Although aquaculture is considered an old tradition, modern aquaculture is essentially a post-1950 phenomenon. The perception that tilapia (O. mossambicus) would be a potential panacea to the growing animal protein deficiency in Asia (Lin 1977) began to be dismissed relatively early for reasons indicated earlier. O. niloticus became the preferred tilapia species for aquaculture in the region (Smith and Pullin, 1984). Although it is difficult to assess whether this species has made a significant contribution to the animal protein needs of rural Asian communities, it certainly had a major impact on aquaculture developments in Asia and the Pacific since the 1970s.
Aquaculture of tilapias provides a classic example of a success story of a species group outside its natural range of distribution. The group currently contributes about 3.8 percent to the cultured fish and shellfish production of about 40 million tonnes globally (FAO FishStat). The current aquaculture production (2002) of tilapias is about 1.5 million tonnes, the great bulk of which takes place in Asia (Figure 6) accounting for nearly 80 percent of the total world production. It is important to note, however, that tilapia culture in Africa and South America is also increasing.
Prior to the mid-1990s, the yield of tilapia from capture fisheries was greater than that from aquaculture. Currently, the later accounts for approximately 2.5 times the production from capture fisheries. Tilapia aquaculture production increased from
28 000 tonnes to 1.504 million tonnes globally from 1970 to 2002; in Asia and the Pacific, production increased from 23 000 tonnes to 1.192 million tonnes (Figure 6) equivalent to an annual growth rate of 13.2 percent and 13.1 percent, respectively. In contrast, capture fisheries for tilapias have grown at the rate of 3.5 percent per annum.
The growth of tilapia culture can be exemplified by comparing production with cyprinids and salmonids in different time periods (Figure 7). The trend shows that in all three groups of fish the annual rate of increase declined with time, as in the case of the aquaculture industry as a whole (De Silva, 2001b). However, the rate of increase in tilapia culture exceeded that of cyprinids and salmonids over all the time periods considered, with tilapia culture recording the highest rate of annual growth over the last two decades among all finfish groups. The growth of tilapia culture in Asia and the Pacific however, lagged slightly behind to that of the world. For example, the percent annual increase in tilapia culture in Asia and the Pacific for the 20 (1982 - 2002), 10 (1992 - 2002) and 5 (1997 - 2002) year periods was 12.5 (vs. 12.6), 10.9 (vs. 11.9) and 7.7 (vs. 10.0), respectively. This lower rate of growth in Asia and the Pacific is more a reflection of increased tilapia production in countries such as Egypt, where O. niloticus production increased from 9 000 tonnes in 1980 to 168 000 tonnes in 2002. Also the rate of growth observed, in all three groups of fish is considerably higher than that witnessed in the sector as a whole (De Silva, 2001b). Although a number of tilapia species has been introduced into the region (Table 1), only a small number of these are cultured (Table 7). Oreochromis mossambicus and O. niloticus are the most widely cultured tilapias in the world. In 15 countries in Asia and the Pacific, only four tilapia species are cultured, dominated by O. mossambicus (five countries) and O. niloticus (10 countries); the later accounts for more than 90 percent of the production and its contribution to aquaculture production has been increasing steadily (Figure 8).
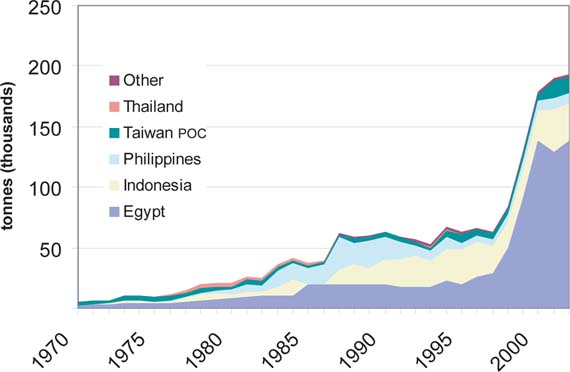
FIGURE 6
Aquaculture production of tilapia by
continent 1970-2002
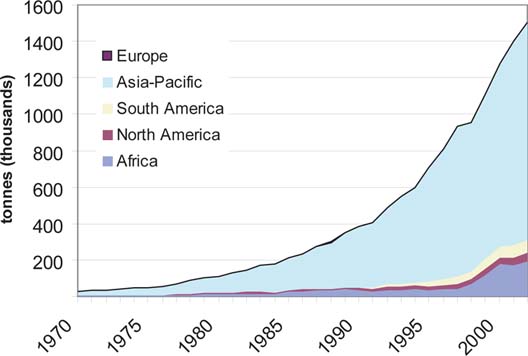
FIGURE 7
Global aquaculture production of salmonids,
cyprinids and tilapia 1970-2002
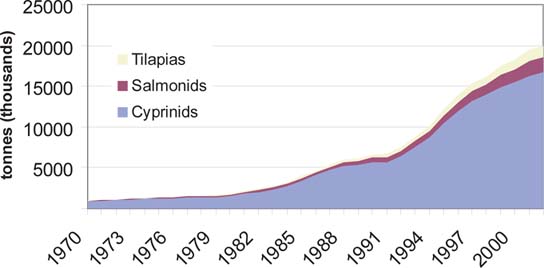
FIGURE 8
Aquaculture production of tilapias by species
in Asia and the Pacific 1970-2002
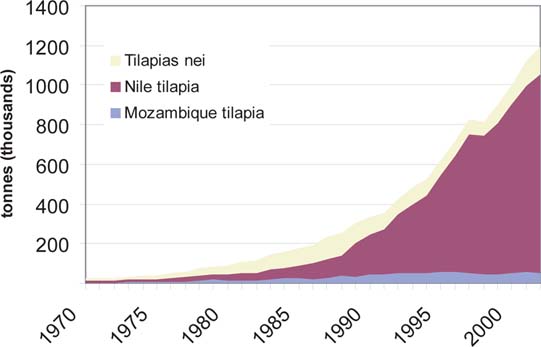
Table 8 lists the top ten countries in cultured tilapia production. In each year considered, six or more Asian nations ranked among the highest producers. Since 1995, some South American nations have become significant contributors to cultured tilapia production. Beginning in 1989, Peoples Republic of China (PR China) has dominated global tilapia aquaculture production, accounting for 47 percent of global production in 2002. In Egypt, cultured tilapia production increased from 9 000 tonnes in 1980 to 168 000 tonnes in 2002, thus attaining the second highest production in global tilapia culture.
The principal nations in Asia and the Pacific which have adopted tilapia culture are PR China, Indonesia, the Philippines, Thailand and Taiwan Province of China. Changes in O. niloticus production in these countries indicate that PR China currently accounts for over 70 percent of the region's production, an increase from 39 percent of Asia and the Pacific production in 1988, before the reported rapid increase in Nile tilapia culture in PR China. This has led to a decreased share of production from the other countries of the region, even when production has exhibited growth. For example, in the Philippines, the proportional contribution to regional Nile tilapia production was 27% in 1988 and only 10% in 2002, even though production increased from 27 000 tonnes to 104 000 tonnes over the period (Figure 9). Indonesia continues to dominate O. mossambicus culture, accounting for nearly 50 000 tonnes and more than 90 percent of the global total in 2002. Over 60 percent of this production is reported as originating in brackish waters.

Farmed red tilapia in Sri Lanka
TABLE 7
The number of countries/territories in each
continent where different species of tilapias are cultured (production greater
than 100 tonnes in 2002). Many countries do not report to species level so
Orechromis spp. is included as well
|
Continent/Region |
not specified (spp.) |
Oreochromis spp. |
Tilapia spp. |
|||||
|
niloticus |
mossambicus |
aureus |
spilurus |
andersonii |
rendalli |
zillii |
||
|
N. America |
4 |
7 |
2 |
3 |
0 |
0 |
0 |
0 |
|
S. America |
3 |
4 |
1 |
0 |
0 |
0 |
0 |
0 |
|
Africa |
4 |
13 |
1 |
0 |
0 |
1 |
0 |
1 |
|
Asia-Pacific |
9 |
10 |
5 |
0 |
1 |
0 |
0 |
0 |
|
Europe |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
TABLE 8
Top ten cultured tilapia producing
countries/territories in the world, 1970-2002
|
1970 |
1980 |
1990 |
1995 |
2000 |
2002 |
||||||
|
Country |
Production (tonnes) |
Country |
Production (tonnes) |
Country |
Production (tonnes) |
Country |
Production (tonnes) |
Country |
Production (tonnes) |
Country |
Production (tonnes) |
|
Taiwan POC |
11 287 |
Taiwan POC |
33 712 |
PR China |
106 071 |
PR China |
314 903 |
PR China |
629 182 |
PR China |
706 585 |
|
PR China |
5 828 |
Indonesia |
14 901 |
Philippines |
76 142 |
Philippines |
81 954 |
Egypt |
157 425 |
Egypt |
167 735 |
|
Egypt |
2 500 |
Philippines |
13 214 |
Indonesia |
53 768 |
Thailand |
76 383 |
Philippines |
92 579 |
Philippines |
122 390 |
|
Nigeria |
2 129 |
PR China |
9 000 |
Taiwan POC |
52 047 |
Indonesia |
74 125 |
Indonesia |
85 179 |
Indonesia |
109 768 |
|
Thailand |
1 732 |
Egypt |
9 000 |
Egypt |
24 916 |
Taiwan |
46 293 |
Thailand |
82 581 |
Thailand |
100 576 |
|
Philippines |
1 417 |
Thailand |
8 419 |
Thailand |
22 895 |
Egypt |
21 969 |
Taiwan POC |
49 235 |
Taiwan |
85 059 |
|
Israel |
1 400 |
Mexico |
6 907 |
Japan |
5 825 |
Colombia |
16 057 |
Brazil |
32 459 |
Brazil |
42 003 |
|
Indonesia |
1 191 |
Nigeria |
2 952 |
Mexico |
5 000 |
Brazil |
12 014 |
Colombia |
22 870 |
Lao PDR |
26 872 |
|
Hong Kong SAR |
450 |
Israel |
2 512 |
Israel |
4 795 |
Malaysia |
8 866 |
Lao PDR |
18 928 |
Colombia |
24 000 |
|
Mexico |
200 |
Japan |
2 392 |
Sri Lanka |
4 500 |
U.S.A. |
6 838 |
Malaysia |
18 471 |
Malaysia |
20 757 |
FIGURE 9
Aquaculture production of tilapia by country
in the Asia-Pacific region 1970-2002
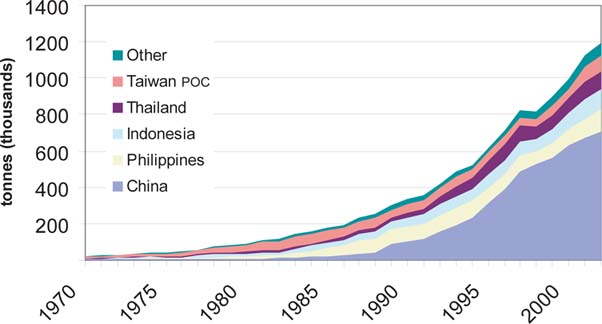
FIGURE 10
Major producers of tilapia in brackishwater
1970-2002
One of the significant changes that has occurred over the last decade is the increase in tilapia culture in brackish waters (Figure 10). For 2002, brackishwater culture production was 193 000 tonnes. This represents a tripling from the relatively constant values (approximately 60 000 tonnes) for the period 1987-1997. O. niloticus accounts for about 73 percent of the brackishwater production, with most of the increase driven by large increases in production in Egypt. On the other hand, brackishwater culture of O. mossambicus has not increased as dramatically over the last decade. Egypt dominates O. niloticus brackishwater culture with 138 000 tonnes produced in 2002 (FAO FishStat).
