Miyagawa Shoji, Tokyo, Japan
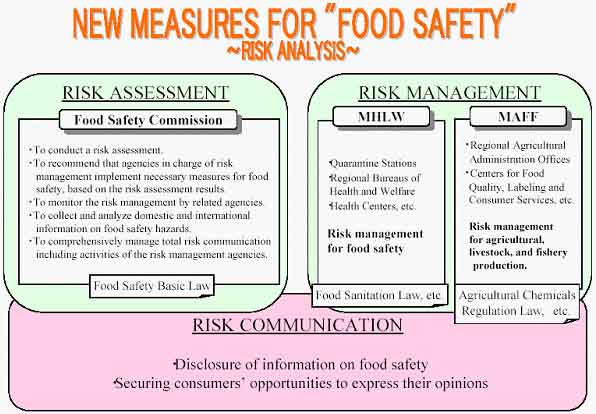
There has been a growing concern and distrust on food safety among the Japanese public, triggered by various problems, notably Bovine Spongiform Encephalopathy (BSE), agricultural chemicals residues in imported foods, and false labelling.
In July 2003 the Japanese Government enacted the Food Safety Basic Law to introduce a risk analysis approach into food safety regulation, focusing on consumer health protection. And it required the Food Safety Commission (FSC), newly established in the Cabinet Office and headed by seven scientific experts, to conduct risk assessment in risk analysis on individual issues on food safety[81]. In addition the Food Sanitation Law is revised with the aim of protecting the public health, through the implementation of well-designed measures to ensure food safety[82]. Food safety regulations under the jurisdiction of the Ministry of Health, Labour and Welfare (MHLW) are a mainstay of risk management measures implemented by the government.
Regulatory Framework on Veterinary Drug Residues in Food
Veterinary drug is defined as any substance that is used for medical treatment of animals including livestock and aquaculture fish and shellfish. The veterinary drugs used in animal production include anthelminthics, chemically-synthetic antimicrobials, and antibiotics. In the Japanese regulatory framework the Food Safety Commission conducts toxicological evaluation as a part of risk assessment under the Food Safety Basic Law. Under the Food Sanitation Law the MHLW establishes the tolerances for residues that may remain in foods as result of the veterinary drug use during animal production with reference to the conclusion of toxicological evaluation from the FSC. The tolerance is normally expressed as Maximum Residue Limit (MRL). The Ministry of Agriculture, Forestry and Fishery (MAFF) authorizes the production of veterinary drugs upon the request and establishes standards for applications of the drug under the Pharmaceutical Affairs Law
a) Residue Standards under the Food Sanitation Law
The Food Sanitation Law prescribes that foods may not contain antibiotics. It also prescribes that meat, milk, eggs, and fish/shellfish may not contain chemically-synthetic antimicrobials. These provisions were established from the viewpoint of human public health, considering safety of veterinary drugs themselves and drug-resistance in micro-organisms, which may result from antibiotics use of these drugs.
Since 1995 the MHLW has been establishing residue standards for veterinary drugs. The standards for 30 veterinary drugs including antibiotics, anthelminthics, and synthetic hormone are currently enforced.
The residue standards like MRLs for veterinary drugs are established in a procedure similar to those for pesticide residues in food. The FSC conducts safety evaluations of the veterinary drug based upon the information from the required toxicity studies such as chronic study, teratotoxicity study, and carcinogenicity study. When the No-Observed-Adverse-Effect-Level (NOAEL) is established, the Acceptable Daily Intake (ADI) is obtained by applying safety factors to the NOAEL.
When the ADI is established the MHLW proposes draft MRLs to the Pharmaceutical Affairs and Food Sanitation Council, an Advisory body to the Minister of Health, Labour and Welfare, based upon the result of residue study in the authorized application of the veterinary drug, and taking into account the Codex standards as well as residue standards in foreign countries. The Theoretical Maximum Daily Intake (TMDI) or Estimated Maximum Daily Intake (EDI) is obtained using the drafted MRLs and the food intake data from the national nutrition survey. When the TMDI or EDI is found to be less than the ADI and that there will be no health problem associated with the residue below the MRLs, the advisory body adopts the MRLs.
