Milani Chaloupka[2], Peter Dutton[3], Hideki Nakano[4]
Abstract
Six species of sea turtles occur in the Pacific Ocean (green turtles, loggerheads, leatherbacks, hawksbills, olive ridleys and flatbacks). All species, except flatbacks, have transboundary distributions. The status of most sea turtle stocks in the Pacific Ocean is poorly understood. Some stocks are increasing, such as in eastern Australia and Hawaii. However, there is evidence that many stocks have been reduced significantly, which is mainly a result of overharvesting of eggs, subsistence or commercial harvest of large turtles and nesting habitat destruction. Incidental capture in coastal and pelagic fisheries can also be an important source of mortality for some stocks. Abundance trends for the six Pacific species of sea turtles are reviewed, using the best available quantitative information.
INTRODUCTION
The status of the sea turtle stocks in the Pacific Ocean basin is poorly understood (Spotila et al., 1996; Meylan and Donnelly, 1999; Chaloupka and Limpus, 2001; Seminoff, 2002). Many stocks have been reduced significantly, which is mainly a result of overharvesting of eggs (Meylan and Donnelly, 1999; Chaloupka, 2001; Seminoff, 2002), subsistence or commercial harvest of large turtles (Horikoshi et al., 1994; Meylan and Donnelly, 1999; Trinidad and Wilson, 2000; Gardner and Nichols, 2001; Limpus et al., 2004) and nesting habitat destruction (Sharma, 2000; Matsuzawa et al., 2002). Other sources of mortality can have local importance, such as fibropapilloma disease (Chaloupka and Balazs, n.d.) or tiger shark attack (Heithaus, Frid and Dill, 2002; Balazs and Chaloupka, unpubl. strandings data for Hawaii). Incidental capture in coastal and pelagic fisheries can also be an important source of mortality for some stocks (Chan, Liew and Mazlan, 1988; Cheng and Chen, 1997; Chaloupka, 2003b).
Most assessments of the status and trends of sea turtle populations have been based on monitoring the seasonal beach nesting activity of adult females (Chaloupka and Limpus, 2001). But monitoring only female nesting activity provides insufficient information for stock assessment because: (1) females skip breeding seasons, and (2) no information is provided on demographic structure because the immature, adult male and non-breeding female components are not sampled (Chaloupka and Limpus, 2001).
Reliable estimates of sea turtle abundance that would be suitable for stock assessment and conservation management planning depend on sampling the entire demographic structure of a population resident in the foraging grounds. Yet such foraging ground abundance estimates are only known for three Pacific sea turtle stocks - the southern Great Barrier Reef green turtle metapopulation (Chaloupka and Limpus, 2001; Chaloupka, 2002b), the Australian loggerhead metapopulation (Chaloupka and Limpus, 2001) and the Hawaiian green turtle metapopulation (Balazs and Chaloupka, 2004).
All previous regional assessments of sea turtle abundance have been based mainly on anecdotal or qualitative information (Spotila et al., 1996; Meylan and Donnelly, 1999; Seminoff, 2002). Here we review briefly the abundance trends for the six species of sea turtle (green turtles, loggerheads, leatherbacks, hawksbills, olive ridleys, flatbacks) that occur in the Pacific Ocean basin using the best available quantitative information. However, most data are based on nesting beach monitoring and so must be viewed with extreme caution. Moreover, little is known about the spatial and temporal trends in coastal or pelagic fishing effort in the Pacific (Wetherall et al., 1993; Robins, 1995; Poiner and Harris, 1996; Lu, Lee and Liao, 1998; Slater et al., 1998; McCracken, 2000; Chaloupka, 2003b; Tuck, Polacheck and Bulman, 2003). Hence there is very little quantitative information available to support any robust risk analysis of fisheries impacts on sea turtle population viability (Slater et al., 1998).
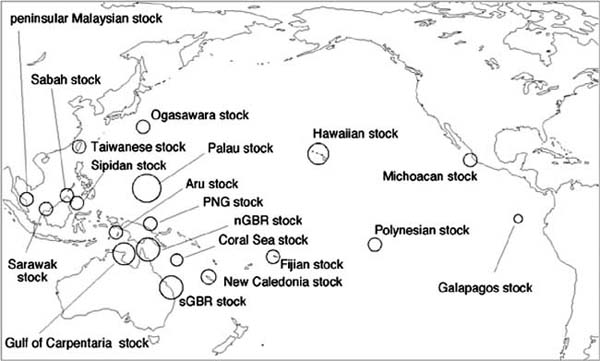
Figure 1. Location of the major regional rookeries for the Pacific green turtle stocks.