Culture practices of tilapias in the world are very diverse, perhaps the most diverse among all aquaculture species in the world. It is a group of fish that could be cultured at many desired intensities, thus, appealing to all socio-economic strata, enabling the culture practices to be adjusted to suit their economic capabilities. Oreochromis niloticus is commonly cultured in backyard and/or home garden ponds to supplement the income of poor households as well as provide a fresh source of animal proteins to the family. In such situations, the cultured stock is often fed with kitchen waste and supplemented by relatively readily available, often low cost agricultural by-products such as rice bran. However, the direct nutritional value of the latter to the stock is not known and in all probability rather low; the inputs act more as a fertilizer. Oreochromis niloticus is cultured in relatively poor quality waters, including: (a) sewage fed ponds (e.g. commercial culture in Calcutta, India) (Edwards. 1990; Edwards et al., 1990); and (b) primary and secondary treated waste effluents (e.g. Egypt) (Khalil and Hussein, 1997). So far, there have not been any reports of detrimental effects of consumption of fish reared in sewagefed farms on human health even as the practice has been in operation since the 1930s (Nandeesha, 2002).

Releasing red tilapia fry, Sri Lanka
Tilapias are generally used in other aquaculture systems. Oreochromis niloticus is often used in integrated-fish culture. The most extreme development in this system is the integration of commercial poultry farming with O. niloticus culture. Cultured stocks are not fed, but depend on poultry waste and the natural food production in eutrophic ponds. Oreochromis niloticus is also being increasingly used in rice-fish culture, a traditional system which is gaining revival in Asia and is reported to enhance the overall yields in practices in PR China (Banghuai and Qianlong, 1995) and Bangladesh (Gupta et al., 2002). Similarly, the potential of using O. niloticus in biogas slurry has been demonstrated with a view to integrating biogas technology in food production (Edwards et al., 1988).
Tilapia pond culture practices are, in almost all cases, conducted in static systems, which require water replenishment between grow-out cycles and aeration is rarely used. Such minimal requirements reduce the initial investment and also keep the recurrent cost relatively low and therefore, more affordable for poorer sectors of the community. Cage culture of tilapias is also relatively popular, the intensity and the sophistication varying from practice to practice and requiring minimal investment. Tilapia cage culture is effective as a means of providing alternative livelihood to displaced persons during reservoir impoundment (e.g. Jatilnuhur reservoir in Indonesia and Batan Ai reservoir in Malaysia). Operations in these cases are done on an industrial scale when cage construction, feed supplies, marketing strategies etc. are provided as an integrated package.

Cage culture of tilapia in Batanai reservoir, Sarawak, East Malaysia
TABLE 9
Inputs used and yields obtained from freshwater
tilapia culture practices in some Asian countries in 1999 (After Dey,
2001)
|
Parameter |
Bangladesh |
PR China |
Philippines |
Thailand |
Viet Nam |
|||
|
Pond |
Pond |
Cage |
Pond |
Cage |
Pond |
Pond |
||
|
Mc |
Mc |
Pc |
Mc |
Mc |
Mc |
Pc |
Mc |
|
|
Inputs * |
|
|
|
|
|
|
|
|
|
Rice bran |
426 |
9 472 |
3 982 |
|
3 172 |
|
445 |
79 |
|
Commercial feed |
|
2 811 |
3 998 |
11 431 |
2 336 |
533 |
3 087 |
62 |
|
Manure |
|
615 |
1 339 |
|
7175 |
|
2 035 |
132 |
|
Fertilizer |
10 |
|
|
|
213 |
|
|
|
|
Yield * |
1 736 |
5 860 |
6 593 |
5613 |
2 959 |
540 |
6 290 |
3 020 |
|
Harvest wt. (g) |
111 |
310 |
340 |
460 |
130 |
175 |
232 |
241 |
Mc- mono culture; Pc- polyculture; * kg/unit area
Most interestingly, tilapia culture is also being conducted in recirculation or closed cycle systems in temperate countries such as Canada, which incorporates expertise on greenhouse operations (Fitzsimmons, 2001). This is an evidence of a growing demand for cultured tilapia in many countries and the subsequent expansion of tilapia culture practices throughout the world.
The diversity of tilapia culture practices is also borne by the fact that tilapias are high salinity tolerant species and could be cultured in brackish waters and in sea cages. For sea cage culture, the appropriate species is O. spilurus (Cruz and Ridha, 1990). As mentioned earlier, tilapia production in brackish waters have grown steadily over the last decade with Egypt being the largest producer.
The returns from tilapia culture differ according to the environment, system and practice of culture. According to Dey (2001), the best tilapia culture practices in Asia and the Pacific are encountered in Taiwan Province of China yielding 12 to 17 tonnes/ ha/yr in ponds, followed by mean yields of 6.6, 6.3, 3.0 and 1.7 tonnes/ha/yr in Thailand, the Philippines, Viet Nam and Bangladesh, respectively. Cage culture operations in the Philippines and in PR China on the other hand, yielded 0.5 and 5.5 tonnes/100 m2, respectively (Table 9). Similarly, the mean size at harvest varied considerably among countries; between culture systems, the highest (460 g) being in cage cultured O. niloticus in PR China (Table 9).
Why have tilapias been successful as a cultured species group throughout the tropical countries? Bearing in mind that the bulk of tilapia production is a result of farming, its success can be primarily attributed to the following:
relative ease of culture under extensive, semi- and/or intensive practices, thus relatively less limited by the economic status of the farmer compared to most other finfish species;
relevant species exhibit many of the desirable traits expected of a species suitable for culture (e.g. relatively high growth rate, wide range of tolerance to physicochemical characteristics, resistance to disease, easiness of propagation, etc.);
moderately high dress-weight ratio;
long shelf-life, and;
as a white fish, tilapia is mild and lends itself to industrial preparations better than most other white fish (Picchietti, 1996).
In addition to all of the above, most of the commonly cultured tilapias are easily weaned on to artificial feeds. The group has the ability to derive its nutrition effectively from the natural food in the rearing systems particularly in ponds. This attribute makes it the foremost choice in home-garden and/or backyard small-scale, subsistence fish culture in developing countries such as Bangladesh, Viet Nam, etc.
TABLE 10
Summary of different hybrid combinations of
tilapias that have known to produce monosex male progeny (from Mair,
2001)
|
Female parent |
Male parent |
Remarks |
|
O. niloticus |
|
Applied commercially but results inconsistent |
|
O. niloticus |
O. macrochir |
Majority of broods are all male; some commercial application too |
|
O. niloticus |
O. urolepis hornorum |
|
|
O. niloticus |
O. variables |
All progenies monosex |
|
O. mossambicus |
O. aureus |
|
|
O. mossambicus |
O. urolepis hornorum |
|
|
O. spilurus niger |
O. macrochir |
|
|
O. spilurus niger |
O. urolepis hornorum |
|
|
O. aureus |
O. urolepis hornorum |
|
|
T. zillii |
O. andersonii |
|
Culture of tilapias has gone through a number developmental phases since the nineteenth century (Lin 1977). In confined environments such as ponds, early maturation of the species, which resulted to overpopulation and eventually stunting, needs to be addressed. The earliest approach to overpopulation was stocking of predators, and few examples include Ophicephalus spp. and Clarias spp. in Asia (Chimitis 1957), L. niloticus and Micropterus salmoides) in Africa (Meschkat, 1967) and Cichalosoma managuense in Central America (Dunseth and Bayne 1978). Such a strategy requires the following: (a) optimization of predator to prey ratios; and (b) appropriate timing of the introduction with the correct number of predators of appropriate size(s). The effectiveness of such a strategy was limited and "fine tuning" is required so that an optimal balance is formed between the fry production of tilapias and the voracity of the predator(s). This strategy does not eliminate the channelling of food energy for egg and fry production thereby decreasing yields that could have been otherwise attained.

Netload of tilapia, Myanmar
Realizing that male tilapias grow faster, further approaches to solving the problem of overpopulation in culture systems was to develop all-male tilapias. Beardmore et al. (2001) summarized the potential advantages of the use of monosex animals in aquaculture:
higher growth rate;
elimination of reproduction;
reduction of sexual/territorial behaviour;
narrower size range at harvest; and
reduction of environmental impacts resulting from escapees.
In view of the above considerations, the initial approach was to produce monosex tilapias through hybridization (Hickling, 1960; 1963). Female O. mossambicus x male O. hornorum (Hickling 1960, 1963) cross generated nearly all-male hybrids. Other cross selection followed which resulted in all-male and/or nearly all-male hybrids (see Table 10). Thus, hybridization strategy minimized overpopulation in the culture environments and also resulted in higher yields because of the faster rate of growth of the males. However, large-scale hybrid production did not always succeed due to instability in production of all-male hybrids and increasing appearance of females among progenies (Wohlfarth, 1994). Only few species of commercial value consistently produced allmale F1 generation. The most notable of these was the O. niloticus x O. aureus cross, which tolerated winter periods in Israel and became a focal point for a fresh wave of distribution of the parent species to other regions (Pullin and Capili, 1988). Except in a few cases, hybridization did not become established as a commercial method for allmale tilapia production
The hybridization phase gave way to hormonal sex-reversal as a method of producing large quantities of all-male seed stock, an approach perceived to be more practical. At the time that hormonal sex reversal was gaining popularity, a "shift" in the species was taking place, where O. niloticus became the preferred species for culture in view of its faster growth rate, attractive coloration and supposedly better taste. Consequently, most of the technological advances on sex-reversal were developed for this species, although the initial discoveries were made on O. mossambicus when Clemens and Inslee (1968) succeeded in producing all-males by oral administration of 17á-methyltesterone (male hormone) to early stage fry. Since the original discovery the effectiveness of 17á-methyltesterone, sex-reversal in a number of tilapia species has been demonstrated, e.g. O. aureus (Eckestein and Spira, 1965; Guerrero, 1975), O. niloticus (Tayamen and Shelton, 1978), O. hornorum (Obi and Shelton, 1983), Tilapia zillii (Yoshikawa and Oguri, 1978), O. spilurus (Ridha and Lone, 1990), among others. In addition, apart from 17á- methyltesterone, the effectiveness of other chemicals incorporated in food and orally administered for sex-reversal in tilapias has also been demonstrated (Varadaraj, 1990).
Hormonal sex-reversal is effective in early fry stages only (Hiott and Phelps, 1993). The hormones are almost always provided with the feed by spraying an alcoholic solution of the hormone on the feed and allowing the alcohol to evaporate. The dosage (generally 40 to 60 ppm) and treatment duration for the different hormones used for tilapias are well documented (McAndrew, 1993; Pandian and Sheela, 1995). It is also important to note that the orally administered masculinizing hormones, in particular 17á-methyltesterone, are eliminated (converted into polar metabolites) within 72 h of administration and that by day 10 only traces remained (Johnstone et al., 1983; Curtis et al., 1991). Indeed, even in instances when 17á-methyltesterone was used as a growth promoter, the chemical was eliminated from muscle within a very short period of time (Rothbard et al., 1990), thus enablling the continued use of the technique without restrictions on the marketing the final product.
