable. As the coastal waters warm and day length increases towards and during spring and early summer, seawater quality becomes increasingly variable. NTM values begin to climb unpredictably and in some years there will be periods when it is difficult to produce D-larvae from apparently good quality eggs - eggs will develop normally in the artificial control medium but not in the hatchery seawater. The phenomenon applies equally to a wide range of bivalve species, not just to the Pacific oyster.
Unstable seawater quality is generally coincident with intense phytoplankton production in coastal waters during spring bloom conditions. There is no direct evidence that it is the metabolites or break down products of the phytoplankton that cause deterioration in water quality. Rather, it may be the bacteria associated with blooms or the metabolites, including the exotoxins, they produce.
A situation of this kind is illustrated in Figure 74 at a hatchery location where the dominant algal species towards the end of the spring bloom in coastal waters was the colonial flagellate, Phaeocystis pouchetti. Comparison of the NTM values for the different years shows that the quality of hatchery seawater was at its poorest when numbers of bacteria forming colonies on TCBS agar were at their highest. Bacteria that form colonies on this agar include species of the genus Vibrio. They include known opportunistic pathogens, such as V. anguillarum, which have frequently been reported as among the more dominant bacteria in hatchery systems at that time of year. They are often implicated in disease situations. V. anguillarum is known to produce potent exotoxins including a low molecular weight ciliostatic toxin that inhibits the beating of cilia of the velum in larvae and gills in juveniles.
Seawater quality for embryo development can be improved by various chemical pretreatments for 24-hour periods before use. This is in addition to the usual filtration and UV disinfection measures. Frequently effective treatments include the addition of 1 mg per l EDTA (ethylenediamine-tetraacetic acid) and 20 mg per l sodium metasilicate (Na2SiO3.9H2O) to tanks containing filtered seawater which are then vigorously aerated until required for use 24 hours later. The percentage of eggs that develop to the D-larva stage can often be significantly improved by such treatment. For example, in 28 trials with Pacific oyster embryos, yields of D-larvae from weekly spawnings throughout the hatchery season (March to September) were improved from a mean of 36.6% to 52.9%. The improvement through use of chemical pre-treatment compares with a mean yield of 54.6% in the artificial control medium.
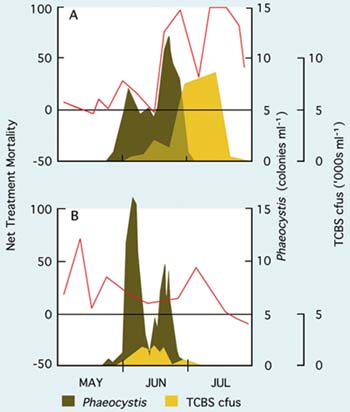
Figure 74: The relative survival (as net treatment mortality - red line) in bioassays comparing development to D-stage larvae of fertilized Pacific oyster eggs in artificial and in normally treated hatchery seawater during the period May to July, 1977 (A) and 1978 (B). The horizontal black line, equivalent to a net treatment mortality of zero, denotes equality in survival in both the tested seawater and the control medium. The blooming of the colonial flagellate, PhaeocyZstis pouchetti, (as colonies per ml) and the number of bacteria colonies (cfus - colony forming units - as thousands per ml) growing on TCBS agar in samples taken from the adjacent coastal waters are superimposed. Adapted from Utting and Helm (1985) including previously unpublished data.
The growth rate of larvae from the D-stage is also influenced by variability in seawater quality in much the same way and for the same reasons as is embryo development. Again, effects on growth have been evident in all species of bivalves tested. Comparative growth of Pacific oyster larvae over a 6-day period from the D-stage when cultured at the beaker scale at 25ºC in normal hatchery water and in an artificial seawater preparation (Lyman and Fleming formula, from Sverdrup et al. (1942)) is shown in Figure 75. Differences in growth rate are expressed as Growth Index (GI), where:
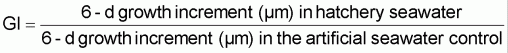
Growth indices >1.0 are indicative of periods when growth was superior in hatchery water; a GI of 1.0 shows parity in performance between the two media and GIs of <1.0 denote times when growth rate was inferior in hatchery water compared with the artificial medium.
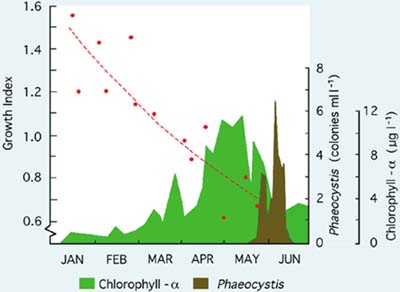
Figure 75: The comparative growth of Pacific oyster larvae over a 6-day period at 25ºC in normal hatchery seawater and artificial seawater calculated as a growth index (see text). Chlorophyll - á and the numbers of Phaeocystis pouchetti colonies at the hatchery intake are shown as indicators of phytoplankton production in the coastal waters adjacent to the hatchery. (M.M. Helm, unpublished)
Results shown in Figure 75 imply a progressive deterioration in seawater quality from the beginning of the hatchery season in January until trials ended in late May when - for a period of approximately 6 weeks - larvae failed to survive the 6-day experimental period in either medium. Until the end of April, batches of larvae reared for spat production developed normally to settlement in hatchery seawater and provided good yields of spat. Large-scale culture was problematic beyond that time with poorer survival and eventual failure of larvae to reach settlement. The same trend is apparent for European flat oyster, Ostrea edulis, larvae where deteriorating performance both in terms of growth (Figure 76) and survival often culminated in disease and the total mortality of broods of larvae in culture in May and June. Again, seawater quality was more variable in some years than in others.