Source: Bowen, 1992; Dutton, Broderick and Fitzsimmons, 2002
Green turtles (Chelonia mydas)
Background
The green turtle comprises 20 management units or stocks in the Pacific (Dutton, Broderick and Fitzsimmons, 2002), which are shown in Figure 1. Many Pacific stocks are declining (Seminoff, 2002) but some are stable or increasing (Chaloupka and Limpus, 2001). Stable stocks include the Terengganu rookery (Liew, 2002), Ko Khram rookery (Charuchinda, Monanunsap and Chantrapornsyl, 2002), Sabah and Philippine Turtle Island rookeries in the Sulu Sea (Chaloupka, 2001; Basintal, 2002), Guam rookery (Cummings, 2002), Raine Island rookery of the northern Great Barrier Reef stock (Limpus et al., 2004), Heron Island rookery of the southern Great Barrier Reef stock (Chaloupka and Limpus, 2001), East Island rookery of the Hawaiian stock (Balazs and Chaloupka, 2004), Galapagos rookery (Seminoff, 2002) and the Playa Colola rookery of the Michoacan stock (Seminoff, 2002).
Table 1. Summary of nesting seasons for 20 Pacific green turtle stocks shown in Figure 1
|
Stock |
Nesting location |
Season (peak) |
Source |
|
sGBR |
Heron Island |
Oct-Jan |
Chaloupka & Limpus (2001) |
|
nGBR |
Raine Island |
Oct-Jan |
Limpus et al. (2004) |
|
Sipidan |
Sipidan Island |
year round (Oct-Jan) |
Basintal (2002) |
|
Sulu Sea |
Philippine Turtle Islands |
year round (Jul-Sep) |
Chaloupka (2001) |
|
Sarawak |
Sarawak Turtle Islands |
year round (Jul-Sep) |
Chaloupka (2001) |
|
Malaysia |
Terengganu |
year round (Jun-Jul) |
Chaloupka (2001) |
|
Taiwan (Prov. of China) |
Wan-an Island |
Jul-Aug |
Cheng (2002) |
|
Ogasawara |
Ogasawara Islands |
May-Aug |
Suganuma (1985) |
|
Hawaii |
French Frigate Shoal |
May-Jul |
Balazs & Chaloupka (2004) |
|
Michoacan |
Playa Colola-Maruata |
Sep-Jan |
Alvarado-Diaz, Arias-Coyotl & Delgado-Trejo (2003) |
|
Revillagigedo |
Isla Clarion |
Dec-April |
Sarti, Roldan & Dutton (2002) |
|
Galapagos |
Isabella, St.Cruz, Fernandina |
Dec-April |
Zarate, Fernie & Dutton (2004) |
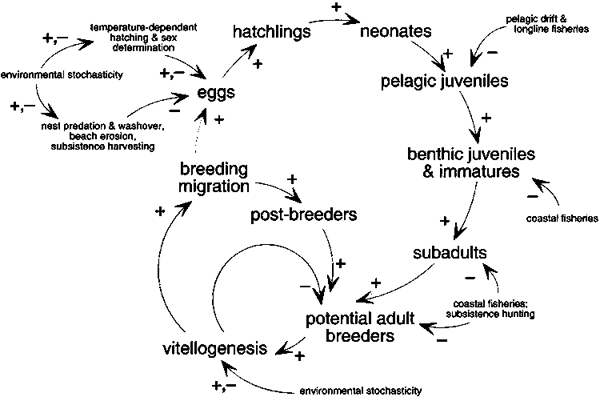
Figure 2. Lifecycle graph or causal loop model based on developmental phases and reproductive status (source: Puccia and Levins, 1985) for Pacific green turtles. The demographic structure and feedback mechanisms depicted here are included in the stochastic simulation model to explore Pacific green turtle metapopulation dynamics subjected to various hazards (e.g. nesting beach erosion, nest inundation by wave washover, egg and turtle harvesting, or incidental capture and drowning in coastal or pelagic fisheries) - see Chaloupka (2002a, 2004)
The main reason for the decline of some green turtle stocks in the Pacific Ocean is the overharvesting of eggs and large turtles (Horikoshi et al., 1994; Limpus, Couper and Read, 1994; Chaloupka, 2002a; Seminoff et al., 2003b - see Figure 3d). There is extensive demographic information available for the southern Great Barrier Reef stock (Limpus, Couper and Read, 1994; Limpus and Chaloupka, 1997; Chaloupka and Limpus, 2001; Chaloupka and Limpus, 2002; Chaloupka, 2002a; Chaloupka, 2002b). There is also extensive demographic information available for the Hawaiian stock including foraging ground abundance estimates (Balazs and Chaloupka, 2004; Balazs and Chaloupka, in press; Chaloupka and Balazs, n.d.). Some important demographic information such as survival probabilities and somatic growth dynamics is available for the Baja California population (Seminoff et al., 2002; Seminoff et al., 2003b). Demographic data are not available for any other Pacific green turtle stock.