The technology for mass production of sex-reversed juveniles was initially developed by Rothbard et al. (1983) and has been extended into many hatcheries in the region. On the other hand, Beardmore et al. (2001) pointed out the main disadvantages of the method particularly in relation to practical difficulties in providing a uniform dose to all of the stock.
Following hormonal sex-reversal of tilapias and the greater understanding of sexdetermination systems in a number of key species (Mair et al., 1991a; 1991b), it became possible to develop techniques for the production of genetically male tilapia (GMT) initially on O. niloticus (Scott et al., 1989; Mair et al., 1991b). This technique is based on creation of males with two Y chromosomes (supermales) that when mated with other genotypes usually to give all-males and generally only a small percentage of females. Genetically male has also been produced, in this instance, through gynogenesis of XY neofemales (Vardaraja and Pandian, 1989).
The technique of producing GMT, particularly in the case of O. niloticus, is well established and is commercially adopted now, achieved through several generations of breeding (Mair and Little, 1991). Beardmore et al. (2001) claimed that the YY/GMT technology in O. niloticus is the only genetic technology adopted by the aquaculture industry for the production of all-males. Mair and Little (1991) considered the relative advantages, including commercial viability, of producing genetically all-male tilapia and its, as opposed to hormone induced sex- inversion to be: (a) all genetically male progeny; (b) potential to produce 100 % male progeny; (c) potential that no reproduction will occur in the growout phase; (d) applicability to most fry production systems; (e) not labour intensive after the initial phase; (f) lack of consumer resistance; (g) no centralization of fry production; and (h) comparatively higher growth rate.
Perhaps the best established dissemination programme of the GMT technology is in the Philippines done through an organization named Phil-Fishgen (Mair et al., 2002). Phil-Fishgen is also involved in research and evaluation of the technology and has an accreditation scheme for private hatcheries. It is estimated that the 32 accredited hatcheries currently hold about 40 000 broodstock sets (1YY male: 3 normal females). According to Mair et al. (2002), the broodstocks held in the accredited hatcheries are capable of producing 50 milion GMT fry per year.
Using on-station and on-farm trials, Mair et al. (1995) demonstrated that the yield from GMT was 30 - 40 percent higher compared to normal mixed-sex tilapia (existing Philippine strains) and the mean grow-out period for GMT was 4.6 months, compared to 4.8 and 5.1 months for GIFT (Genetic Improvement of Farmed Tilapia - see next sectionj) fish and sex-reversed tilapia, respectively (Mair et al. 2002). The comparatively lower price of fry and fingerlings of GMT compared to prices of sex - reversed and GIFT fish appears to lure more growers, particularly the newer ones, to GMT farming (Mair et al., 2002). However, it must be noted that there is increasing evidence that the sex determining mechanisms in fish in general (tilapias in particular) are very plastic and often influenced by environmental factors such as naturally occurring exogenous steroids, temperature and other physical variables and pollution (see review by Devlin and Nagahama, 2002). The environmental factors are often the main cause for the presence of females observed in GMT fish, which at times are potentially capable of mitigating growth advantage otherwise attributed to GMT fish.
Genetic improvement of most cultured aquatic species lags far behind that of farmed plants and animals. Availability of genetically improved seeds is considered as the single most important factor in the green revolution that became responsible for averting a famine in the developing world during the second half of the last century (Gjedrem, 2002). Gjedrem (2002) in reviewing the degree of response of aquaculture species to selection concluded that the mean genetic gain per generation among ten species was 13.3 percent generation with a range of 9.0 to 17.5 percent in clams and channel catfish, respectively. The gain in tilapia was estimated to be about 13.5 percent.
While developments are taking place on all-male tilapia production (hormone treated or genetically), it was increasingly recognised that tilapia genetic resources in its native habitats need to be conserved, wild stocks protected and an international research programme on tilapia genetics be established (Pullin, 1988). Pullin and Capili (1988) also took into consideration the possible bottleneck effects of tilapia introduction to Asia and addressed the need for genetic improvement of cultured tilapias, particularly O. niloticus. The increasing interests in tilapia culture and the almost unanimous acceptance that cultured tilapia stocks needed genetic assessment and improvement led to the birth of the regional research and development programme "Genetic Improvement of Farmed Tilapia - GIFT", under the leadership of the WorldFish Centre - WFC, based in Penang, Malaysia (then referred to as the International Centre for Living Aquatic Resources Management - ICLARM, based in Manila, Philippines).
The "GIFT Fish" was the result of this carefully conducted genetic selection and improvement programme based on broodfish collected from four African countries (Egypt, Ghana, Kenya and Senegal) and four commercial O. niloticus strains (from Israel, Singapore, Taiwan Province of China and Thailand) used in the Philippines (Eknath et al., 1993; Dey and Gupta, 2000). In the initial phase of the research, it was evident that the gain in growth and survival through crossbreeding was less than expected. This was followed by a pure-breeding strategy among the best performing purebred and crossbred groups that led to the build-up of a genetically-mixed base population. This population formed the basis for the final selection programme through a combined family and within-family selection strategy (Eknath, 1995). Subsequent selection resulted in the emergence of the GIFT strain, which is purported to have an 85 percent cumulative genetic gain compared to the base population (Eknath et al., 1993).
The development of a better strain by itself does not complete the task particularly in regions where tilapia culture is widespread, often rural and very diverse, unless the findings are extended to practitioners to enable them to reap the benefits. This was achieved through another regional project 'Dissemination and Evaluation of Genetically Improved Tilapia Species in Asia or DEGITA' coordinated by WorldFish Centre (ICLARM) and involving five Asian countries. The project aims to ascertain the following:
genetic, socio-economic and environmental aspects of the production of GIFT strain in different agro-ecological conditions and culture systems;
the overall impact of the GIFT strain on different socio-economic groups (e.g. farmers, consumers, etc.); and
disseminate the strain among small farmers if found to be superior to locally available strains (Dey and Gupta, 2000).
The detailed findings which basically evaluated the usefulness of the GIFT strain for improvement of the productivity and the socio-economic well-being of smallscale farmers in Asia are given in Dey (2000). Table 11 summarizes the results of trials conducted in five countries comparing the performance of the GIFT strain with other commercially available strains (after Dey et al., 2000). The GIFT strain performed better in all countries. For example, on an average farm, the harvesting weight of the GIFT strain was 18 percent higher in PR China and 58 percent higher in Bangladesh. It was suggested that the better performance of the GIFT fish, after accounting for the wide heterogeneity of production environments, input levels and other factors was solely a result of the superiority of the strain. The study also demonstrated that the break-even price to be 7 - 36 percent lower for the GIFT strain (Dey et al., 2000).
The production of the GIFT tilapias and the continuation of the selection strategy for desirable traits are conducted now through a non-profit private foundation, the GIFT Foundation International, incorporated in the Philippines. The Foundation functions through a system of private hatcheries that enter into a mutual agreement that commits the latter to the following:
supporting the selective breeding and research work of the GIFT Foundation through research and development contributions;
following the standards established by the GIFT Foundation for hatchery operations and procedures, customer service and support and marketing and promotions; and
working with the GIFT Foundation and other GIFT hatcheries to improve the entire GIFT network operations, technical expertise, technical support to farmers and, marketing efforts.
Unfortunately, there is no information available on the impact of the Foundation on tilapia culture in general. However, what is common knowledge is that GIFT tilapia has been introduced into a number of other Asian countries. Oreochromis niloticus is widely distributed in Asia already and there are no reports, even anecdotal ones, that it has been responsible for the decline of indigenous species. As such it may be argued that the "GIFT Fish" may not cause any negative impacts on the environment when introduced and/or established. In contrast, it could also be suggested that "GIFT Fish", because of its genetic superiority, it could be more invasive and would increase its range of distribution and thereby bring about detrimental environmental impacts which were not evident with O. niloticus. The reverse also could occur because of its rather specialized traits (e.g. fast growth) that may have reduced fitness in the wild.
Cultured tilapia became an international commodity relatively recently, when frozen farmed tilapia fillets found their way into the mainstream food services and retail establishments of the American foodchain followed by expansion into Europe (Picchietti, 1996). Initially, frozen fillet imports to America exceeded fresh fillet imports by 30 percent and the author predicted that with time, increasing acceptance of tilapia will eventually outweigh frozen fillet imports. It has been suggested that tilapia would have a place as a generic white fish because of its mild taste and lends itself to industrial preparations better than most other white fish (Picchietti, 1996).
TABLE 11
Results of on-farm trials on the GIFT (G)
strain and non-GIFT (NG) strains of tilapias in different culture systems
(Modified after Dey et al., 2000)
|
System |
Strain/trials |
Duration (days) |
SD (no.m-2) |
Weight (g) |
Male% |
Recovery% |
Yield (kg/ha) |
|
|
Initial |
Final |
|||||||
|
Bangladesh |
||||||||
|
Pond |
NG/21 |
173 |
20 000 |
2.99 |
60.7 |
38.3 |
74.1 |
896 |
|
G/23 |
174 |
20 000 |
3.35 |
107.6 |
40.4 |
74.4 |
1 593 |
|
|
Difference |
|
|
|
46.9*** |
2.1*** |
0.2 |
697*** |
|
|
PR China |
||||||||
|
Cage |
NG/10 |
111 |
1 000 050 |
56.8 |
263.8 |
56.1 |
88.5 |
310 967 |
|
G/10 |
111 |
1 000 050 |
55.2 |
308.0 |
56.2 |
94.6 |
389 346 |
|
|
Difference |
|
|
|
44.2*** |
0.1 |
6.1*** |
78 380*** |
|
|
Pond |
NG/7 |
127 |
20 686 |
15.55 |
220.5 |
63.8 |
92.5 |
4 275 |
|
G/7 |
127 |
20 257 |
16.43 |
245.6 |
49.6 |
91.8 |
4 645 |
|
|
Difference |
|
|
|
25.1 |
-14.2** |
-0.7 |
370 |
|
|
Philippines |
||||||||
|
Cage |
NG/5 |
118 |
213 880 |
6.33 |
135.4 |
55.9 |
58.5 |
15 285 |
|
G/5 |
118 |
213 880 |
6.33 |
161.1 |
55.4 |
68.3 |
23 551 |
|
|
Difference |
|
|
|
25.7 |
-0.5 |
9.8 |
8 266** |
|
|
Pond |
NG/50 |
127 |
21 394 |
0.76 |
52.1 |
46.9 |
55.2 |
912 |
|
G/58 |
126 |
23 976 |
0.76 |
67.0 |
48.2 |
70.0 |
1 361 |
|
|
Difference |
|
|
|
14.9** |
1.3 |
14.8** |
448* |
|
|
Thailand |
||||||||
|
Pond |
NG/2 |
256 |
62 500 |
2.14 |
80.2 |
65.0 |
40.2 |
2 044 |
|
G/13 |
250 |
49 231 |
1.22 |
119.6 |
69.6 |
47.6 |
2 829 |
|
|
Difference |
|
|
|
39.4 |
4.6 |
7.4 |
786 |
|
|
Viet Nam |
||||||||
|
Pond |
NG/6 |
139 |
20000 |
1.01 |
52.7 |
52.7 |
45.9 |
558 |
|
G/7 |
152 |
20429 |
1.31 |
52.3 |
52.3 |
47.8 |
743 |
|
|
Difference |
|
|
|
-0.4 |
-0.4 |
1.9 |
185 |
|
***, ** and * denotes significance of the differences between selected parameters at 1, 5 and 10 levels, respectively.
The main export market for cultured tilapia is the United States of America. In 2000, imports of tilapia (whole frozen, fresh and frozen fillet forms) in the United States amounted to 40 553 tonnes valued at US$ 101 377 853 and has since been increasing steadily. According to Harvey (2001), tilapia imports to the United States of America increased by 394 percent by between 1993 and 2000. The main tilapia exporting countries are Taiwan Province of China, PR China, Ecuador and Costa Rica. Fresh fillet are currently supplied by Ecuador, Costa Rica and Honduras; frozen fillet and whole frozen fish comes from Asia (Vannuccini, 2001). Chinese imports have been increasing almost exponentially over the past few years. Harvey (2001) predicts that PR China will become the leading exporter of tilapia to the United States.