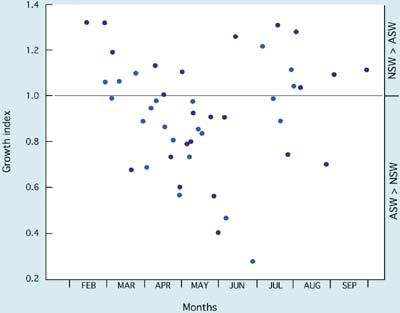
Figure 76: Growth indices of samples of broods of European flat oyster, Ostrea edulis, larvae grown at the beaker scale in hatchery and artificial seawater for a 4-day period from the time of release at 24+1ºC over a hatchery season. Results cover a 2-year period - differentiated by the shade of the data points. From M.M. Helm (1971) and previously unpublished data.
The quality of eggs in terms of their quantitative and qualitative biochemical composition also has a bearing on subsequent performance of larvae. Studies on this topic have mainly focussed on lipid content and, in particular, on the importance and role of highly unsaturated fatty acids (HUFAs) either donated by the female during oogenesis or mobilised directly from the diet during the period of egg maturation prior to spawning.
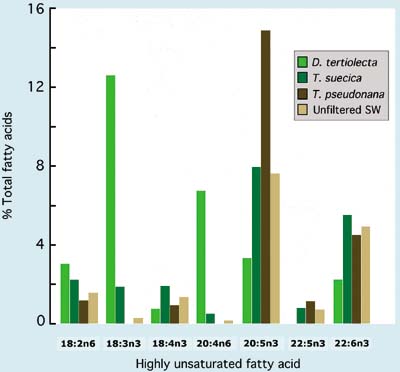
Figure 77: The highly unsaturated fatty acid composition of Manila clam, Tapes philippinarum, eggs from a hatchery-held broodstock provided with different diets during conditioning. Control clams were kept in unfiltered seawater while others were supplied a 3% ration of either Dunaliella tertiolecta, Tetraselmis suecica or Thalassiosira pseudonana in 2µm filtered seawater. (I. Laing, A. R Child and M. M. Helm - previously unpublished data).
The conditions to which females are exposed during oogenesis and egg maturation can have a profound effect both on fecundity and the quality of eggs subsequently spawned. Diet composition and abundance of food are of great importance and apply equally to stocks in their natural habit and in the hatchery broodstock conditioning environment. The HUFA composition of freshly spawned eggs can be significantly altered by the diet provided during conditioning (Figure 77) but there is no evidence that such changes exert a readily discernable effect on the viability and vigour of the larvae developing from the eggs. Neither is there any evidence to suggest that larvae from wild stock European flat oysters are any more or less viable than from a hatchery conditioned stocks although their HUFA profiles may be very different (Figure 78). The differences may be too subtle to be obvious in the context of hatchery culture where efforts are made to provide near optimal conditions for the larvae.
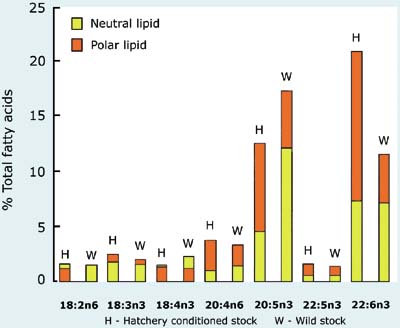
Figure 78: A comparison of the highly unsaturated fatty acid composition of hatchery conditioned and wild stock European flat oyster, Ostrea edulis, larvae. Fatty acids are differentiated into neutral (triacylglycerols) and polar (structural) components. Modified from M.M. Helm et al. (1991).
What tends to be more important is the total lipid content of newly spawned eggs, or of newly liberated larvae in the case of the European flat oyster. Total lipid as a proportion of the ash-free dry (organic) weight of Pacific oyster eggs is positively correlated with the percentage that develop to the D-larva stage (Figure 79). Even when using a standard broodstock conditioning protocol, lipid content can vary widely according to time of the year and from year to year (Figure 80A). This may be explained by the quantity, diversity and nutritional value of food present in unfiltered seawater supplied to conditioning tanks before cultured algae is added (Figure 80B). It may also help to explain why some years in the hatchery are more productive and suffer fewer disruptions than others.
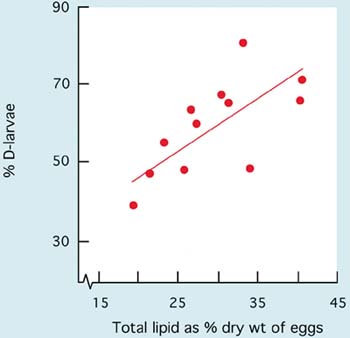
Figure 79: Relationship between total lipid as a percentage of dry weight and the percentage of Pacific oyster, Crassostrea gigas, eggs that develop to the D-larva stage. From S.D. Utting and M.M. Helm (1985) and previously unpublished data.
In larviparous oysters, e.g. Ostrea edulis, the growth increment of larvae in the 4-day period following liberation from adults is significantly correlated with lipid content at the time of release, suggesting the importance of maternally donated reserves during early larval development (Figure 81). Again there are indications of seasonality and differences between years. However, such effects become less pronounced as larvae continue to develop when diet and ration fed day by day are of overriding influence.
There is close similarity in the relationship between shell length and the ash-free dry weight of larvae amongst most of the bivalve species commonly cultured when they are each grown in near optimum conditions (Figure 82A). The exception among species investigated is the larviparous oyster, Ostrea edulis, where there is increasing divergence in shell length related ash-free dry weight in later stage larvae compared with larvae of Crassostrea sp. This is explained by the more than 3-fold difference in the rate at which lipid is accumulated as larvae grow towards metamorphosis (Figure 82B). Studies suggest that lipid is much more important as an energy source during metamorphosis in Ostrea edulis than it is in the oviparous oysters.