When a substance is found display carcinogenicity together with genotoxicity the ADI for the substance cannot be established. In this case the tolerance for the residue from the substance is established as "Not Detected (ND)" at the level of detection (LOD) in the official analytical method.
b) Control of Veterinary Drug Residue in Food
The MHLW has a national monitoring programme on residues in animal origin foods. The purpose of the monitoring programme is to confirm the compliance of products in the marketplace. The quarantine stations under the jurisdiction of the MHLW monitors imported products and the local governments monitor the products in the domestic markets. The tests on veterinary drug residues are performed by the official laboratories in quarantine stations and local governments.
In 2002, 7,912 samples from domestic products and 10,871 samples from imported products were analyzed and 3 samples in domestic products and 18 samples in imported products were found not to comply with the Japanese requirement of veterinary drug residues[83]. Products not meeting the specifications and standards of the Food Sanitation Law are discarded, reshipped, or otherwise disposed of. The analyzed samples were fresh meat, poultry, egg, milk, honey, and aqua cultured fish and shellfish like eel, salmon and shrimp.
Positive List System for agricultural chemical residues in food
Japan has stipulated the introduction of a new system for agricultural chemical residues in the revised Food Sanitation Law, for instance, considering a recent increase in imported foods. The system, the so-called positive list system, is aimed at prohibiting the distribution of foods that contain agricultural chemicals such as pesticides, veterinary drugs and feed additives unless MRLs for them are established under the Food Sanitation Law. This system will come into effect within three years after the publication of the revised law on 30th May 2003.
Under this system, Japan is going to clarify quantitative limits of chemicals that are allowed to remain in foods. It will prohibit foods from being distributed in the domestic market when these chemicals are detected at levels exceeding the established limits in the foods. A certain level that is unlikely to pose adverse health effects is uniformly applied to chemicals for which MRLs are not established. This would be similar to the level known as a "default level" in the Western countries.
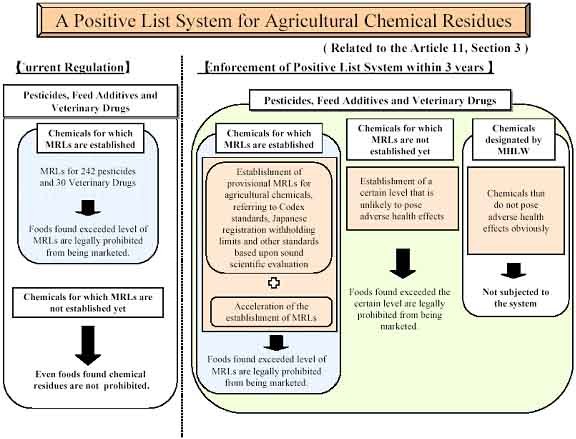
Japan works to develop provisional MRLs as an interim measure to protect consumer health as well as to facilitate food distribution. The provisional MRLs are applied in the period from the enforcement of the positive list system to the time when their formal MRLs are established based on the regular procedure. This measure is taken to prevent the possibility that foods that are currently allowed to be distributed would be unfairly treated after introduction of the system because no MRLs are established. Provisional MRLs are targeted at chemicals for which certain MRLs, such as Codex MRLs, have already been established based on sound science. These chemicals include pesticides that are permitted for use in Japan under the Agricultural Chemicals Regulation Law.
In October 2003 the MHLW proposed the first draft of the provisional MRLs for the positive list system and requests for the comments on the draft[84]. The provisional MRLs for 647 agricultural chemicals including veterinary drugs are proposed. Around 1,200 comments from domestic and foreign parties were received. The MHLW is going to publish the second draft of the provisional MRLs, incorporated with some comments to the first draft, in Summer 2004 and to request comments on the draft from the foreign governments, consumers and industry sectors. The MHLW will also notify the proposed MRLs to the World Trade Organization (WTO) in accordance with the WTO SPS agreement early next year.