The European market for tilapia is still rather limited; UK is considered to be the major outlet. Vannuccini (2001) reckoned that the main markets in Europe are the big cities where large communities of African, Chinese and Asian communities live. However, an increase in tilapia consumption has been a recent trend also among non-ethnic communities. European markets prefer larger-sized frozen tilapia. There are growing markets for tilapia in Canada, the Middle East and even in a rather sophisticated market such as in Japan (Vannuccini, 2001). In Japan, high quality tilapias are being used for sashimi and as a substitute to sea bream in traditional Japanese cooking.
It is thus evident that tilapias, commonly considered as the poor man's fish, are now beginning to make major entry to the sophisticated markets of the world. It has also become a popular and sought fish after freshwater fish in countries such as the
Philippines and Indonesia, replacing milkfish and gouramy, respectively. These trends are positive signs for all producers and can potentially influence a significant upsurge in tilapia culture worldwide.
TABLE 12
Predicted global and Asia and the Pacific
cultured tilapia production (tonnes) for the years 2003 to 2010. Estimates are
based on the approximate rate of increase between 2001 and 2002 (6%) and on the
approximate average rates for the period 2001-2002 (9%)
|
Year |
Global Production - Rate of mean percent annual growth |
Asia and the Pacific Production - Rate of mean percent annual growth |
||
|
6 percent |
9 percent |
6 percent |
9 percent |
|
|
2003 |
1 595 000 |
1 640 000 |
1 263 000 |
1 299 000 |
|
2004 |
1 690 000 |
1 787 000 |
1 339 000 |
1 416 000 |
|
2005 |
1 792 000 |
1 948 000 |
1 420 000 |
1 544 000 |
|
2006 |
1 899 000 |
2 124 000 |
1 505 000 |
1 683 000 |
|
2010 |
2 398 000 |
2 998 000 |
1 900 000 |
1 375 000 |
Future prospects
It is believed that there is potential for further tilapia culture development in the Asia and the Pacific, as well as in Africa and South America. The trends in the growth of tilapia aquaculture production, taking into consideration the mean annual increase in production, over different time periods are summarized in Figure 11. More importantly, the observed decline of the annual growth rates for tilapia production is significantly lower than that for total finfish as described by De Silva (2001b) and is also lower than for any other cultured finfish groups. Tilapia culture in North America is still a young industry and the market for tilapias is being steadily expanded in the United States, Japan and even Europe. This situation might provide an added impetus to increased tilapia culture in developing countries in the immediate future.
In view of the decreasing annual rate of growth in production (note that production is increasing but the rate of increase is beginning to decrease), cultured tilapia production in the world and in the Asia and the Pacific was projected using two rates of increase. The first rate, 6 percent increase per year, approximates the smallest increase in production in the last few years, observed from 2001 to 2002. the second rate, a 9 percent annual increase, approximates the average rate of increase observed for 2000-2002 (Table 12). Taking average rates of increase over longer time periods would result in a larger growth rate and, hence, larger projected production. By 2006, even at a rather modest rate of increase of 6 percent per year, the world cultured tilapia production would reach 1 899 000; whereas, with a 9 percent rate of growth per year production would be over 2.1 million tonnes. By the same year, tilapia production in the Asia and the Pacific is estimated to be 1 505 000 and 1 683 000 tonnes at growth rates of 6 and 9 percent, respectively. The present analysis also indicates that the contribution from the region to the world cultured tilapia production would continue to be nearly 80 percent.
Table 12 also extends the projections beyond 2006 to 2010 for information, but obviously caution should be used when projecting too far into the future. The above computations are based on production trends over the last few years. They do not take in to account plausible changes that could occur in tilapia aquaculture practices, which would be likely to have a significant positive impact on production, and the industry as a whole. An example of this is the relatively recent, but the increasing emphasis of tilapia culture in brackish waters. This trend is likely to continue with the possible adoption of tilapia culture in unused or abandoned shrimp ponds - a viable proposition for many Asian farmers. Possible increases in production through expanded integrated rice-fish farming of tilapias may also be realized.
The upsurge in culture-based fishery activities in Asia and in the Americas is also likely to contribute to significant increases in production. Considering prospects for tilapia aquaculture in the Americas, Fitzsimmons (2001) believes that by 2010 total production will exceed 500 000 tonnes/year. This might be possible as most developing nations are embarking on tilapia aquaculture and there is an increasing emphasis on high density, intensive culture in recirculating systems in nations such as the United States of America and Canada.
The growth in cultured tilapia production will also likely be augmented by technological developments and more effective extension of the technologies that are already commercialized or being commercialized. A good example of this is the production and availability of genetically improved seed such as GMT. Most of these improved seed types (specifically GMT), as yet, are not readily available to most rural farmers in Asia, except perhaps in Thailand and to a certain extent in the Philippines. Ready availability of such seed to rural farmers throughout Asia is bound to have a positive impact on production.
The potential development of strains with a better growth rate, such as the "GIFT fish" and indeed the greater popularization of already developed better performing strains are also likely to impact tilapia culture. Tilapia culture is currently restricted to warm tropical climates. Tilapia culture in northern Viet Nam requires facilities for over-wintering. Progress has been made with the development of cold resistant O. niloticus strain (Dr T. M. Thien, personal communication, 2003). Development and popularization of a cold resistant strain of O. niloticus would provide the opportunity to extend the range of its culture in relatively cold climates, thereby positively impacting overall production. These developments will, however, be affected to varying degrees by policies which countries would adopt with regard to the use of genetically improved organisms in food production.
Commercial feed manufacturers in developing countries in Asia often view tilapias as a suitable group of fish to market their feeds through community-based subsidy schemes. Tilapia's robust characteristics, such as fast growth rate and relative resistance to disease, satisfy the aspirations of rural farmers who are new to aquaculture, thus would be willing to consider such schemes. With the current trend towards increasing competition among industrial suppliers of "aquaculture goods" and primarily feeds, such feed subsidy schemes are likely to become popular among rural communities and can thus contribute to the overall increases in production.
In Asia, tilapia cage culture has been one of the primary ways of providing alternate livelihoods to displaced persons from dam building and reservoir impoundment in inland areas. Although dam building, particularly across major rivers, is subjected to increasing community disapproval (Roy, 1999), it goes on unabated in most developing countries (McCully, 1995) primarily for purposes other than fisheries. It is being considered in many instances that tilapia cage culture could be an alternative livelihood for displaced persons, and such programmes might contribute to the overall tilapia production in the coming years.
Twenty-five years ago, predictions were made with regard to sewage treatment plants becoming large centres for food production (Borgstrom, 1978), and that excreta use in aquaculture would become an increasingly important form of waste disposal and food production (IRCWD, 1985). Use of excreta for food production is not a new phenomenon; night soil is used for agricultural production in some countries, although this practice has now become controvercial on food safety grounds and is gradually diminishing. These predictions have not yet been fully realized. As we progress through the new millennium, there will be more demand for waste management and recycling of biological wastes for food production, directly and indirectly, will become paramount. Tilapias have shown to be ideal candidates in this regard. As pointed out earlier, tilapias are already cultured in sewage ponds as well as in wastewater treatment plants. With increasing pressures on communities to recycle nutrient resources, there is real prospect for expansion of such activities and the potential for contributing to the overall cultured tilapia production in the tropics.
Prospects for tilapia culture will remain an attractive proposition both to the poor sectors of communities and those who are embarking on aquaculture, either to supplement the household income or as an alternative livelihood, because of the following characteristics: (a) need for only modest investments; (b) relatively low recurrent costs for the culture of the tilapias; (c) ease of breeding and growout; and (d) ability to do well in waters of relatively low quality. The fact that tilapia culture can also be conducted on a large-scale, on an intensive basis, or integrated with other forms of agriculture such as rice-fish farming or livestock-fish farming, also provides greater scope for expansion of its culture practices.
The distinction between culture-based fisheries, stock enhancement and aquaculture is often difficult to determine. It is considered that culture-based fisheries fall into the realm of aquaculture with the following characteristics:
it is a farming activity;
it involves intervention in the lifecycle;
more often than not ownership (singly and/or collectively) is defined.
Public perception on relatively intensive forms of aquaculture is not favourable and environmental lobby groups are becoming increasingly opposed to aquaculture development (De Silva et al., 2001b). In such a climate, it is likely that there could be an upsurge in culture-based fishery activities in the region and elsewhere (Quiros and Mari, 1999) depending on the availability of small water bodies. A conservative estimate of the water surface area classified as small-scale irrigation schemes in developing countries in Asia is estimated to be 66 710 052 ha (FAO, 1999), a huge resource that has been established for purposes other than fisheries. Utilization of such water resources for culture-based fisheries has the added advantage of not being dependent on feed inputs, but, and by and large, depends on the natural food productivity of the systems, thereby becoming a non-polluting aquaculture activity.

A day's harvest of tilapia from a culture-based fishery in Mandalay, Myanmar
Culture-based fisheries are in a development phase in Asia. The social and institutional implications and economic viability are being evaluated (Lorenzen et al., 1998a; 1998b). The general consensus is that it is a viable practice and has a major role to play in poverty alleviation in Asia. It is currently recognized that all forms of stock enhancement of existing water bodies is a major development strategy that would significantly increase the world's food fish supplies, especially in developing countries (Welcomme and Bartley, 1998).
Culture-based fisheries generally use species combinations with O. niloticus as the common species. In initial trials conducted in Sri Lanka, it was demonstrated that the stocked tilapias accounted for a production range of 32 - 91 percent, in systems that yielded 838 to 1 030 kg/ha/cycle, where tilapia accounted for the highest production (Chakrabarty and Samaranayake, 1983). On the other hand, culture-based fishery activities in northeast Thailand, tilapia (O. niloticus) gave median yields per fingerling stocked, as compared to Chinese carps, but yields per fingerling of tilapia spanned a wide range up to 0.83 kg seed/fish, considerably higher than the financial break-even level. This indicates that there is great scope for improvement (Lorenzen et al., 1998a).
With the envisaged developments and expansion in tilapia culture and the trend towards intensification of farming systems, feed supply and cost of production will become issues for consideration. Tilapia has some advantages in view of its omnivorous feeding habit which requires less protein as compared to other species cultured using intensive systems (De Silva et al., 1989). Although there have been considerable advances in laboratory research on partial replacement of fish meal in tilapia diets, it has never been demonstrated in any cultured fish species that effective diets can be prepared without fish meal. Inclusion of fish meal in feeds remains essential.