A stochastic simulation model of the metapopulation dynamics of the Hawaiian stock was developed for National Oceanic and Atmospheric Administration (NOAA) Fisheries to help evaluate the impact of competing risks on green turtle population viability (Chaloupka, 2003, unpubl. - see Figure 2). A stochastic simulation model for the Australian stock was described in detail in Chaloupka (2002a) and a spatially explicit extension of that model was described in Chaloupka (2004). A more comprehensive stochastic simulation model was developed for the Great Barrier Reef Marine Park Authority to evaluate potential impacts of indigenous harvests on the viability of the southern Great Barrier Reef stock (Chaloupka, 2003a; see also Dobbs and Limpus, in press). A Bayesian surplus production stock assessment model has been developed recently for the Hawaiian green turtle metapopulation (Chaloupka, unpubl.).
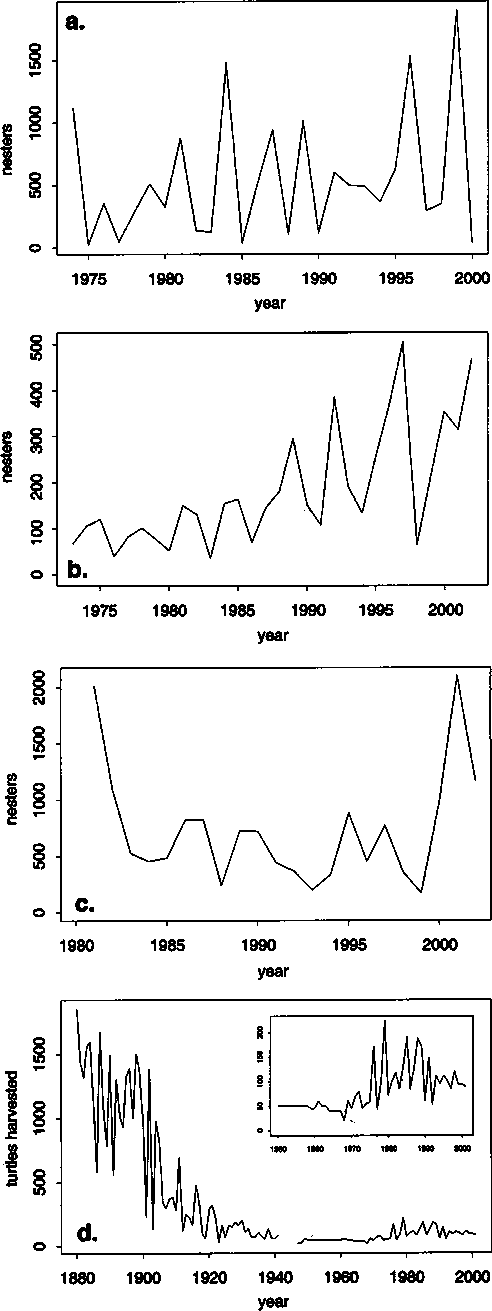
Figure 3. Trends in nesting abundance of three Pacific green turtle populations (a, b and c) and the harvest history of the Ogasawara population (d). Panel (a) shows the annual nesting census of green turtles at the Heron Island rookery, southern Great Barrier Reef (source: Chaloupka and Limpus, 2001; Limpus and Limpus, 2003). Panel (b) shows the annual nesting census of green turtles at the East Island rookery, French Frigate Shoals, Hawaii (source: Balazs and Chaloupka, 2004). Panel (c) shows the annual nesting census of green turtles at the Colola rookery, south Michoacan, Mexico (source: USA Biological Opinion, 2004). Panel (d) shows the number of adult green turtles harvested each year around the Ogasawara Islands, Japan (source: Horikoshi et al., 1994 with updates from Dr Suganuma, pers. comm.). The inset shows the same data for the period 1950-2000.
Hazards
Green sea turtles account for a small proportion of the incidental take in the Queensland east coast otter trawl (Robins, 1995; Slater et al., 1998) and Australian northern prawn fishery (Poiner and Harris, 1996). Green turtles are commonly caught in pelagic fisheries in the North Pacific (McCraken, 2000) and in some coastal fisheries off the California coast (Julian and Beeson, 1998).
Green turtles have also been the preferred target species for a wide range of subsistence harvests throughout the Pacific (Chaloupka, 2002a; Seminoff, 2002) and for egg collection (Chaloupka, 2001). The main hazards for the species are (Figure 2):
egg harvesting;
harvest of large turtles in the foraging grounds and on nesting beaches;
nesting habitat destruction;
incidental capture in coastal and pelagic fisheries.