Consideration of Default Level
It is generally recognized that the agricultural chemicals such as pesticides and veterinary drugs are only allowed to be used during the production with an authorization based upon an evaluation concerning human health and in some case the use of substances is subjected to appropriate application to meet tolerance of residues in foods. The default level in the Japanese positive list system for agricultural chemicals will apply in cases like residues resulting from the use of an unauthorized substance or an unauthorized application.
The Pharmaceutical Affairs and Food Sanitation Council has discussed the default level as a risk management option for agricultural chemical residues in foods. It evaluates the toxicological thresholds of various substances and exposures through food consumption. In the discussion of the toxicological thresholds the Council reviews the report of the forty-fourth meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA) concerning the safety evaluation of flavoring substances in 1995[85] and the US Federal Registers on threshold of regulation for substances used in food contact articles in 1995[86]. These two documents conclude that the thresholds of human exposure to substance as less than 1.5µg/person/day would be reasonable to protect human health from substances in foods.
The council also reviews the ADIs set by the Joint FAO/WHO Meetings on Pesticide Residues (JMPR) and the MHLW as well as exposures to the substances. While the Council has not reached any conclusion, 0.01 ppm as the default level in the Japanese positive list system for agricultural chemicals would not result the human exposure to a substance exceeded from 1.5µg/person/day.
The Commission of the European Communities proposed a regulation on MRLs of pesticides in products of plant and animal origin in 2003[87]. The regulation states that the products shall not contain any pesticides residue exceeding 0.01mg/kg for active substances for products for which no specific MRL is set.
Risk Assessment of Semicarbazide
In response to the information from the European Union the MHLW began to examine AOZ and semicarbazide, which are metabolites from Nitrofurans, in powdered egg exported from Belgium in April 2003.
The preliminary toxicological evaluation on nitrofurans (furazolidone and nitrofurazone) and their metabolites (AOZ and semicarbazide respectively) was conducted by the Pharmaceutical Affairs and Food Sanitation Council in June 2003. The Council concluded that the nitrofurans and their metabolites may be carcinogenic and genotoxic and ADI for the substances are inappropriate to be established. Based on the evaluation the council recommended that the food should not be distributed when AOZ and semicarbazide are detected in the food.[88]
As Semicarbazide was reported to be in wide range of foods packed in glass jar in the studies in Europe the MHLW is planning to ask the Food Safety Commission for the risk assessment of the substance. In addition the MHLW initiated a study to analyze the presence of Semicarbazide in baby food packed in glass jar.
| [81] Food Safety Commission;
http://www.fsc.go.jp/english/index.html [82] Ministry of Health, Labour and Welfare (MHLW); Revision of Food Sanitation Law (in Japanese), http://www.mhlw.go.jp/topics/bukyoku/iyaku/syoku-anzen/kisei/index.html [83] MHLW; Results on Monitoring the Residues in Animal Origin Foods in 2002 (in Japanese), http://www.mhlw.go.jp/topics/yunyu/1-8/index.html [84] MHLW; Request for Comments on the First Draft of Provisional Maximum Residue Limits for Agricultural Chemicals in Foods, 28th October 2003, http://www.mhlw.go.jp/english/topics/mrls/index.html [85] Joint FAO/WHO Expert Consultation on Food Additives (JECFA); Evaluation of Certain Food Additives and Contaminants - Forty-forth report of the Joint FAO/WHO Expert Consultation on Food Additives, 1995 [86] Food and Drug Administration (FDA); Food Additives: Threshold of Regulation for Substances Used in Food Contact Articles; Final Rule, 21 CFR Part 5, et al, 1995 [87] European Commission; Proposal for a Regulation of the European Parliament and of the Council on maximum residue levels of pesticides in products and animal origin, COM(2003) 117 final, 2003/0052(COD) [88] MHLW; Residues of Nitrofurans Metabolites in Powdered Egg from Belgium and India, 27th June 2003 (in Japanese), http://www.mhlw.go.jp/houdou/2003/06/h0627-3.html |