Future growth in tilapia culture will be triggered primarily by export markets to the United States of America, Japan and some European countries, and even to the Near East. These countries have stringent quality requirements concerning chemical and veterinary drug residues. Therefore, in order to be competitive, developing country producers have to satisfy such requirements and endeavour to produce high quality products that are safe to eat and satisfy consumer demand and expectations. Tilapia culture practices will need a general uplift in facilities and attitudes in dealing with a commodity that is not considered as high value, profit margins are relatively narrow and market demand is a possible constraint.
There are increasing concerns on the potential threats to biodiversity in countries where tilapias have been introduced. It was also shown that most, if not all, of the perceived effects of tilapias on biodiversity are primarily due to factors other than tilapias and explicit evidence in this regard, at least to date, are wanting.
The overall impact of tilapias in the region can best be assessed in the following context: (a) its present and future contribution to the well-being of the society; (b) perceived impact as a group of aliens that have become an integral component of the current ichthyo-fauna of the region; (c) present and future impacts that tilapias have on biodiversity. The positive outcomes of introduced tilapias in the Asia and the Pacific are:
establishment and sustenance of capture fisheries in certain countries in the region;
an important aquaculture species group in most countries in the region, appropriate for a wide range of aquaculture operations including integrated aquaculture operations;
a source of inexpensive animal protein in developing countries; and
a source of money through local and international trade.
The above have, undoubtedly and significantly, impacted the socio-economic milieu of various sectors of the communities involved. There had been direct and indirect beneficiaries from these impacts; the former being those involved in the production, both capture and culture of the tilapias as well as aspects of marketing of the produce; the latter being the general populace through the access to an affordable animal protein source. The benefits in exact economic terms, considering the value of the produce, are difficult to estimate. The same will be true of the impact of tilapias on the overall nutritional status of rural communities, which in turn could have a profound influence on their wellbeing.
In a similar fashion, tilapias are beginning to make a marked impact on food fish production in parts of the tropical belt in North and South America, through the establishment of self-sustaining fisheries in both culture-based fisheries and aquaculture sectors. However, unlike in the Asia and the Pacific, these developments are beginning to take root and are likely to reach a peak in the coming decade. As in Asia, the impacts are widespread favouring a vast range of socio-economic strata in the community.
There is no other alien aquatic species or a species group in the tropics that currently makes a contribution of such high magnitude to fish food production as tilapias do. The significance of tilapias as food fish is doubly important because it is an affordable animal protein source to most poor communities in developing countries. For example, in Bangladesh, tilapias are regularly stocked and maintained in the home-garden ponds together with major carps. More often than not, these small-scale aquaculture operations produce tilapias for household consumption and carps for generating cash income (Barman et al., 2002; see also Table 8). On the other hand, tilapia capture fisheries in lakes and reservoirs, which in most instances happened to be located in rural areas (e.g. in Sri Lanka and Indonesia), tend to provide an accessible and an affordable animal protein source to communities living in the vicinity of these water bodies.

Nile tilapia from ponds and rice fields in Yunnan province, China
As such, tilapias play an important role in food security and poverty alleviation. It will be hard to replace this position with another indigenous group of fish. The success of tilapias in food production is similar to other sectors where total food supplies are mainly derived from alien species. Approximately 95 percent of livestock products are based on five species while plant products (e.g. cereals, fruits, etc.) are based on 100 or so species (Prescott-Allen and Prescott-Allen, 1990).
It is instructive to look at the introductions of tilapia into the region in context with other introductions of inland fishes. Beverton (1992) examined several threats to aquatic species, e.g. overfishing, habitat loss, alien species, and disruption of waterflows. He evaluated the records in Welcomme (1988) on 1 354 piscine introductions in inland waters throughout the world and reported that:
22 percent disappeared without a trace (considered to be an underestimate due to non-reporting of failed introductions)
51 percent had little or no effect on the ecosystem(s), or depended on regular stocking for continued presence,;
17 percent established self-sustaining populations, either with beneficial or neutral effects;
7 percent had harmful effects that could obviously be discerned; and
3 percent rapidly increased and then declined, either through natural means or deliberate eradication.
The overall conclusion from Beverton's analysis was that the major threats to inland fishes were habitat deterioration and changes in waterflows. The majority of introduced species have proved either "non-viable or ecologically neutral". However, some general colonisers and powerful predators (e.g. Nile perch) seriously harmed the native fish fauna. It was also shown that negative effects could manifest 50 to 100 years after the introduction as in the case of the accidental spread of the sea-lamprey (Petromyzon marinus) in the Laurentian Great Lakes of North America. However, as Beverton (1992; page 139) points out, "... it is usual for two or more... threats to operate together;... the result may be more dangerous than the sum of their independent effects."

Tilapia cages in Lake Maninjau, West Sumatra
Species of tilapia, especially Nile tilapia, have become domesticated and genetically altered to increase production. This has also raised concerns over their interaction with native con-specifics or close relatives. For example tilapia genetically altered and moved around Africa would inter-breed with native varieties thus contaminate native gene pools or they may even enter fish farming facilities and compromise selective breeding programmes. Similarly salmon and trout that have been introduced or stocked into the Pacific Northwest of North America may interbreed with native salmonids and reduce the fitness of those populations. What is needed is to keep a careful track of events when and if newly developed strains of tilapias are introduced into the natural ranges of distribution, and try to minimize the risks of uncontrolled breeding, for example by using all male fish.
Freshwater fish are considered to be one of the most threatened groups of vertebrates used by humans (Bruton, 1995; Moyle and Leidy, 1992). In general, alien piscine introductions are cited as a major contributing factor leading to the threatened and endangered status of indigenous species (Moyle and Leidy, 1992).
The balance of currently available evidence in the Asia and the Pacific suggests that, as far as we are aware, tilapias have had no major negative impact on biodiversity per se. Welcomme and Vidthayanon (1999) examined the impacts of alien species in the lower Mekong Basin and found no direct evidence of environmental harm arising from tilapias. However, in some instances it may be too early to judge negative impacts. Therefore, there is a need for vigilance. With increasing emphasis on biodiversity issues (see for example Holdgate, 1996; Maclean and Jones 1995; Beveridge et al., 1994), it is understandable that there are schools of thoughts emerging and indeed gaining momentum, expressing the view for a shift in emphasis of culturing aliens (i.e. the tilapias) to indigenous species (Jensen, 1999; Bakos, 1997; Bartley, in press). This may not be the complete solution to the hypothetical problem of tilapias impacting biodiversity with time.
Tilapias are already present in most of the major watersheds in the region and it will be difficult to remove them completely from natural and quasi-natural water bodies in which they are already established. Previous attempts to eradicate established alien fish species (e.g. common carp in Australia) provide compelling evidence of the inherent difficulties that are encountered in such an exercise (Roberts and Tilzey, 1997). The analysis of Beverton (1992) on introductions concluded that only rarely has an unwanted alien been eradicated once it has become established. Evidence has been presented that tilapias are established in the Asia and the Pacific as well as in the Americas and will continue to contribute to the world's animal protein supplies, as well as have significant societal impacts in developing countries. It will be difficult, if not impossible, to eradicate them.
In the context of increasing but often unsubstantiated views on tilapia impacts on biodiversity, a pragmatic strategy would be to prevent the spread of tilapias to environmentally sensitive areas, or areas with productive inland fisheries. Such a strategy should go in parallel with prevention of further deterioration of the immediate environments and their catchments. A good example is the Great Lake, also known as the Tonle Sap, in Cambodia with its unique hydrology and a very rich and diverse piscine fauna. To date, tilapias have not been reported to inhabit this lake. The fishers in fishing communes around this lake increased from 0.36 to 1.20 million, and catches surged from 125 000 to 235 000 tonnes, a 1.9 fold increase from 1940 to 1995 - 1996, with an overall 44 percent decrease in catch per fishing commune inhabitant (Sverdrup- Jensen, 2002). In this productive system tilapia are not needed and should be prevented from becoming established. If the Tonle Sap becomes more degraded and overfished tilapia may find an opportunity to invade as there are tilapia farms in the floodplain of the lack. More often than not, aliens get established in undesired waters when the environment is degraded and its diversity and complexity are reduced as a consequence (Maclean and Jones, 1995; Moyle and Leidy, 1992).
Pillay (1977) expressed the view that haphazard introduction of species between nations, watersheds and continents, for all intents and purposes, is over and future introductions will be more considered from an ecological viewpoint. Unfortunately, it is common knowledge that currently many introductions and transfers of fish, crustaceans and newly developed strains are still being made. The general concerns on introductions led to the development of guidelines for introductions (ICES 1994; Turner 1988), and culminated in the development of codes of conduct for responsible fisheries (FAO 1995). Alien species in general and tilapia specifically have played and will continue to play a role in fishery development in developing countries in Asia and the Pacific. The task at hand is to ensure that this important group of fish is used responsibly and that native biodiversity is conserved in the process.
Amarasinghe, U.S. 2002. The fishery and population dynamics of Oreochromis mossambicus and Oreochromis niloticus (Osteichthyes, Cichlidae) in a shallow irrigation reservoir in Sri Lanka. Asian Fisheries Science 715: 7 - 20.
Amarasinghe, U.S. & De Silva, S.S. 1992. Population dynamics of Oreochromis mossambicus and O. niloticus (Cichlidae) in two reservoirs in Sri Lanka. Asian Fisheries Science 5: 37 - 61.
Amarasinghe, U.S. & De Silva, S.S. 1996. Effect of Oreochromis mossambicus x O. niloticus (Pisces: Cichlidae) hybridization on population reproductive potential and long-term influence on a reservoir fishery. Fisheries Management and Ecology 3: 239 - 249.
Amarasinghe, U.S. & De Silva, S.S. 1999. The Sri Lankan reservoir fishery: a case for introduction of a co-management strategy. Fisheries Management and Ecology 6: 387 - 400.
Amarasinghe, U.S., De Silva, S.S. & Moreau, J. 1989. Spatial changes in growth and mortality and effects on the fishery of Oreochromis mossambicus (Pisces: Cichlidae) in a man-made lake in Sri Lanka. Asian Fisheries Science 3: 57 - 68.
Amarasinghe, U.S., De Silva, S.S. & Nissanka, C. 2002. Evaluation of the robustness of predictive yield models based on catchment characteristics using GIS for reservoir fisheries in Sri Lanka. Fisheries Management and Ecology 9: 292 - 302.
Andrews, S. 1985. Aquatic species introduced to Fiji. Domodomo 111: 67 - 82.
Anonymous. 1995. National Fisheries Development Plan 1995 - 2000. Ministry of Fisheries and Aquatic Resources Development, Colombo.
Arthington, A.H. 1991. Ecological and genetic impacts of introduced and translocated freshwater fishes in Australia. Canadian Journal of Fisheries and Aquatic Sciences 48 (Suppl. 1): 33 - 43.
Arthington, A.H. & Blühdorn, D.R. 1994. Distribution, genetics and ecology of the introduced cichlid, Oreochromis mossambicus, in Australia. Mitteilungen Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 24: 53 - 62.
Arthington, A.H., Mckay, R.J., Russell, D.J. & Milton, D.A. 1984. Occurrence of the introduced cichlid Oreochromis mossambicus (Peters) in Queensland. Australian Journal of Marine and Freshwater Research 35: 267 - 272.