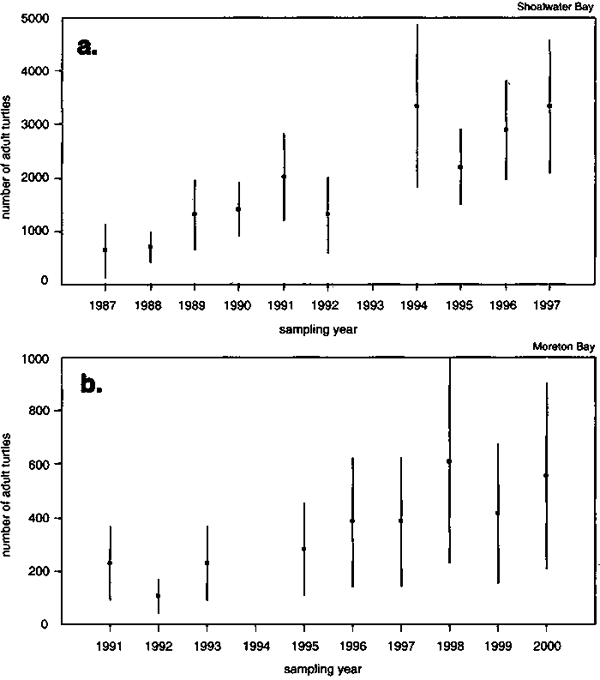
Figure 4. Trends in Horvitz-Thompson type population abundance (solid squares) for green turtles resident in two major foraging grounds of the southern Great Barrier Reef stock. Panel (a) shows adult abundance estimates for the Shoalwater Bay foraging ground population of the sGBR metapopulation. Panel (b) shows adult abundance estimates for the Moreton Bay foraging ground population of the sGBR metapopulation. Vertical bar = approximate 95 percent confidence interval. Source: Chaloupka, 2002b
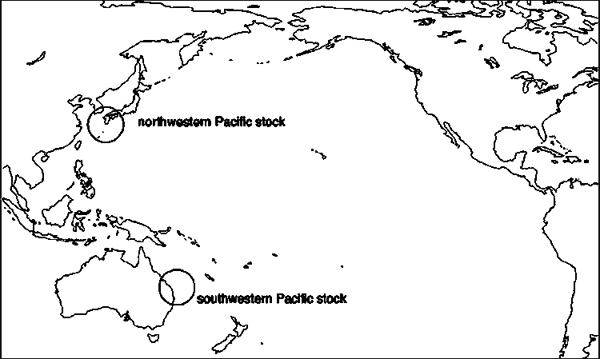
Figure 5. Location of the major regional rookeries for the Pacific loggerhead turtle stocks. Source: Bowen et al., 1994; Kamezaki et al., 2003; Limpus and Limpus, 2003
Loggerheads (Caretta caretta)
Background
The loggerhead sea turtles resident in Pacific waters comprise two distinct stocks (Figure 5). Ongoing genetic and tagging studies are beginning to define breeding stocks on a finer scale within these broad regions. Within Australia, the cluster of rookeries in the east and west is recognized as two distinct management units by genetic studies (see Dutton, Broderick and Fitzsimmons, 2002). Limpus and Limpus (2003) also suggest an additional unit, encompassing the small rookeries in New Caledonia (1 300 km distant from Australian nesting beaches). Similar genetic studies in Japan (Hatase et al., 2002) indicate the presence of at least four discernible management units and provide evidence that all loggerheads found in the northern Pacific originate in Japan. The transition from hatchling to young juvenile in this species occurs in the open ocean. Juvenile foraging areas occur off Baja California, Mexico, approximately 10-12 000 km from their nearest nesting beaches in Japan (Figure 6). Pacific loggerhead stocks have declined significantly over the last 50 years (Figure 7). This is apparent for all nesting populations (Figure 7a,c) and many foraging ground populations (Chaloupka and Limpus, 2001), but not for all foraging ground populations of the Australian stock (Figure 7b). There is extensive demographic information available for the Australian stock (Chaloupka and Limpus, 2002; Chaloupka, 2003b; Limpus and Limpus, 2003). There is very little demographic information available for the Japanese stock (Kamezaki and Matsui, 1997) but there are comprehensive nesting data available for this stock (Sato et al., 1997; Kamezaki et al., 2003). A stochastic simulation model of the population dynamics of the Japanese stock was developed for NOAA Fisheries to help evaluate the impact of competing risks on loggerhead population viability (Chaloupka, unpubl.). A stochastic simulation model for the Australian stock was described in detail in Chaloupka (2003b), which suggested the dramatic decline in the Australian loggerhead stock was a result of foxes feeding on nest contents at mainland rookeries and incidental capture in coastal and pelagic fisheries.