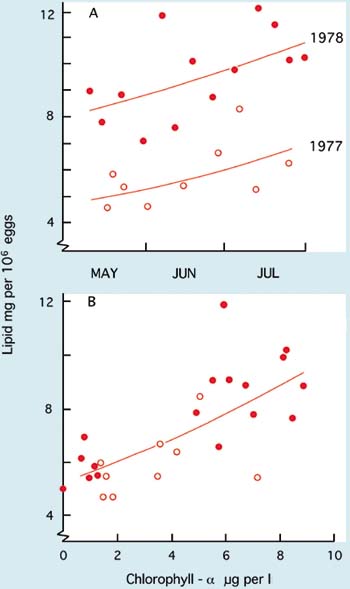
Figure 80: Relationships between the total lipid content of freshly spawned Pacific oyster eggs (expressed as lipid per million eggs) and, (A) months of the year in two different years and (B), the chlorophyll - á content of unfiltered seawater supplied to broodstock in a hatchery when employing a standard conditioning protocol. From Utting and Helm (1985) and previously unpublished data.
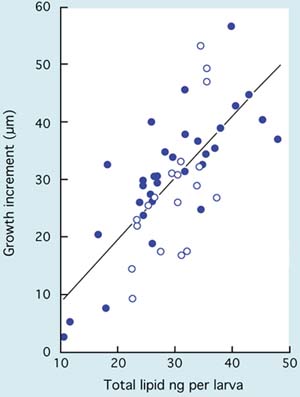
Figure 81: The relationship between the growth increment of Ostrea edulis larvae in a 4-day period from liberation and total lipid content at liberation from hatchery conditioned broodstocks. Each data point represents a specific brood of larvae over a 2-year period - each year differentiated according to the shading of the data point. Larvae were cultured on the beaker scale in artificial seawater and provided with the same diet and ration to provide standardized conditions throughout the sequence of trials. From M.M. Helm (1971) and previously unpublished data.
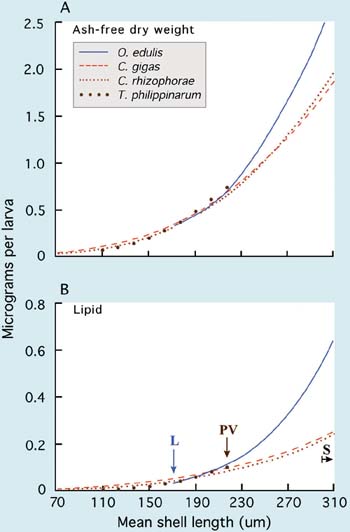
Figure 82: A comparison of increases in both (A) ashfree dry (organic) weight and (B) lipid content per larva relative to mean shell length in larvae of four bivalve species. L - denotes mean size at liberation of Ostrea edulis larvae; PV - the onset of the pediveliger stage in Tapes philippinarum and S - the onset of settlement in the three oysters. Source: M.M. Helm - previously unpublished data.
Mention was made in 5.3.3 of the implication of bacteria of the genus Vibrio in mass mortalities of larvae, which occur from time to time in the best run hatcheries. Vibrio sp. may not always be the direct cause of abnormal rates of mortality, nor are they the only group of opportunistic or obligate pathogens that can contaminate cultures and present problems. Potentially pathogenic species are present within the hatchery environment year round but are for the most part held in check by being only a minor part of the bacterial flora. At other times of the year, as indicated in 5.3.3, they may proliferate and dominate the microbial flora, posing a serious threat to production.
Before ascribing mass mortalities of larvae to a disease outbreak other potential causes need to be investigated. For example, the cleanliness of pipelines and filters needs to be inspected. Likewise, equipment such as pumps and air blowers that may have corroded or be leaking oil needs to be thoroughly examined. Algal cultures may have become badly contaminated or a technician may have made an error of judgement or miscalculation and grossly overfed a culture, or have forgotten to switch on the air flow to a tank or tanks, or not rinsed a tank after bleach treatment. Only after every avenue has been investigated and discounted should the possibility of disease be considered.
Unlike disease in larval fish, the onset of disease in bivalve larvae is swift and catastrophic. Larvae rarely show protracted symptoms leading to a mass mortality situation. They might appear perfectly normal in terms of colouration and behaviour the night before, but by the next morning be on the bottom of the tank, either dead or moribund with their shells almost devoid of tissue and filled with ciliate protozoans as opportunistic scavengers. Often there is prior warning in terms of larvae not grazing the food to the extent that would normally be expected the day before a mass mortality occurs. This highlights the importance of maintaining thorough records.
Once larvae have dropped to the bottom of a tank there is little the hatchery operator can do other than add a powerful sterilizing agent such as bleach to the tank. Even though a small percentage of the larvae may still be active and appear normal, they will invariably die before they reach metamorphosis if a pathogen is implicated. The objective is to try and contain the disease and eliminate the source of infection. This may mean closing the hatchery for thorough fumigation, ensuring that all equipment is cleaned and sterilized. The hatchery is then allowed to lie fallow for a week or two before production is resumed. The use of antibiotics is inadvisable during such outbreaks. They rarely improve the situation and there is always the risk that the pathogens will gain resistance to them.
Many hatcheries concentrate their production during periods of the year when mass mortalities are unlikely to occur. In temperate regions the most reliable period is winter and early spring, i.e. before the onset of phytoplankton blooming. Late June through to the end of September is often suitable for uninterrupted production.
Further reading on disease in bivalve larvae is to be found at the end of Part 5.
Larvae swim freely in the water column for much of the larval phase (Figure 83A). Typically, they will swim upwards to the water surface and then retract the swimming/ feeding organ (the velum), close their shell valves and sink towards the bottom to resume swimming activity again. As they develop towards the end of the larval phase, feeding activity slows, less food is consumed, and larvae spend an increasing period of time towards the bottom and on the bottom of the tank. This marks the beginning of metamorphosis, a critical stage in development during which extensive mortalities can occur. Considerable anatomical changes take place during metamorphosis. Successful transformation and survival to the juvenile form is dependent on a number of factors, not least of which is the availability of energy reserves accumulated during the larval phase. The importance of producing healthy larvae with large energy reserves cannot be over stressed.