ARTI 1998 - 99. Weekly food commodities bulletin. A publication of the food policy division of the Agrarian Research and Training Institute (Ed. H. Kobbekaduwa), Colombo, Sri Lanka.
Averhoff, O.R.L. 1999. Fish yield in Cuban reservoirs and relationships with some morphometric and edaphic parameters. Lakes and reservoirs: Research and Management 4: 75 - 83.
Aypa, S.M. 1993. The present status and ecology of sinarapan (Mistichthys luzonensis) in Lake Buhi, Camarines Sur Province. Paper Presented at the National Symposium on Lake Fisheries Management, October 1993, PCARRD, Los Banos, Laguna.
Bakos, J., 1997. Exotic fish species: can they be managed? Mekong Fish. Catch and Culture 3: 1 - 4.
Baluyut, E.A. 1983. Stocking and introduction of fish and lakes and reservoirs in the ASEAN countries. FAO Fisheries Technical Paper 236: 82pp.
Baluyut, E.A. 1999. Introduction and fish stocking in lakes and reservoirs in South East Asia: a review. In W.L.T. van Densen & M.J. Morris, eds. Fish and fisheries of lakes and reservoirs in Southeast Asia and Africa, pp. 117 - 141. Otley, United Kingdom, Westbury Publishing.
Banghuai, W. & Qianlong, Z. 1995. Cultivating different breeds of fish in ricefields. In K. T. MacKay, ed. Rice-fish culture in China, pp. 139 - 146. Ottawa, Canada. DRC.
Barman, B.K., Little, D.C. & Edwards, P. 2002. Small-scale fish culture in northwest Bangladesh: a participatory appraisal focusing on the role of tilapia. In P. Edwards, D.C. Little & H. Demaine, eds. pp. 227 - 252. Rural aquaculture. Oxford, United Kingdom. CABI Publishing.
Bartley, D.M., Bhujel, R.C., Funge-Smith, S., Olin, P.G., Phillips, M.J. (eds and comps). 2004. International Mechanisms for the Control and Responsible Use of Alien Species in Aquatic Ecosystems. FAO Non Serial Publication. Rome FAO 2004. (In press).
Bartley, D.M. & C.V. Casal. 1998. Impact of introductions on the conservation and sustainable use of aquatic biodiversity. FAO Aquaculture Newsletter 20: 15-19.
Bartley, D. M., Subasinghe, R. & Coates, D. 1996. Draft framework for the responsible use of introduced species. EIFAC/XIX/96/Inf. 8, June 1996.
Bartley, D.M., Crespi V., Fleisher I.J., & Subasinghe R. In press. Aquatic Alien Species and Their Contribution to Aquatic Production, Food Security and Poverty Alleviation: an overview of data from ASEAN countries. Proceedings of the International workshop, building capacity to combat impacts of aquatic invasive alien species and associated transboundary pathogens in ASEAN countries. NACA, Bangkok, Thailand.
Beardmore, J.A., Mair, G.C. & Lewis, R.I. 2001. Monosex male production in finfish as exemplified by tilapia: applications, problems, and prospects. Aquaculture 197: 283 - 301.
Bernacsek, G.M. 1997. Large dam fisheries of the lower Mekong countries: review and assessment. Data-base. MKG/R, 97023, 2: 145 pp. Phnom Penh, Cambodia Mekong River Commission.
Beveridge, M.C.M., Ross, L.G. & Kelly, L.A. 1994. Aquaculture and biodiversity. Ambio 23: 497 - 502.
Beverton, R.J.H. 1992. Fish resources; threats and protection. Netherlands Journal of Zoology 42: 139 - 175.
Blühdorn, D.R., & Arthington, A.H. 1990. The incidence of stunting in Australian populations of the introduced cichlid, Oreochromis mossambicus (Peters). In R. Hirano & I. Hanyu, eds. Proceedings of the Fourth Asian Fisheries Forum, pp. 41 - 44. Manila, Philippines, Asian Fisheries Society.
Borgstrom, G. 1978. The contribution of freshwater fish to human food. In S. D. Gerking, ed. Ecology of freshwater fish production, pp. 469 - 491. Oxford, United Kingdom, Blackwell Science Publishers.
Bowen, S.H. 1981. Digestion and assimilation of periphytic detrital aggregate by Tilapia mossambica. Transactions of the American Fisheries Society 100:239 - 245.
Bruton, M. 1995. Have fishes had their chips? The dilemma of threatened fishes. Environmental Biology of Fishes 43:1 - 27.
Chakrabarty, R.D. & Samaranayake, R.A.D.B. 1983. Fish culture in seasonal tanks. Journal of Inland Fisheries, Sri Lanka 2:125 - 140.
Chervinski, J. 1982. Environmental physiology of tilapias. In R.S.V. Pullin & R. H. Lowe- McConenell, eds. The biology and culture of tilapias, pp. 119 - 128. ICLARM Conference Proceedings No.7, Manila, Philippines, ICLARM.
Chimitis, P. 1957. The tilapia and their culture, a second review and bibliography. FAO Fisheries Bulletin 10:1 - 24.
Clemens, H.P. & Inslee, T. 1968. The production of unisexual broods by Tilapia mossambica sex-reversed with methyltesterone. Transactions of the American Fisheries Society 97: 18 - 21.
Coates, D. 1985. Fish yield estimates for the Sepik River, Papua New Guinea, a large floodplain system east of "Wallace's Line". Journal of Fish Biology 27: 431 - 443.
Coates, D. 1987a. Consideration of fish introductions to the Sepik River, Papua New Guinea. Aquaculture and Fisheries Management 19: 231 - 241.
Coates, D., 1987b. The inland fisheries in Papua New Guinea. FAO Fisheries Report 371: 119 - 124.
Cruz, E.M. & Ridha, M. 1990. Production of marketable-size tilapia, Oreochromis spilurus (Günther), in seawater cages using different production schedules. Aquaculture and Fisheries Management 21: 187 - 194.
Curtis, L.R., Diren, F. T., Hurley, M.D., Seim, W.K. & Tubb, R.A. 1991. Disposition and elimination of 17 á-methyltesterone in Nile tilapia (Oreochromis niloticus). Aquaculture 99: 193 - 201.
De la Cruz, C. 1998. Social, economic and cultural aspects in implementing inland fishery enhancements in the Philippines. FAO Fisheries Technical Report 374: 323 - 336.
De Silva, S.S. 1985a. Status of the introduced cichlid, Sarotherodon mossambicus (Peters) in the reservoir fishery of Sri Lanka: a management strategy and ecological implications. Aquaculture and Fisheries Management 16: 91 - 102.
De Silva, S.S. 1985b. Body condition and nutritional ecology of Oreochromis mossambicus (Pisces, Cichlidae) populations of man-made lakes in Sri Lanka. Journal of Fish Biology 27: 621 - 633.
De Silva, S.S. 1988. Reservoirs of Sri Lanka and their fisheries. FAO Technical Paper 298, 126 pp.
De Silva, S.S. (ed.), 1989. Exotic aquatic organisms in Asia. Proceedings of the Workshop on Introduction of exotic Aquatic Organisms in Asia. Asian Fisheries Society, Special Publication 3, Manila, Philippines, Asian Fisheries Society., 154 pp.
De Silva, S.S. 2001a. Reservoir fisheries: broad strategies for enhancing yields. In S.S. De Silva, ed. Reservoir and culture-based fisheries: biology and management, pp. 7 - 15. ACIAR Proceedings No. 98, Canberra, Australia, ACIAR.
De Silva, S.S. 2001b. A global perspective of aquaculture in the new millennium. In R. P. Subasinghe, P. B. Bueno, M.J. Phillips, C. Hough, S. E. McGladdery, & J. R. Arthur, eds. Aquaculture in the Third Millennium, pp. 431 - 459. Bangkok, Thailand, NACA, and Italy, Rome, FAO.
De Silva, S.S. & Chandrasoma, J. 1980. Reproductive biology of Sarotherodon mossambicus, and introduced species in an ancient man-made lake in Sri Lanka. Environmental Biology of Fishes 5: 253 - 259.
De Silva, C.D. & Ranasinghe, J. 1989. Biochemical evidence of hybrid gene introgression in some reservoir populations of tilapia in southern Sri Lanka. Aquaculture and Fisheries Management 20: 269 - 277.
De Silva, S.S. & Sirisena, H.K.G. 1988. Observations on the nesting habits of Oreochromis mossambicus (Peters) (Pisces: Cichlidae) in Sri Lankan reservoirs. Journal of Fish Biology 33: 689 - 696.
De Silva, S.S., Amarasinghe, U.S., Nissanka, C., Wijesooriya, W.A.D.D. & Fernando M.J.J. 2001. Use of geographical information systems as a tool for fish yield prediction in tropical reservoirs: case study on Sri Lankan reservoirs. Fisheries Management and Ecology 8: 47 - 60.
De Silva, S.S., Gunasekera, R.M. & Atapattu, D. 1989. The dietary protein requirements of young of tilapia and an evaluation of the least cost dietary protein levels. Aquaculture 80: 271 - 284.
De Silva, S.S. & Senaratne, K.D.W. 1988. Oreochromis mossambicus is not universally a nuisance species: the Sri Lankan experience. In R.S.V. Pullin, T. Bhukaswan, T. Tonguthai, & J.L. Maclean, eds. The Second International Symposium on Tilapia, pp. 445 - 450. ICLARM Conference Proceedings, 15. Bangkok, Thailand, Department of Fisheries, and Manila, Philippines, International Centre for Living Aquatic Resources Management.
Devlin, R.H. & Nagahama, Y., 2002. Sex determination and sex differentiation in fish: an overview of genetic, physiological and environmental influences. Aquaculture. 208: 191 - 364.
Dey, M.M. (ed.). 2000. Special issue: socio-economics of tilapia culture in Asia. Aquaculture Economics and Management 4: 1 - 124.
Dey, M.M., 2001. Tilapia production in South Asia and the Far East. In Subasinghe, S. & Singh, T., eds. Tilapia: production, marketing and technological development, pp. 17 - 27. Kuala Lumpur, Malaysia, INFOFISH.
Dey, M.M., & Gupta, M.V. 2000. Socioeconomics of disseminating genetically improved Nile tilapia in Asia: introduction. Aquaculture Economics and Management 4: 5 - 11.
Dey, M.M., Eknath, A.E., Sifa, L., Hussain, M.G., Thien, T.M., Nguyen, V.H., Aypa S. & Pongthana, N. 2000. Performance and nature of genetically improved farmed tilapia: a bio-economic analysis. Aquaculture Economics and Management 4: 83 - 106.
Dunseth, D.R. & Bayne, D.R. 1978. Recruitment control and production of Tilapia aurea (Steindachner) with the predator Cichlosoma managuense (Günther). Aquaculture 14: 383 - 390.
Eckestein, B. & Spira, M. 1965. Effect of sex hormones on gonadal differentiation in a Cichlid, Tilapia aurea. Biological Bulletin 129: 482 - 489.
Edwards, P. 1990. General discussion on wastewater-fed aquaculture. In P. Edwards, R.S. V. Pullin, eds. Wastewater-fed aquaculture, pp. 281 - 291. Proceedings of he International Seminar on Wastewater Reclamation and Re-use for Aquaculture. Bangkok, Thailand, Asian Institute of Technology.