Table 2. Summary of nesting seasons for Pacific loggerhead turtle stocks shown in Figure 5
|
Stock |
Nesting location |
Season (peak) |
Source |
|
Southwest Pacific |
New Caledonia, southern GBR/Queensland |
Oct-Mar (Dec) |
Limpus & Limpus (2003) |
|
Northwest Pacific |
Southern Japan, Ryukyu Islands |
Apr-Aug (Jul) |
Kamezaki et al. (2003) |
Hazards
Nearly 51 percent of sea turtles caught in the Queensland east coast otter trawl fishery were found to be loggerheads (Robins, 1995; Slater et al., 1998) while around 10 percent of the incidental capture of sea turtles in the Australian northern prawn fishery (Gulf of Carpentaria) was loggerheads (Poiner and Harris, 1996). Loggerheads are also commonly caught in pelagic fisheries in the northern Pacific (Wetherall et al., 1993; McCraken, 2000) and some coastal fisheries off the California coast (Julian and Beeson, 1998). The loggerheads caught in these high seas and coastal fisheries in the North Pacific all belong to the Japanese breeding stock (Figure 6). Recently, juvenile loggerheads of Australian stock origin have been found foraging in the southeast Pacific off the coast of Peru and Chile, suggesting that this stock is distributed across the entire southern Pacific Ocean (Dutton, in prep; see also Donoso et al., 2000, and Alfaro-Shigueto et al., in press) and is impacted by high seas and coastal fisheries operating in this region of the eastern Pacific (Donoso and Dutton, in press).

Figure 6. Genetic stock composition (based on mitochondrial DNA haplotypes) of loggerheads in the Pacific. Two regional nesting stocks are shown (Japan and Australia). The turtles in the North Pacific foraging areas belong to the Japanese nesting stock. Source: Bowen et al., 1995
The main hazards for loggerheads are (see also NMFS and USFWS, 1998a):
incidental capture in coastal and pelagic fisheries;
nesting habitat destruction, including beach armourment;
feral animal predation on nests.
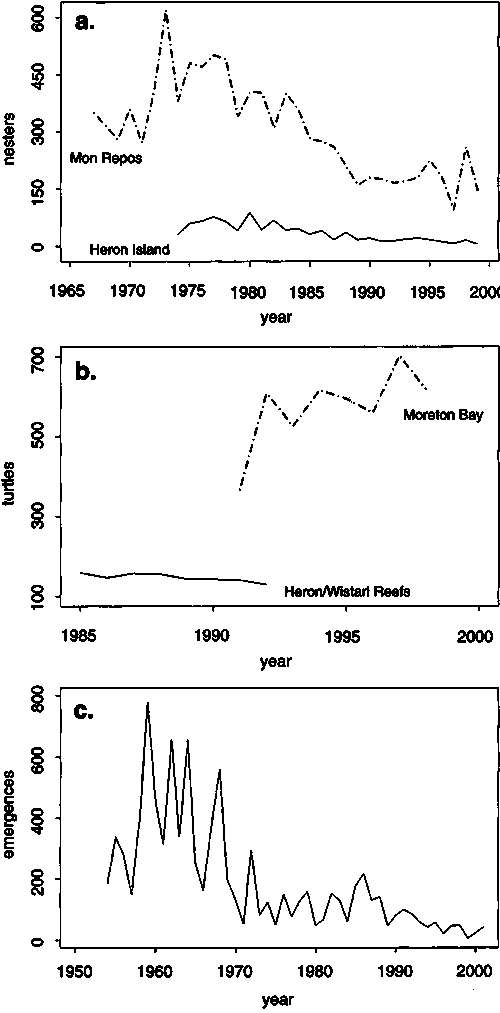
Figure 7. Trends in nesting abundance for the two Pacific loggerhead stocks. Panel (a) shows number of nesters recorded each year for the southwestern Pacific loggerhead rookeries at Mon Repos (source: Limpus and Limpus, 2003) and Heron Island (source: Chaloupka and Limpus, 2001). Panel (b) shows abundance estimates for loggerheads at two foraging ground populations of the southwestern Pacific stock based on Horvitz-Thompson type estimates using Cormack-Jolly-Seber capture probabilities (source: Chaloupka and Limpus, 2001). Panel (c) shows the number of female beach emergences or haul-outs recorded each year for the northwestern Pacific loggerhead rookery at Kamouda, Japan (source: Kamezaki et al., 2003)

Figure 8. Location of the major regional rookeries for the Pacific hawksbill turtle stocks. Source: Broderick et al., 1994; Dutton, Broderick and Fitzsimmons, 2002
Hawksbills (Eretmochelys imbricata)
Background
The hawksbill turtle comprises five stocks or management units in the Pacific (Dutton, Broderick and Fitzsimmons, 2002), which are shown in Figure 8. The hawksbill is critically endangered with some Pacific stocks in decline (Meylan and Donnelly, 1999; Seminoff et al., 2003a). However, stable stocks include the Ko Khram rookery in the Gulf of Thailand (Charuchinda, Monanunsap and Chantrapornsyl, 2002) and the Sabah Turtle Islands rookery in the Sulu Sea (Basintal, 2002). The eastern Pacific stock was abundant but is now only occasionally found along the Baja and Pacific Mexico coast (Seminoff et al., 2003a). Long-term monitoring of nesting abundance is only available for the northern Australian stock (Figure 9a) and the Sabah stock (Figure 9b). The Australian stock has declined in recent years but there are no foraging ground abundance estimates for any Pacific population. There are only limited demographic data available for hawksbills (Chaloupka and Limpus, 1997; Pilcher and Ali, 1999). There are no reliable demographic models of hawksbill population dynamics (Chaloupka and Musick, 1997) but a stochastic simulation model is in development for the Western Pacific Regional Fishery Management Council (Hawaii).