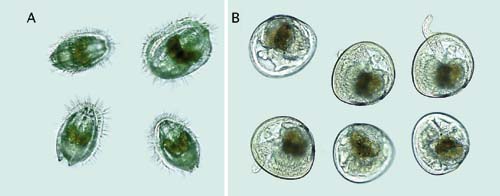
Figure 83: Photomicrographs of (A) swimming Argopecten gibbus larvae showing the ciliated swimming/feeding organ, the velum, and (B) eyed pediveligers of the same species. The foot can be seen extending between the shell valves in three larvae and the small, black eye-spot is visible below the digestive gland, particularly clear in the upper left larva in (B).
Metamorphosis can be divided into two stages, settlement which is reversible (except in oysters) and metamorphosis which is irreversible.
Settlement is the initial stage of metamorphosis. Larvae begin to drop out of the water column onto a substrate, crawl around on the substrate using their foot with the shell upright and search the surface for a suitable place to settle (Figure 83). If the surface is unsuitable they will move off or swim away and seek a more suitable location. This process can be repeated several times and metamorphosis can be delayed for some time if a suitable surface is not found.
Metamorphosis is the second stage and it is irreversible. Factors that trigger it remain unknown but type of substrate along with physical, chemical and biological cues are undoubtedly important. Considerable morphological and physiological changes occur in the animal at this time as it changes from a swimming larva to a spat. Metamorphosis can occur quickly but can be delayed if suitable conditions are not met. In hatcheries it can sometimes be delayed if water temperature is reduced.
Indication that substrate searching behaviour, preparatory to settlement (sometimes termed fixation or setting) and metamorphosis has, or is about to begin, is the appearance - in many species - of a pair of darkly pigmented eye-spot, one on either side between the surface of the digestive gland and the shells valves (Figure 83). What role the eye-spot actually plays is a matter for conjecture. The appearance of the eyespot is size-related (see later) and coincides with "stringing" or "funneling" behaviour of the larvae en masse when they aggregate together with mucous secretions when transferred from screens to buckets at water change (Figure 84). These are clear signs that larvae are ready to set.
Figure 84: "Stringing" (or "funneling") behaviour of mature larvae prior to settlement. The black mass is numerous larvae aggregated together at and just below the water surface in a bucket.
At this time, or a day or two later, larvae will also be seen probing a newly developed foot between the shell valves (Figure 83B). This foot has a ciliated tip and numerous sensory receptors and is used in substrate searching when larvae are seeking a suitable niche to settle and either form a byssus or cement attachment with the chosen location. The foot provides them with the mobility to crawl across surfaces and may also have a feeding ("pedal feeding") function in some species. It is also the site of byssus or cement glands, depending on species. Oysters form cement attachments with the surface while other bivalves attach with byssus threads. Larvae are referred to as pediveligers at this stage.
There has been considerable controversy as to whether bivalve - and other invertebrate larvae - settle and metamorphose on a rigid schedule or if they select a particular substrate and require a specific cue(s) before the process begins. At present the general belief is that environmental cues influence settlement and metamorphosis and that larvae require specific chemical stimuli before the processes of settlement and metamorphosis are initiated. Studies show these cues are chemicals called neurotransmitters and they must be present to initiate settlement and metamorphosis.
Strategies to facilitate and enhance settlement of pediveligers vary widely among hatcheries according to species and to the methods that will be employed to grow the early juveniles. Hatchery managers want larvae to settle on a convenient substrate (cultch - see 5.4.3.2) and to begin metamorphose as quickly as possible. Studies have shown that several methods, including both physical and chemical stimuli help initiate these processes. The most common physical method used is temperature shock, chilling mature larvae (sometimes in a refrigerator) and then placing them in warm water in setting tanks. Results have been variable but there is an indication that the success rate of metamorphosis can be improved when this method has been used.
A common method to stimulate and increase the success of metamorphosis is the use of chemicals. Several have been tried including ammonia and a group of chemicals known as neurotransmitters including L-DOPA (L-3-4-dihydroxyphenylalanine), epinephrine, norepinephrine and yohimbine.
Many hatchery managers question the use of chemicals to stimulate and increase the success of metamorphosis and they are not used at many hatcheries. Managers believe that high rates of successful metamorphosis of hatchery produced bivalve larvae can be attained at either a hatchery or a remote setting site if the larvae are of high quality with good food reserves and are handled properly. They believe addition of neurotransmitters may initially yield higher rates of successful metamorphosis than untreated larvae, but little if any difference is observed in the number of juveniles that grow to 5 to10 mm in treated or untreated larvae. Neurotransmitters have permitted some larvae to metamorphose that ordinarily would not have been able to do so but they do not have sufficient reserves to develop further into juveniles.
The material used to settle larvae on at hatcheries or remote setting facilities is termed cultch and it can be a variety of materials. Two important criteria for cultch are that it must be a suitable surface for larvae to settle on and it must be easily handled.
Oyster hatcheries on the West Coast of North America do not always settle the pediveligers themselves but supply growers with eyed larvae to be set remotely at locations adjacent to the oyster farms (Figure 85). This methodology is dealt with in Part 6.2

Figure 85: A remote, oyster setting system located on Vancouver Island, British Columbia, Canada. Eyed Pacific oyster, Crassostrea gigas, larvae are received from West Coast hatcheries and are set in concrete tanks packed with net bags filled with clean, aged Pacific oyster shells. Once the shells have a sufficient set - a few days later - they are moved to nursery growout at the farm.
The following is a synopsis of the more common methods employed to set mature, eyed larvae of the various bivalve groups.