Edwards, P., Polprasert, C., Rajput, V.S. & Pacharaprakiti, C. 1988. Integrated biogas technology in the tropics. 2. Use of slurry for fish culture. Waste Management and Research 6: 51 - 61.
Edwards, P., Pacharaprakiti, C., & Yomjinda, M. 1990. Direct and indirect reuse of septage for culture of Nile tilapia Oreochromis niloticus, pp. 165 - 168. In Hirano, R. & Hanyu, I., eds. The Second Asian Fisheries Forum. Manila, Philippines Asian Fisheries Society.
Eidman, H.M. 1989. Exotic aquatic species introductions into Indonesia. In De Silva, S.S., ed. Exotic aquatic organisms in Asia, pp. 57 - 62. Asian Fisheries Society Special Publication No. 3. Manila, Philippines, Asian Fisheries Society.
Eknath, A.E. 1995. Managing aquatic genetic resources. Management example 4: the Nile tilapia. In J. Thorpe, ed. Conservation of fish and shellfish resources: managing diversity, pp. 176 - 194. London, Academic Press.
Eknath, A.E., Tayamen, M.M., Palada-de Vera, M.S., Danting, J.C., Reyes, R.A., Dionisio, E.E., Capili, J.B., Bolivar, H.L., Abella, T. A., Circa, A.V., Bensten, H.B., Gjerde, B., Gjedrem, T. & Pullin, R. S. V. 1993. Genetic improvement of farmed tilapias: the growth performance of eight strains of Oreochromis niloticus tested in different farm environments. Aquaculture 111: 171 - 188.
FAO. 1995. Code of Conduct for Responsible Fisheries, Rome, Italy, FAO, 41 pp.
FAO. 1999. Irrigation in Asia in figures. Water reports 18, Rome, Italy, FAO, 228 pp.
Ferdouse, F. 2001. Tilapia in Asian markets... can we sell more? INFOFISH International 5/2001, 23-26.
Fernando, C.H. 1991. Impacts of fish introductions in tropical Asia and America. Canadian Journal Fisheries and Aquatic Sciences 48 (Suppl. II): 24 - 32.
Fernando, C.H. & De Silva, S. S. 1984. Man-made lakes; ancient heritage and modern biological resource. In Fernando, C. H., ed. Ecology and biogeography in Sri Lanka, pp. 431 - 451. Hague, The Netherlands. W. Junk.
Fernando, C.H. & Holcik, J. 1982. The nature of fish communities: a factor influencing the fishery potential of tropical lakes and reservoirs. Hydrobiologia 97: 127 - 140.
Fernando, C.H. & Indrasena, H.H.A. 1969. The freshwater fisheries of Ceylon. Bulletin of the Fisheries Research Station, Ceylon, 20: 101 - 134.
Fitzsimmons, K. 2001. Tilapia production in the Americas. In Subasinghe, S. & Singh, T., eds. Tilapia: production, marketing and technological development, pp. 7 - 16. Kuala Lumpur, Malaysia, INFOFISH.
Frey, D.G. 1969. A limnological reconnaissance of Lake Lanao. Mitteilungen Internationale Vereinigung Für Theoretische Und Angewandte Limnologie 15: 112 - 127.
Gindelberger, B. 1981. Why sinarapan almost disappeared from Lake Buhi. ICLARM Newsletter 4: 3 - 5.
Gille., R. 1989. Tilapia in the Pacific Island; are there lessons to be learned. Asian Fisheries Science 2: 37 - 43.
Gjedrem, T. 2002. Selective breeding. Essential for further productivity, sustainability in aquaculture. The Advocate 5 (1): 46 - 47.
Glucksman, J., West, G. & Berra, T.M. 1976. The introduced fishes of Papua New Guinea with special reference to Tilapia mossambica. Biological Conservation 9: 37 - 44.
Gupta, M.V., Sollows, J.D., Mazid, M.A., Rahaman, A., Hussain, M.G. & Dey, M.M. 2002. Economics and adoption patterns of integrated rice-fish farming in Bangladesh. In P. Edwards, D. C. Little & H. Demaine, eds. Rural Aquaculture, pp. 41 - 54. Oxford, UK, CABI Publishing.
Guerrero, R.D. 1975. Use of androgen for the production of all-male Tilapia aurea (Steindachner). Transactions of the American Fisheries Society 104: 342 - 348.
Guerrero, R.D. 1999. Impacts of tilapia introductions on the endemic fishes in some Philippine lakes and reservoirs. In W.L.T. Van Densen & M.J. Morris, M.J., eds. Fish and fisheries of lakes and reservoirs in Southeast Asia and Africa, 151 - 157. Otley, UK, Westbury Publishing.
Harvey, D. 2001. Domestic production: imports/exports expected higher in 2001. Aquaculture Magazine 27 (3): 33 - 38.
Hickling, C.F. 1960. The Malaca tilapia hybrid. Journal of Genetics 57: 1 - 10.
Hickling, C.F. 1963. The culture of tilapias. Scientific American 208: 143 - 151.
Hio., A.E. & Phelps, R.P. 1993. Effect of initial size on sex reversal of Oreochromis niloticus fry using methytesterone. Aquaculture 112: 301 - 308.
Holdgate, M. 1996. The ecological significance of biological diversity. Ambio 25: 409 - 416.
Hussain, G.H. 1996. Progress of DEGITA project activities in Bangladesh. Paper Presented at the Third Steering Committee Meeting, International Network on Genetics in Aquaculture (INGA), July 1996, Cairo, Egypt, 17 p. (unpublished).
ICES. 1984. Guidelines for implementing the ICES Code of Practice concerning introductions and transfers of marine species. Cooperative Research Report, No. 130, 28pp.
IRCWD. 1985. Health aspects of wastewater and excreta use in agriculture and aquaculture: the Engelberg Report. WHO International Reference Centre for Waste Disposal, IRCWD News No. 23: 11 - 18.
Jensen, J. 1999. Why the tilapia? Mekong Fish: Catch and Culture 5: 4 - 6.
Johnstone, R., Macintosh, D.J. & Wright, R.S. 1983. Elimination of orally administered 17 á - methyltesterone by Oreochromis mossambicus (tilapia) and Salmo gairdneri (rainbow trout) juveniles. Aquaculture 35: 249 - 257.
Khalil, M.T. & Hussein, H.A. 1997. Use of wastewater for aquaculture: an experimental field study at a sewage treatment plant in Egypt. Aquaculture Research 28: 859 - 865.
Lin, S.W. 1977. Aquaculture in South east Asia: A historical overview. Washington Sea Grant Publication, 108 pp.
Lobel, P.S. 1980. Invasion by the Mozambique tilapia (Sarotherodon. mossambicus: Pisces; Cichlidae) of a Pacific atoll marine ecosystem. Micronesica 16: 349 - 355.
Lorenzen, K., Juntana, J., Bundit, J. & Tourongruang, G. 1998a. Assessing culture fisheries practices in small waterbodies: a study of village fisheries in northeast Thailand. Aquaculture Research 29: 211 - 234.
Lorenzen, K., Garaway, C.J., Chamsingh, B. & Warren, T. 1998b. Effects of access restrictions and stocking on small water body fisheries in Laos. Journal of Fish Biology 53 (Suppl. A): 345 - 357.
Macaranas, J.M., Taniguchi, N., Pante, M-J. R., Capili, J.B. & Pullin, R.S.V. 1986. Electrophoretic evidence for extensive hybrid gene introgression into commercial Oreochromis niloticus (L.) stocks in the Philippines. Aquaculture and Fisheries Management 17: 249 - 258.
Maclean, R.H. & Jones, R.W. 1995. Aquatic biodiversity: a review of current issues and efforts. Strategy for International Fisheries Research, Ottawa, Canada, 56 pp.
Mair, G. C. 2001. Genetics in tilapia aquaculture. In Subasinghe, S., Singh, T., eds. Tilapia: production, marketing and technological development, pp. 136 - 148. Kuala Lumpur, Malaysia, INFOFISH.
Mair, G.C. 2002. Domestication and broodstock management. Implications for longterm quality of cultured stocks. The Advocate 5: 39 - 42.
Mair, G.C. & Little, D.C. 1991. Population control in farmed tilapias. NAGA, the ICLARM Quarterly 14: 8 - 13.
Mair, G.C., Abucay, J.S., Beardmore, J.A. & Skibinski, D.O.F. 1995. Growth performance trials of genetically male tilapia (GMT) derived from YY- males in Oreochromis niloticus L.: on station comparisons and mixed-sex and sex reversed male populations. Aquaculture 137: 313 - 322.
Mair, G.C., Clarke, G.J.C., Morales, E.J. & Seveilleja, R.C. 2002. Genetic technologies focussed on poverty? A case study of genetically improved tilapia (GMT) in the Philippines. In P. Edwards, D. C. Little & H. Demaine, eds. Rural Aquaculture, pp. 197 - 225. Oxford, UK, CABI Publishing.
Mair, G.C., Scott, A.G., Penman, D.J., Beardmore, J.A. & Skibinski, D.O.F. 1991a. Sex determination in the genus Oreochromis. 1. Sex reversal, gynogenesis and triploidy in O. niloticus. Theoretical and Applied Genetics 82: 144 - 152.
Mair, G.C., Sco., A.G., Penman, D.J., Skibinski, D.O.F. & Beardmore, J.A. 1991b. Sex determination in the genus Oreochromis. I. Sex reversal, gynogenesis and triploidy in O. aureus Steindachner. Theoretical and Applied Genetics 82: 153 - 160.
Maitipe, P. & De Silva, S.S. 1985. Switches between zoophagy, phytophagy and detritivory of Sarotherodon mossambicus (Peters) adult populations in twelve man- made Sri Lankan lakes. Journal of Fish Biology 26: 49 - 61.
Mather, P.B. & Arthington, A.H. 1991. An assessment of genetic differentiation among feral Australian populations. Australian Journal of Marine and Freshwater Research 42: 721 - 728.
Mathson, N.S., Balavong, V., Nilsson, H., Phounsavath, S. & Hartmann, W.D. 2001. Changes in fisheries yield and catch composition at the Nam Ngum reservoir, Lao PDR. In S. S. De Silva, ed. Reservoir and Culture Based Fisheries: Biology and Management, pp. 48 - 55. Canberra, Australia, ACIAR.
McAndrew, B.J. 1993. Sex control in Tilapiines. In J. F. Muir & R. J. Roberts, eds. Recent Advances in Aquaculture IV, pp. 87 - 98. London, Blackwell.
McCully, P. 1995. Silenced rivers. The ecology and politics of large dams. London, Zed Books. 350 pp.
McDowall, R.M. 1981. The relationships of Australian freshwater fishes. In A. Keast, Ed. Ecological Biogeography of Australia, pp. 1253 - 1273. The Hague, Dr. W. Junk.
Meschkat, A. 1967. The status of warm-water fish culture in Africa. FAO Fisheries Report 44: 88 - 122.
Michaelis, F.B. 1989. Australian Government position: introduction of exotic species. In S. S. De Silva, ed. Exotic Organisms in Asia. Proceedings of the Workshop on Introduction of exotic Aquatic Organisms in Asia, pp. 125 - 132. Asian Fisheries Society, Special Publication 3, Manila, Philippines, Asian Fisheries Society.