Table 3. Summary of nesting seasons for Pacific hawksbill turtle stocks shown in Figure 8
|
Stock |
Nesting location |
Season |
Source |
|
Malaysian |
Terengganu |
Apr-Aug |
Chan & Liew (1996) |
|
Sabah |
Gulisaan Island |
Feb-Apr, Jun-Aug |
Basintal (2002) |
|
Australian |
Milman Island |
Dec-Apr |
Loop, Miller & Limpus (1995) |
A Bayesian surplus production stock assessment model has been developed for the Cuban hawksbill turtle population (Chaloupka, unpubl., for the IUCN review of the application of the Convention on International Trade in Endangered Species [CITES]), and this is being applied to the central Pacific hawksbill stock.
Hazards
Hawksbills appear to be rarely caught in either pelagic fisheries (Wetherall et al., 1993; McCracken, 2000) or coastal fisheries (Robins, 1995; Poiner and Harris, 1996; Slater et al., 1998). The main hazards for hawksbills are (Meylan and Donnelly, 1999; see also NMFS and USFWS, 1998c):
commercial harvesting for bekko (tortoiseshell);
egg harvesting;
nesting habitat destruction.
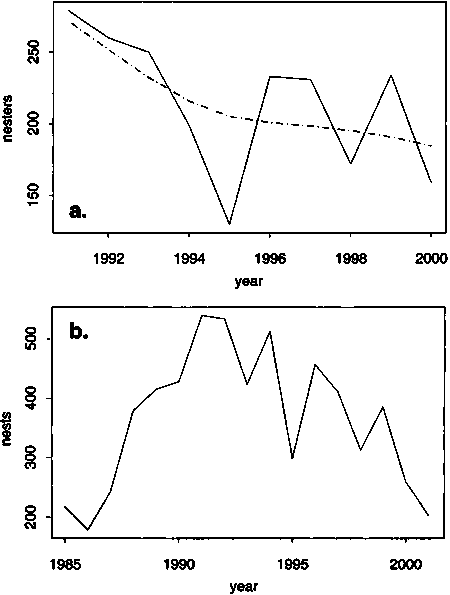
Figure 9. Trends in nesting abundance of two Pacific hawksbill populations. Panel (a) shows the estimated number of hawksbills nesting each year (solid curve) on Milman Island, northern Great Barrier Reef (source: Miller et al., in prep.). The underlying long-term trend in the nester series is shown by a robust cubic spline smooth fit (dashed curve), which suggests that the nester series declined most rapidly during the early 1990s and has since slowed. Panel (b) shows the estimated number of hawksbills nesting at the Gulisaan rookery in the Sabah Turtle Islands (source: Pilcher and Ali, 1999; Basintal, 2002)

Figure 10. Location of the major rookeries for the Pacific leatherback turtle stocks. Source: Chua, 1988; Hirth, Kasu and Mala, 1993; Sarti et al., 1996; Spotila et al., 1996; Dutton et al., 1999; Suarez, Dutton and Bakarbessy, 2000
Leatherbacks (Dermochelys coriacea)
Background
The leatherback sea turtles resident in Pacific waters comprise two main distinct stocks (Figure 10), (with possibly a third “Indo-Pacific” stock whose status is unclear, in Malaysia). All stocks in the Pacific are apparently in decline (Sarti et al., 1996; Spotila et al., 1996) although perhaps not as seriously for the population that nests along the north Vogelkop coast of Papua near Jamursba-Medi (Hitipeuw and Maturbongs, 2002). However, there are no reliable estimates of the long-term status and trend in Pacific leatherback abundance. Many leatherbacks are apparently caught in coastal fisheries operating in Malaysian (Chan, Liew and Mazlan, 1988) and Californian waters (Julian and Beeson, 1998). Leatherbacks are also caught in pelagic fisheries in Chilean (Eckert and Sarti, 1997; Donoso and Dutton, 2000, and in press) and north Pacific waters (McCraken, 2000). A stochastic simulation model of the metapopulation dynamics of the western Pacific stock has been developed for NOAA Fisheries to help evaluate the impact of competing risks on leatherback metapopulation viability (Chaloupka, unpubl.).