(i) Oysters
Surfaces are provided for settlement either in the tanks in which the larvae are grown - directly in the broodstock tanks in the special case of Tiostrea larvae - or in special-purpose settlement tanks. This is when 50% or more of the larvae are at the eyed stage and applies equally to Ostrea and Crassostrea species. Hatcheries will often grade out the largest larvae in a batch on a 240 µm mesh (retaining larvae of 300 to 340 µm shell length) for settlement, leaving the remainder to grow and develop further. Appropriate densities of oyster larvae per unit volume at settlement are within the range 2 000 to 5 000 per l although the area of settlement surface is the more important criterion. Types of materials in common usage to provide large surface areas for settlement include the following:
a) Sheets of slightly roughened PVC, which may be vertically stacked in the water column with each sheet separated by a spacer, or be a single sheet lying on the base of the tank. (PVC sheet formed in the shape of semi-cylindrical roof tiles are also sometimes used).
b) Layers of shell chips and particles prepared by grinding aged, clean oyster shell spread over the base of settlement trays or tanks. The particulate material is graded so that only pieces that pass through a 500 µm but are retained by a 250 µm screen are used.
c) Bundles, bags or strings of aged clean oyster shells dispersed throughout the water column, usually in settlement tanks.
d) Various plastic or ceramic materials coated with cement (lime/mortar mix). For example, stacks of cement-coated, plastic Chinese "hat" collectors are sometimes used to settle oyster spat in large tanks. Once grown to a suitable size spat can be removed by bending and flexing the collectors to break apart the cement coating.
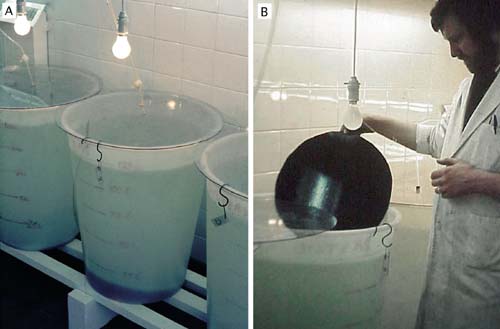

Figure 86: A and B - In this example, matt surfaced PVC sheets used as settlement substrate for oyster spat are placed on the base of larval culture tanks (A). The tanks are illuminated by overhead, tungsten filament lamps to aid rapid settlement. Spat collectors are checked several times each day (B) and when the set density is sufficient, the newly settled spat are gently removed with a razor blade. C and D - Staff at a Cuban oyster hatchery stringing mangrove oyster shells on lengths of nylon twine (C). These strings are placed in concrete settlement tanks with sufficient eyed larvae to provide the required set density (D). Large-volume larval culture tanks can be seen behind the settlement tanks in photograph D.
Larvae tend to settle and attach more prolifically on the shaded under surface of substrate materials in shallow tanks. A low intensity tungsten filament lamp (60W), mounted above deeper tanks, will also encourage larvae to settle towards the bottom in the more shaded areas (Figure 86A and B). The attractiveness of large surface area collectors can be enhanced by painting them with an aqueous extract of homogenized oyster flesh. They are then allowed to air-dry before being placed in the settlement tanks. The reason is that larvae exhibit gregarious behaviour and will tend to attach where others have attached before. PVC collectors improve in their ability to attract settlement with usage over time. When sufficiently "aged" they do not require coating with the aqueous extract.
Methods (a) and (b) above are used to produce what are known as "cultchless" spat. Cultchless oyster spat (spat that are no longer attached to a substrate or are attached to a shell particle) can then be grown as separate individuals through to marketable size to service the half-shell trade. In contrast, survivors of those set on whole shells will eventually grow together, their shells fuse and form clusters, and are suitable only for meat extraction when harvested.
To provide cultchless spat when using PVC collector sheets, the newly set spat need to be removed from the surfaces with a razor blade within 24 hours of attachment. This is done by immersing the sheet in a shallow tray of seawater and gently scrapping the razor blade, mounted in a suitable holder, across the surface while spraying the blade with a jet of seawater. The number of removed spat can be estimated using the same method as for larvae (section 5.1.2.3). They are then moved to the within hatchery, early juvenile culture system.
As previously mentioned, eyed oyster larvae can be encouraged to undergo metamorphosis without cementing themselves to a substrate by the use of the neurotransmitter, epinephrine. This involves dissolving 0.1832 g of epinephrine (adrenalin) in a little 10% hydrochloric acid and then diluting it in 10 l of filtered seawater, which is a sufficient volume to treat 2 million eyed larvae. Larvae of settlement size are exposed to this treatment for 60-90 minutes and are then returned to the culture tanks. At the next water change, larvae that have metamorphosed and have begun growth as spat are graded from those that are still larvae by retaining them on a 270 µm mesh sieve. Only larvae ready for imminent settlement will respond to this treatment and will complete metamorphosis without fixation. Larvae that do not respond are unharmed and can be treated again one or two days later. This method of treatment can be used with or without the provision of settlement surfaces (usually with).
Post-settlement survival rates in oysters are generally high with 50 - 70% of those that set reaching 2 mm shell length.
(ii) Scallops
In contrast to oyster larvae, eyed scallop pediveligers form a byssus attachment to surfaces upon which they settle. They will attach to filamentous red algae, hydrozoans, bryozoans and polychaete tubes in nature, amongst other suitable living and non-living substrates. Polyethylene netting, nylon mesh and a variety of other similarly filamentous materials provide satisfactory substitutes in the hatchery. Eyed pediveligers can be set in larval tanks or in purpose-provided settlement tanks, either in static water conditions or in flow-through. In the latter, a mesh screen on the outflow is essential to retain larvae within the tank. Since scallop larvae, pediveligers and early juveniles are particularly fragile and delicate the tendency is to remove them from the larvae tanks when eyed and to transfer them to settlement tanks. This is at a considerably smaller shell length than for oyster larvae - 220 to 240 µm compared with 300 to 340 µm. Examples of suitable types of settlement tank and collector materials are shown in Figures 87 and 88.

Figure 87: Scallop pediveligers can be set at a density of up to 2 000 per l in cultch filled tanks equipped for static water, recirculation or flow-through. The system illustrated is at the Bermuda Biological Station of Research, Inc. and is used for both Argopecten gibbus and Pecten ziczac. See text for an explanation of the steps involved.