Moreau, J. & De Silva, S.S. 1991. Predictive fish yield models for lakes and reservoirs in the Philippines, Thailand and Sri Lanka. FAO Fisheries Technical Paper 319, 42 pp.
Moreau, J., Bambino, C. & Pauly, D. 1986. Indices of overall growth performance of 100 tilapia (Cichlidae) populations. In J.L. Maclean, L.B. Dizon & L.V. Hosillos, eds. The First Asian Fisheries Forum, pp. 201 - 206. Manila, Philippines, Asian Fisheries Society.
Moyle, P.E. & Leidy, R.A. 1992. Loss of biodiversity in aquatic ecosystems: evidence from fish faunas. In P.L. Fiedler and S.K. Jain, eds. Conservation Biology: the theory and practice of nature conservation, preservation and management, pp. 128 - 169. UK, Chapman and Hall.
Murray, F.J., Koddithuwakku, S. & Little, D.C. 2001. Fisheries marketing systems in Sri Lanka and their relevance to local fishery development. In S. S. De Silva, ed. Reservoir and Culture Based Fisheries: Biology and Management, pp. 287 - 308. Canberra, Australia, ACIAR.
Nandeesha, M.C. 2002. Sewage fed aquaculture systems of Kolkata, a century - old innovation of farmers. Aquaculture Asia VII (2): 28 - 32.
Nelson, S. G. & Eldredge, L. G., 1991. Distribution and status of introduced cichlid fishes of the genera Oreochromis and Tilapia in the islands of the South Pacific and Micronesia. Asian Fisheries Science 4: 11 - 22.
Obi, A. & Shelton, W.L., 1983. Androgen and estrogen sex reversal in Tilapia hornorum. In L. Fishelson & Z. Yaron (eds.). Proceedings of the First International Symposium on Tilapia in Aquaculture, pp. 165 - 173. Tel-Aviv University, Israel.
Oreihaka, E. 2001. Characteristics and status of the Lake Tegano fishery. In S.S. De Silva, ed. Reservoir and culture-based fisheries: biology and management, pp. 66 - 70. ACIAR Proceedings No. 98. Canberra, Australia. ACIAR.
Pandian, T.J. & Sheela, S.G. 1995. Hormonal induction of sex reversal in fish. Aquaculture 138: 1 - 22.
Pet, J.S., Van Densen, W.L.T. & Vijverberg, J. 1999. Management options for the exploitation of introduced tilapia and small indigenous cyprinids in Sri Lankan reservoirs. In Van Densen, E.L.T. & Morris, M. J., eds. Fish and fisheries of lakes and reservoirs in Southeast Asia and Africa, pp. 159 - 185. UK, Westbury Publishing.
Pethiyagoda, R. 1994. Treats to indigenous freshwater fishes of Sri Lanka and remarks on their conservation. Hydrobiologia 285: 189 - 201.
Phan, P.D. & De Silva, S.S. 2000. The fishery of Ea-Kao reservoir, southern Viet Nam; a fishery based on a combination of stock and recapture and self-recruiting populations. Fisheries Management and Ecology 7: 251 - 264.
Picchietti, M. 1996. Tilapia marketing maturity. Aquaculture Magazine 22: 19 - 26.
Pillay, T.V.R. 1977. Planning of aquaculture development - an introductory guide. FAO, Rome, Italy, 70 p.
Prescott-Allen, R. & Prescott-Allen, C. 1990. How many plants feed the world? Conservation Biology 4: 365 - 374.
Pullin, R.S.V. (ed.). 1988. Tilapia genetic resources for aquaculture. ICLARM Conference Proceedings 16, Manila, Philippines. 108 pp.
Pullin, R.S.V. & Capili, J.B. 1988. Genetic improvement of tilapia: problems and prospects. In R.S.V. Pullin, T. Bhukaswan, K. Tonguthai & J.L. Maclean, eds. The Second International Symposium on Tilapia in Aquaculture, pp. 259 - 266. Bangkok, Thailand, Department of Fisheries and Manila, Philippines, International Centre for Living Aquatic Resources Management.
Pullin, R.S.V., Palomares, M., Casal, C., Dey, M. & Pauly, D. 1997. Environmental impacts of Tilapia. In Fitzsimmons, K., ed. Tilapia Aquaculture: Proceedings of the Fourth International Symposium on Tilapia in Aquaculture, pp.554 - 570. Northeast Regional Aquacultural Engineering Services Publication No. NRAES- 106, Ithaca, N.Y.
Quiros, R. & Mari, A. 1999. Factors contributing to the outcome of stocking programmes in Cuban reservoirs. Fisheries Management and Ecology 5: 241 - 254.
Ranoemihardjo, B.S. 1981. Nauru: eradication of tilapia from fresh- and brackishwater lagoons and ponds with a view to promoting milkfish culture. A report prepared or the tilapia eradication project. FI: DP/NAU/78/001. Field Document 1. FAO, Rome, 15 pp.
Riedmiller, S. 1994. Lake Victoria fisheries: the Kenyan reality and environmental implications. Environmental Biology of Fishes 39: 329 - 338.
Roberts, J. & Tilzey, R. (eds.). 1997. Controlling carp; exploring the options for Australia. CSIRO Land and Water, Canberra.
Ross, L.G., 2000. Environmental physiology and energetic. In M.C.M. Beveridge & B. J. McAndrew, eds. Tilapias: Biology and Exploitation, pp. 89 - 123. Fish and Fisheries Series 25, London, Kluwer Academic Press.
Rothbard, S., Solnik, E., Shabat, S., Amado, R., & Grabie, I. 1983. The technology of mass production of hormone sex-reversed all- male tilapias. In L. Fishelson & Z. Yaron (ed). International Symposium on Tilapia in Aquaculture, pp. 425 - 434. Tel Aviv, Israel, Tel Aviv University Press.
Rothbard, S., Zohar, Y., Zmora, N., Levavi-Sivan, B., Moav, B. & Yaron, Z. 1990. Clearance of 17.methyltesterone from muscle of sex-inversed tilapia hybrids treated for growth enhancement with two doses of the androgen. Aquaculture 89: 365 - 376.
Roy, A. 1999. The cost of living. Flamingo, London, 162 pp. Sarnita, A. S. 1999. Introduction and stocking of freshwater fishes into inland waters of Indonesia. In E.L.T. Van Densen & M.J. Morris, M.J. eds. Fish and fisheries of lakes and reservoirs in Southeast Asia and Africa, pp. 143 - 150. UK, Westbury Publishing.
Sco., A.G., Penman, D.J., Beardmore, J.A.B. & Skibinski, D.O.F. 1989. The 'YY' supermale in Oreochromis niloticus (L.) and its potential in aquaculture. Aquaculture 78: 237 - 251.
Smith, I.R. & Pullin, R.S.V. 1984. Tilapia production booms in the Philippines. ICLARM Newsletter 7: 7 - 9.
Sreenivasan, A. 1976. Fish production and fish population changes in some South Indian reservoirs. Indian Journal of Fisheries 23: 134 - 152.
Sreenivasan, A. & Sundararajan, D. 1967. A note on the tilapia fishery of an impoundment in Madras State. Science and Culture 33: 145 - 146.
Stickney, R.R. 1986. Tilapia tolerance of saline waters: a review. The Progressive Fish- Culturist 48: 161 - 167.
Sugunan, V.V. 1995. Reservoir fisheries of India. FAO Fisheries Technical Paper No. 345, 414 pp.
Sverdrup-Jensen, S. 2002. Fisheries in the Lower Mekong Basin: status and perspectives. MRC Technical Paper No. 6, Mekong River Commission, Phnom Penh, Cambodia. 103 pp.
Tayamen, M.M. & Shelton, W. L. 1978. Inducement of sex-reversal in Sarotherodon niloticus (Linnaeus). Aquaculture 25: 59 - 65.
Termvidchakorn, A., Vidthayanon, C., Getpetch, Y., Sorrak, P. & Paradonpanichakul, P. 2003. Alien aquatic species in Thailand. Department of Fisheries, Bangkok, Thailand. 74 pp.
Turner, G.E. (ed) 1988. Codes of Practice and Manual of Procedures for Consideration of introductions and Transfers of marine and Freshwater Organisms. EIFAC Occasional Paper 23, 38 pp.
Turner, G.S. & Robinson, R.L., 2000. Reproductive biology, mating and parental care. In: M. C. M. Beveridge & B. J. McAndrew, eds. Tilapias: biology and exploitation, pp. 33 - 58. Fish and Fisheries Series 25, London, Kluwer Academic Press.
United Nations Conference on Environment and Development (UNCED). 1992. Convention on biological diversity, Article 2. 3 - 14 June. Rio de Janerio. United Nations, New York, USA.
Uwate, K.R., Kunatuba, P., Raobati, B. & Tenakanai, C. 1984. A review of aquaculture activities in the Pacific Island region. Pacific Islands Development Program, East-West Centre, Honolulu, Hawaii.
Vannuccini, S. 2001. Global markets for tilapia. INFOFISH International 6/2001:16 - 21.
Varadaraj, K. 1990. Producing monosex male Oreochromis mossambicus (Peters) by administering 19-norethisterone acetate. Aquaculture and Fisheries Management 21: 133 - 135.
Vardaraja, K. & Pandian, T. J. 1989. First report on production of supermale tilapia by integrating endocrine sex reversal with gynogenetic techniques. Current Science 58: 434 - 441.
Welcomme, R.L. 1988. International introductions of inland aquatic species. FAO Fisheries Technical Paper 213, 120 pp.
Welcomme, R.L. 1984. International transfers of inland fish species. In W. R. Courtneay and J. R. Stauffer, eds. Distribution, biology and management of exotic species, pp. 22 - 40. Baltimore, U.S.A. The John Hopkins University Press.
Welcomme, R.L. & Bartley, D.M. 1998. Current approaches to the enhancement of fisheries. Fisheries Management and Ecology 5: 351 - 382.
Welcomme, R.L. & Vidthayanon, C. 1999. Report on the impacts of introductions and stocking in the Mekong Basin and policies for control. Management of Reservoir Fisheries in the Mekong Basin, Phase I. Phnom Penh, Cambodia, Mekong River Commission, 62 pp.
Werry, L.P. 1998. A review of freshwater fish introductions in Papua New Guinea. Science in New Guinea 24: 33 - 38.
West, G.J. & Glucksman, J. 1976. Introduction of exotic fish in Papua New Guinea. PNG Agricultural Journal 27: 19 - 48.
Wijeyaratne, M.J.S. & Perera, W.M.D.S.K. 2001. Trophic relationships among the exotic and indigenous fish co-occurring in some reservoirs in Sri Lanka. Asian Fisheries Science 14: 333 - 342.
Winkler, H. 1983. The ecology of cormorants (genus Phalacrocorax). In F. Schiemer, ed. Limnology of Parakrama Samudra, Sri Lanka, pp. 193 - 199. Developments in Hydrobiology No. 150, The Netherlands, Dr. W. Junk.
Wohlfarth, G.W. 1994. The unexploited potential of tilapia hybrids in aquaculture. Aquaculture and Fisheries Management 25: 781 - 788.
Yoshikawa, H. & Oguri, M. 1978. Effects of steroid hormones on sex differentiation in a Cichlid fish, Tilapia zillii. Bulletin of the Japanese Society for Scientific Fisheries 44: 313 - 318.