Table 4. Summary of nesting seasons for Pacific leatherback turtle stocks shown in Figure 10
|
Stock |
Nesting location |
Season |
Peak |
Source |
|
West Pacific |
Terengganu (Malaysia) |
Apr-Sep |
Jul |
Chua (1988) |
|
Jamursba-Medi (Papua) |
Apr-Oct |
Aug |
Suarez, Dutton & Bakarbessy (2000) |
|
|
War Mon (Papua) |
Nov-Feb |
Dec |
Suarez, Dutton & Bakarbessy (2000) |
|
|
Piguwa (Papua New Guinea, PNG) |
Nov-Mar |
Jan |
Hirth, Kasu & Mala (1993) |
|
|
Kamiali-Huon Coast (PNG) |
Nov-Mar |
Dec |
Dutton et al., unpubl |
|
|
Solomon Islands |
Nov-Mar |
Dec |
Ramohia, Pita & da Wheya (2001) |
|
|
East Pacific |
Playa Mexiquillo (Mexico) |
Oct-Mar |
Dec |
Eckert & Sarti (1997) |
|
Cahuitan (Mexico) |
Oct-Mar |
Dec |
Sarti-Marinez (2002) |
|
|
Tierra Colorada (Mexico) |
Oct-Mar |
Dec |
Sarti-Marinez (2002) |
|
|
Playa Grande (Costa Rica) |
Oct-Mar |
Dec |
Steyermark et al. (1996) |
|
|
Playa Langosta (Costa Rica) |
Oct-Mar |
Dec |
|
|
|
Chococente (Nicaragua) |
Oct-Mar |
Dec |
Dutton et al., unpubl. |
Although Atlantic populations appear to be stable or even increasing, leatherback populations are declining at all major Pacific basin nesting beaches, especially in the past two decades (NMFS and USFWS, 1998b; Spotila et al., 2000; Dutton, in press; Figure 14). The major decline of these nesting populations was probably brought about by a severe overharvest of eggs coupled with incidental mortality from fishing (Eckert and Sarti, 1997), especially the high seas driftnet fishery in the 1980s (Sarti et al., 1996).
Remaining breeding assemblages occur on both sides of the Pacific. In the western Pacific region they occur at low and scattered densities in Papua New Guinea, Solomon Islands, Fiji, Thailand, Vanuatu, China and Australia (east and northeast) (Limpus and McLachlan, 1996; Márquez, 1990; Hirth, Kasu and Mala, 1993). In the western Pacific the last remaining major rookery is limited to Papua (formerly Irian Jaya, Indonesia). Prior to 1990 a major rookery was located in Malaysia (Terengganu), but this population has collapsed in the last decade (Chan and Liew, 1996). In the eastern Pacific, the largest rookeries occur along the coasts of Mexico and Costa Rica. Scattered nesting has been reported in Panama, Colombia, Ecuador and Panama (Márquez, 1990; Spotila et al., 1996).
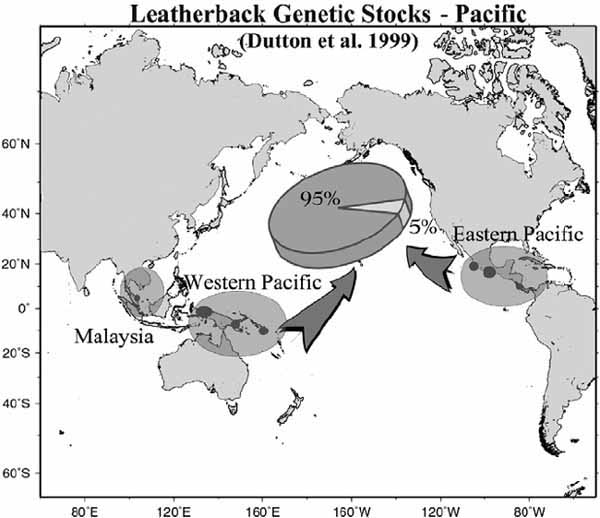
Figure 11. Stock composition of leatherbacks encountered in the north Pacific based on mitochondrial DNA (mtDNA) analysis (17 samples collected from the Hawaii-based longline fishery). The eastern Pacific genetic stock includes nesting populations in Costa Rica and Mexico, while the western Pacific stocks include populations in Papua (Indonesia), Papua New Guinea and the Solomon Islands, and a distinct stock in Malaysia. Source: Dutton et al., 1999
Mitochondrial DNA (mtDNA) sequences can be used to distinguish western Pacific from eastern Pacific genetic stocks (Figure 11). The eastern Pacific genetic stock includes Mexican and Costa Rican breeding assemblages, and the western Pacific stock contains populations in the Solomon Islands, Papua (Indonesia), and Papua New Guinea (Dutton, Broderick and Fitzsimmons, 2002). Genetic results, coupled with tag-recapture and satellite telemetry data suggest that the nesting stocks in the western Pacific primarily use the north Pacific for development and foraging, while animals from eastern Pacific stocks generally forage in the Southern Hemisphere, including the waters off Peru and Chile (Dutton, Broderick and Fitzsimmons, 2002). However, there are exceptions to this pattern, since animals of western Pacific stock origin have been found off Chile (Donoso et al., 2000), and likewise, some leatherbacks of eastern Pacific stock origin have been found in the north Pacific (Dutton, Broderick and Fitzsimmons, 2002).