Scallop pediveligers can be set at densities of between 1 000 and 2 000 per l in cultchfilled tanks equipped for static water, recirculation or flow-through. The example shown in Figure 87 utilizes circular, fibreglass fish tanks of 450 l volume (A) fitted with bottom drains and standpipes. Bundles of plastic mesh netting (B) are loosely packed in the tank(s) (C) or are enclosed in fine mesh "onion" bags suspended in the water column (D). Spat set mainly on the black mesh (E). The plastic pipe arrangement above the water surface, clearly visible in D, is part of an air-lift driven upwelling system. Each vertical limb has an air-line fitted at the base. With the air flow turned on, water is lifted from the base of the tank to be sprayed from drilled holes in the above-water delivery pipes and back into the tank. In operation the water level in the tank half covers the delivery pipes.
Settlement tanks are treated as larval culture tanks for the first 6 to 8 days once pediveligers have been added. Water is changed 3 times during this period by draining water through a sieve to retain the remaining swimming larvae (note the drain valves visible in Figure 87A). At the same time, filtered seawater is added at a rate to balance with the out-flow in order to maintain the water level constant, which prevents the cultch and attached larvae from being exposed to air. This water exchange is continued for 30 to 45 minutes. Numbers of larvae retained on the sieve, their survival and the numbers of metamorphosed but unattached spat are estimated before returning them to the tank. Tanks are gently aerated during this period and are supplied with food in exactly the same way as in larval culture.
After this first week, the air-lift driven upwelling system is switched on and the temperature of the water in the tank is gradually lowered over a period of days to the ambient. The tanks are then operated on flow-through by turning on a continuous and sufficient in-flow of ambient temperature seawater to exchange the tank volume 3 or 4 times each day. The air-lift driven upwelling is maintained and food is added continuously. Three weeks after introduction of the pediveligers the largest attached spat measure 2 mm shell height (Figure 87E).
In essence, the process described above is a hatchery adaptation of the widespread use of plastic mesh contained in "onion" bags to capture natural spat in the sea. A different approach is to set pediveligers in trays or cylinders with suitable aperture mesh bases (120 or 150 µm aperture). The trays are held in shallow tanks through which food supplemented water is recirculated or flows continuously to waste (Figure 88).
Figure 88: A - cylindrical, nylon mesh based trays used to set scallop pediveligers at the Bermuda Biological Station for Research, Inc. The trays are partially immersed in shallow raceway tanks through which seawater can either be recirculated or flow to waste. Each tray receives a downwelling flow of cultured food supplemented seawater. B - the appearance of 3-week old Argopecten gibbus spat growing attached to the mesh base of the tray. A grid marked in 1 cm squares has been placed beneath the mesh to indicate the density of set and enables estimates of numbers to be determined.
Pediveligers are stocked in the trays at a density no greater than 100 per cm2 of the base area. For example, a 25 cm internal diameter cylinder has a bottom mesh area of approximately 500 cm2 and can be stocked with up to 50 000 pediveligers. Space availability for settlement of the pediveligers and for the growth of those that attach and metamorphose to juveniles is critical in determining stock density. Spat are mobile and will respond to overcrowding by detaching their byssus attachment and swimming in search of a less crowded area to re-attach. Soft tissue damage resulting in mortality will occur if spat collide with their neighbours and interlock their shell valves.
Various adaptations of the concept of setting scallop pediveligers in shallow, meshbased trays are used in Europe for Pecten maximus.
Post-settlement survival rates in scallops are usually not very high; 15 to 30% from the initial number of pediveligers to 2 mm shell height is considered normal. Survival tends to be greater using the set in trays method (Figure 88) but growth rate is superior when using bundles or bags of mesh (Figure 87). This is probably because spatial separation of the attached spat is much improved over the large surface area provided throughout the volume of the tank in the latter case.
(iii) Clams and mussels
Clam larvae begin substrate search behaviour at a similar shell length to scallop larvae (at 220 to 240 µm). They also attach to surfaces and to each other, with byssus threads. A convenient way to handle them at this stage is to transfer them to a settlement tank, such as in the example shown in Figure 88, until metamorphosis is complete. Otherwise, they can remain in the larval tanks until settlement is complete. Since they are of similar size and behaviour to scallop pediveligers, similar densities can be set per unit area of the trays. Although adult clams bury themselves in the substrate in nature there is no need to provide substrate until the spat exceed 7 mm shell length. Settled spat can be removed from surfaces with a water jet.
Mussels also attach by means of byssus threads, but more strongly than scallops and clams and they retain their ability to form such attachments throughout their life. Because of their low per unit value compared with oysters, scallops and most of the commercial clams, hatchery culture is less common. Mussel spat are usually collected in nature although interest in hatchery production is now being shown on the West Coast of the USA and in New Zealand. Panels or coils of the same materials used to catch wild spat can be used in the hatchery, including rope, netting and panels of plastic mesh. The type of system with deeper tanks shown in Figure 87 is equally as appropriate for the settlement of mussel larvae as it is for scallops.
How spat are grown once they have settled is dealt with in the next section.
Bayne, B.L. 1969. The gregarious behaviour of the larvae of Ostrea edulis L. at settlement. J. Mar. Biol. Assoc. UK, 49: 327 - 356
Bourne, N., Hodgson, C.A. & Whyte, J.N.C. 1989. A Manual for Scallop Culture in British Columbia. Canadian Tech. Rep. Fish and Aquatic Sciences, No. 1694: 215 pp.
Breese, W.P. & Malouf, R.E. 1975. Hatchery manual for the Pacific oyster. Sea Grant Program Publ., No. ORESU-H-75-002. Oregon State Univ., Corvallis, Oregon, USA: 22 pp.
