Criselda P. Pagluanan, The Philippines
Introduction
In the Philippines, the National Meat Inspection Commission (NMIC), Department of Agriculture, is responsible for the quality and safety of meat and meat products for consumption.
Organizational Flow Chart
|
Department of Agriculture |
|
|
¯ |
|
|
National Meat Inspection Commission |
|
|
¯ |
¯ |
|
Central Meat Laboratory |
14 Satellite Laboratories |
Coordinating Laboratories
|
1. |
Drug Assay Laboratories |
|
|
Bureau of Animal Industry |
|
|
Department of Agriculture |
|
2. |
SGS Philippines, Inc. |
Residue Monitoring Program
The Residue Monitoring Program is carried out nationwide in slaughtered food animals with the following objectives:
- To determine the prevalence of veterinary drug residues in slaughtered animals by random sampling. This includes banned veterinary drugs that may enter the food chain.
- To identify farms those violate laws on veterinary drug using trace-back mechanism.
- To formulate an action plan for high risk animal species currently having a high usage of veterinary drug.
Legislation
Philippine legislation on the use of veterinary drugs is bounded by Republic Act (R.A.) 3720, R.A. 1556 for the Registration of Veterinary Drugs and Products.
The laws and regulations on residues in meat is incorporated in R.A. 9296 or the Meat Inspection Code of the Philippines.
Enforcement
The enforcement of veterinary drug abuse is jointly monitored by the Bureau of Animal Industry (for feeds and livestock) and the National Meat Inspection Commission (for slaughtered food animals).
Veterinary drugs are regularly monitored and traced back to the source of animals found positive for a veterinary drug. Surveillance of animals coming from the identified farm is being carried out.
Program Strategy
The residue program is designed based on the following criteria:
- Level of toxicity of specific drug or chemical
- Species of animal (high residue value)
- Commonly used drugs
- Frequency of detection
- Testing methods/facilities
- Banned products
Sampling
Sampling for residue monitoring is done quarterly in all "AA" and "AAA" accredited meat plants (abattoir, poultry dressing plants). Five samples are collected randomly in each meat plant and brought to the laboratory for examination. These samples are examined for antimicrobials and banned veterinary drugs. Laboratory Report of Analysis is sent to the Inspection Officer to inform the animal owner or meat dealer of the result of examination and advice for whatever findings occur.
Some samples are provided by other sources like the meat industry, consumers and research students.
For imported meat, samples are collected at cold storage upon arrival of the shipment by our Inspection Officers.
Inter-Linkage in Sampling
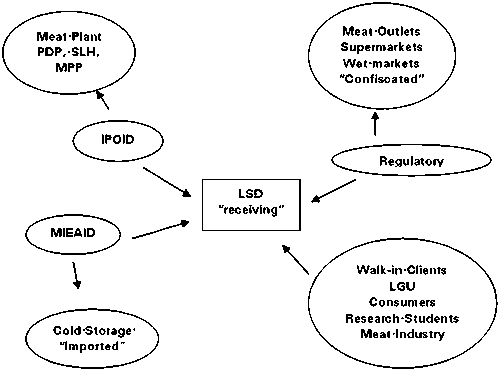
Laboratory Services Centers
The NMIC has 14 Regional Satellite Laboratories and one Central Meat Laboratory. The Satellite Laboratories are capable to conduct screening test for antimicrobials while the Central Laboratory is programmed to be the reference laboratory.
The Central Meat Laboratory is currently performing screening test (Four Plate Test) for antimicrobials and ELISA test for banned products (â-agonists, chloramphenicol, nitrofurans). It is also programmed to conduct analytical method using HPLC and GC.
Commodities Tested
Meat:
- Poultry
- Pork
- Beef
- CarabeefMilk
Eggs
Prawns
Types of Drugs Tested
Aminoglycosides
Macrolides
Tetracyclines
Penicillins
Sulfadrugs
Chloramphenicol
Nitrofurans
ß-agonists
Methods for Testing
1. Screening Method
4 Plate Test
ELISA Kits2. Analytical Methods for confirmation
HPLC
GC/MS
Future Programs
The Department of Agriculture is advocating for the full implementation of residue monitoring program. This program is to ensure the quality and safety of foods of animal origin and other food commodities for public health safety.
These are the activities to be addressed:
- Adoption of new analytical methods.
- Capability development of laboratory staff on analytical methods.
- Networking - collaboration with other laboratories on residue work.
- Evaluation of proposed MRLs of veterinary drugs by International Organizations.
- Evaluation of priority list of drugs to be monitored in the program.
Number of Animals Tested
Table 1. Summary of total number of samples from different species for four years.
|
SPECIES |
Number of Samples Tested |
|||
|
2001 |
2002 |
2003 |
2004 |
|
| |
|
|
|
|
|
PIG |
463 |
828 |
990 |
322 |
|
CATTLE |
53 |
8 |
22 |
67 |
|
CARABAO |
9 |
|
12 |
9 |
|
POULTRY |
247 |
444 |
372 |
216 |
|
|
|
|
|
|
|
TOTAL |
772 |
1'280 |
1'396 |
614 |