Brown, C. & Roland, A. 1984. Characterization of exotoxin produced by a shellfish pathogenic Vibrio sp. J. Fish Diseases, 7: 1 - 10
Brown, C. & Russo, D.J. 1979. Ultraviolet light disinfection of shellfish hatchery sea water: (1) elimination of five pathogenic bacteria. Aquaculture, 17: 17 - 23
Calabrese, A. & Davis, H.C. 1970. Tolerances and requirements of embryos and larvae of bivalve molluscs. Helgol. Wiss. Meeresunters., 20: 553 - 564
Castagna, M. & Manzi, J.J. 1989. Clam culture in North America: hatchery production of nursery stock clams. p 111 - 125. In: Manzi, J.J. & Castagna, M. (eds) Clam Mariculture in North America. Developments in Aquaculture and Fisheries Science, 19. Elsevier, Amsterdam, Oxford and New York.
Chu, F.-L.E., Webb, K.L., Hepworth, D., Barrett, D.B. & Roberts, M. 1983. Growth and fatty acid composition of oyster larvae. In: (eds: Pruder, G.D., Langdon, C. & Conklin, D.) Proceedings of the 2nd International Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition, October 1981, Rehoboth Beach, Delaware. Louisiana State University Press, Baton Rouge: 426 (abstract)
Coon, S.L. & Weiner, R.M. 1985. Induction of settlement and metamorphosis of the Pacific oyster, Crassostrea gigas (Thunberg) by L-DOPA and catecholamines. J. Exp. Mar. Biol. Ecol., 94: 211 - 221
Coon, S.L., Fitt, W.K. & Bonar, D.B. 1990. Competency and delay of metamorphosis in the Pacific oyster, Crassostrea gigas (Thunberg). Mar Biol., 106: 379 - 387
Couturier, C., Dabinett, P. & Lanteigne, M. 1995. Scallop culture in Atlantic Canada. p 297 - 340. In: Boghen, A.D. (ed) Cold-Water Aquaculture in Atlantic Canada. The Canadian Institute for Research on Regional Development, Moncton, Canada: 672 pp.
Crisp, D.J., Yule, A.B. & White, K.N. 1985. Feeding of oyster larvae: The functional response, energy budget and a comparison with mussel larvae. J. Mar. Biol. Assoc. UK, 65: 759 - 783
DiSalvo, L.H., Bleoka, J. & Zebal, R. 1978. Vibrio anguillarum and larval mortality in a California coastal shellfish hatchery. Appl. Environ. Microbiol., 35: 219 - 221
Elston, R., Leibovitz, L., Relyea, D. & Zatila, J. 1981. Diagnosis of vibriosis in a commercial oyster hatchery epizootic: diagnostic tools and management features. Aquaculture, 24: 53 - 62
Elston, R. & Leibovitz, L. 1980. Pathogenesis of experimental vibriosis in larval American oysters, Crassostrea virginica. Can. J. Fish. Aquat. Sci, 37: 964 - 978
Gabbott, P.A. & Holland, D.L. 1973. Growth and metabolism of Ostrea edulis larvae. Nature, London, 241: 475 - 476
Gallager, S.M., Mann, R. & Sasaki, G.C. 1986. Lipid as an index of growth and viability in three species of bivalve larvae. Aquaculture, 56: 81 - 103
Helm, M.M. 1971. The effect of sea water quality on the laboratory culture of Ostrea edulis L. larvae. International Council for the Exploration of the Sea. ICES. CM 1971/K:28: 11 pp.
Helm, M.M. 1977. Mixed algal feeding of Ostrea edulis larvae with Isochrysis galbana and Tetraselmis suecica. J. Mar. Biol. Assoc. UK, 57: 1019 - 1029
Helm, M.M. 1986. Effects of nutrition on larval production in the European oyster, Ostrea edulis. Proceedings 14th Meeting on Environment and Resources: Applications and Perspectives in Aquaculture, Albarella (RO), Italy, 20-28 September 1986: 12 pp.
Helm, M.M. 1990. Moderna proettazione e gestione di schidutitoi per molluschi bivalvi e nuovi sviluppi (Hatchery design and general principles of operation and development). p 65-87. In: Alessandra, G. (ed) Tapes philippinarum: Biologia e Sperimentazione. Ente Svillupo Agricolo Veneto, Venice, Italy: 299 pp. (Italian and English text)
Helm, M.M., 1990. Managing Production Costs - Molluscan Shellfish Culture. p 143-149. Congress Proceedings, Aquaculture International, September 4-7, 1990, Vancouver, BC, Canada: 480 pp.
Helm, M.M. 1991. Development of industrial scale hatchery production of seed of the mangrove oyster, Crassostrea rhizophorae, in Cuba. Food and Agriculture Organization of the United Nations. FAO: TCP/CUB/8958: 46 pp.
Helm, M.M. 1994. Towards reliable bivalve seed supply in Nova Scotia. Bull. Aquacul. Assoc. Canada 94 (4): 9 - 14
Helm, M.M. & Laing, I. 1987. Preliminary observations on the nutritional value of Tahiti Isochrysis to bivalve larvae. Aquaculture 62: 281 - 288
Helm, M.M. & Millican, P.F. 1977. Experiments in the hatchery rearing of Pacific oyster larvae (Crassostrea gigas Thunberg). Aquaculture, 11: 1 - 12
Helm, M.M., Holland, D.L. & Stephenson, R.R. 1973. The effect of supplementary algal feeding of a hatchery breeding stock of Ostrea edulis L. on larval vigour. J. Mar. Biol. Assoc. UK, 53: 673 - 684
Helm, M.M., Holland, D.L., Utting, S.D. & East, J. 1991. Fatty acid composition of early non-feeding larvae of the European flat oyster, Ostrea edulis L. J. Mar. Biol. Assoc. UK, 71: 691 - 705
Holland, D.L. & Spencer, B.E. 1973. Biochemical changes in fed and starved oysters, Ostrea edulis L., during larval development, metamorphosis and early spat growth. J. Mar. Biol. Assoc. UK, 53: 287 - 298
Huggins, W.L., Helm, M.M. & Williams, D.R. 1987. Automatic control of food supply in the culture of filter feeding organisms. Aquacultural Engineering, 6: 259 - 275.
Inamura, H., Nakai, T. & Muroga, K. 1985. An extracellular protease produced by Vibrio anguillarum. Bull. Jap. Soc. Sci. Fish., 51: 1915 - 1920
Jia, J. & Chen, J. 2001. Sea farming and sea ranching in China. FAO Fisheries Tech. Paper, No 418, Food and Agriculture Organization, UN, Rome: 71 pp.
Jeffries, V.E. 1983. Three Vibrio strains pathogenic to larvae of Crassostrea gigas and Ostrea edulis. Aquaculture, 29: 201 - 226
Jespersen, H. & Olsen, K. 1982. Bioenergetics in veliger larvae of Mytilus edulis L. Ophelia, 21: 101 - 113
Knottage, A.S. & Birkbeck, T.H. 1986. Toxicity to marine bivalves of culture supernatant fluids of the bivalve-pathogenic Vibrio strain NCMB 1338 and other marine vibrios. J. Fish Diseases, 9
Lewis, T.E., Garland, C.D. & McMeekin, T.A. 1986. Manual of hygiene for shellfish hatcheries. Department of Agricultural Science, University of Tasmania. University of Tasmania Printing Dept., Hobart, Tasmania: 45 pp.
Loosanoff, V.L. & Davis, H.C. 1963. Rearing of bivalve mollusks. Advances in Marine Biology, 1, Academic Press Ltd, London: 1 - 136
Lovatelli, A. 1985. Conditions for the culture of clam larvae with particular reference to Tapes semidecussatus (Reeve). M.Sc. Thesis. Plymouth Polytechnic, UK: 179 pp.
Malouf, R.E. & Breese, W.P. 1977. Food consumption and growth of larvae of the Pacific oyster, Crassostrea gigas (Thunberg), in a constant flow rearing system. Proc. Nat. Shellfish Assoc., 67: 7 - 16
Manahan, D.T. & Crisp, D.J. 1982. The role of dissolved organic material in the nutrition of pelagic larvae: amino acid uptake in bivalve veligers. Amer. Zool., 22: 635 - 646
Moreno, J.E.A., Moreno, V.J. & Brenner, R.R. 1976. Lipid metabolism of the yellow clam, Mesoderma mactroides: 2 - polyunsaturated fatty acid metabolism. Lipids, 11, 561 - 566
Munn, C.B. 1978. Haemolysin production by Vibrio anguillarum. FEMS Microbiol. Letters, 3: 265 - 268
Roland, W.G. & Broadley, T.A. 1990. A manual for producing oyster seed by remote setting. Province of British Columbia, Ministry of Agriculture and Fisheries, Victoria, BC, Canada: 58 pp.
Strickland, J.D.H. & Parsons, T.R. 1968. A practical handbook of seawater analysis. Bull. Fish. Res. Board of Canada. 167: 1 - 311
Sverdrup, H.U., Johnson, M.W. & Fleming, R.H. 1942. The Oceans: their Physics, Chemistry and General Biology. Prentice-Hall, New York: 1087 pp.
Tubiash, H.S., Chanley, P.E. & Leifson, E. 1965. Bacillary Necrosis: A Disease of Larval and Juvenile Bivalve Mollusks: 1. Etiology and Epizootiology. J. Bacteriol., 90: 1036 - 1044
Utting, S.D. & Helm, M.M. 1985. Improvement of seawater quality by physical and chemical pre-treatment in a bivalve hatchery. Aquaculture. 44: 133 - 144
Utting, S.D. & Spencer, B.E. 1991. The hatchery culture of bivalve mollusc larvae and juveniles. Lab. Leafl., MAFF Fish. Res., Lowestoft, No. 68: 31 pp.
Waldock, M.J. & Holland, D.L. 1984. Fatty acid metabolism in young oysters, Crassostrea gigas: polyunsaturated fatty acids. Lipids, 19: 332 - 336
Waldock, M.J. & Nascimento, I.A. 1979. The triacylglycerol composition of Crassostrea gigas larvae fed on different diets. Marine Biology Letters, 1: 77 - 86
Walne, P.R. 1974. Culture of Bivalve Molluscs. Fishing News (Books) Ltd, Surrey, England: 189 pp.
Webb, K.L. & Chu, F.-L.E. 1983. Phytoplankton as a source of food for bivalve larvae. In: (eds: Pruder, G.D., Langdon, C. & Conklin, D.) Proceedings of the 2nd International Conference on Aquaculture Nutrition: Biochemical and Physiological Approaches to Shellfish Nutrition, October 1981, Rehoboth Beach, Delaware. Louisiana State University Press, Baton Rouge: 272 - 291
Wilson, J.H. 1979. Observations on the grazing rates and growth of Ostrea edulis L. larvae when fed algal cultures of different ages. J. Exp. Mar. Biol. Ecol., 38: 187 - 199
Whyte, J.N.C. 1987. Biochemical composition and energy content of six species of phytoplankton used in mariculture of bivalves. Aquaculture, 60: 231 - 241
Whyte, J.N.C., Bourne, N. & Hodgson, C.A. 1987. Assessment of biochemical composition and energy reserves in larvae of the scallop Patinopecten yessoensis. J. Exp. Mar. Biol. Ecol., 113: 113 - 124
Zaroogian, G.E., Pesche, G. & Morrison, G. 1969. Formulation of an artificial sea water media suitable for oyster larvae development. Amer. Zool., 9: 1144
Zimmer-Faust, R.K. & Tamburri, M.N. 1994. Chemical identity and ecological implications of a waterborne larval settlement cue. Limnol. Oceanogr., 39: 1075 - 1087