Leatherbacks undertake some of the longest migrations of all sea turtles and can travel great distances between feeding and nesting areas (Figure 12). Although leatherbacks do not nest on the United States Pacific coast or territories, they forage in United States waters. Animals that are found in these forage areas are mainly from nesting beaches in the western Pacific, and undertake extraordinary migrations across the Pacific to return to nest in Indonesia, Solomon Islands or Papua New Guinea (Dutton, Benson and Eckert, in press). This migratory behaviour exposes them to several United States and international high seas fisheries where they are taken as bycatch. While some eastern Pacific leatherbacks are found in the north Pacific, most animals that originate in Mexico and Costa Rica migrate south to feed in waters off Peru and Chile and further out in the southeastern Pacific (Dutton, Benson and Eckert, in press; Eckert 1999; Morreale et al., 1996). The juvenile developmental areas remain unknown. Leatherbacks continue to be killed in the artisanal fisheries in Peru (Alfaro-Shigueto et al., in press), and juveniles are caught in the Costa Rican artisanal fisheries (Arauz, unpubl.). Leatherbacks were killed in coastal swordfish gillnet fisheries in Chile (Eckert and Sarti, 1997). However, the size of this fishery has declined considerably since the early 1990s (Donoso and Dutton, in press). The extent of incidental take of leatherbacks by the international fleets that operate on the high seas in the eastern Pacific is unclear. The only data available are for the Chilean swordfish longline fishery, which indicate that leatherbacks are caught, although there have been no observed mortalities (Donoso and Dutton, in press).
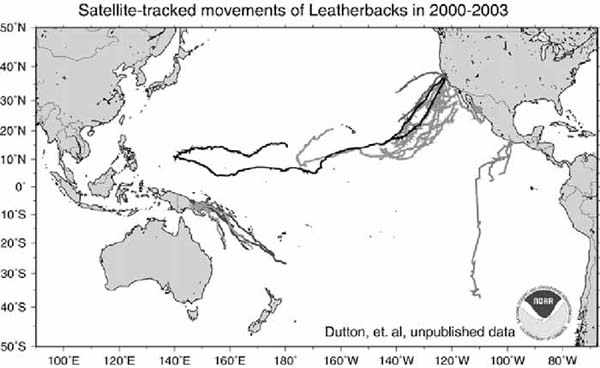
GMT Map created by Denise Parker 06/13/03
Figure 12. Satellite-tracked movements of adult leatherbacks in the Pacific. Tracks include turtles captured and released in a foraging area in Monterey Bay, California (Dutton, Eckert and Benson, unpubl.); females from nesting beaches in Papua New Guinea (Dutton, Benson, Rei and Ambio, unpubl.); and nesting females in Mexico (Sarti, Dutton and Eckert, unpubl.). Additional studies (not depicted here) have tracked southward post-nesting movements of female leatherbacks from Costa Rica passing by the Galapagos Islands (Morreale et al., 1996), and females from Mexico that have travelled to waters off Peru and Chile (Eckert and Sarti, 1997)

Figure 13. Developmental-phase-based and reproductive-status-based lifecycle graph or causal loop model (source: Puccia and Levins, 1985) for Pacific leatherback turtles constructed using information in Eckert (1999). The demographic structure and feedback mechanisms shown here are included in stochastic simulation models to explore western Pacific leatherback turtle metapopulation dynamics subject to various hazards, e.g. nesting beach erosion, nest inundation by wave washover, egg and turtle harvesting, or incidental capture and drowning in coastal or pelagic fisheries
Hazards
The main hazards for leatherbacks are (Figure 13, see also NMFS and USFWS, 1998b):
egg harvesting;
pig and veranid predation of eggs at coastal rookeries;
incidental capture in coastal and pelagic fisheries;
subsistence harvest of large turtles in the foraging grounds and on nesting beaches.
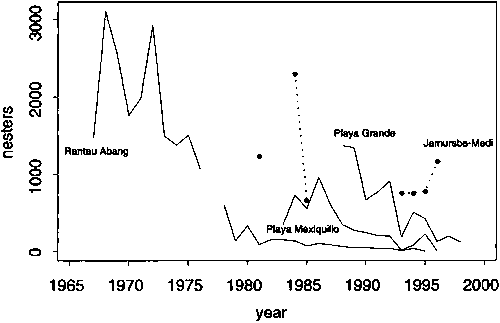
Figure 14. Trends in the nesting abundance of four major Pacific leatherback populations. The western Pacific stock comprises the Rantau Abang and Jamursba-Medi rookeries while the eastern Pacific stock comprises the Mexiquillo and Playa Grande rookeries. The data has been derived in various ways from Chua, 1988; Chan and Liew, 1996; Spotila et al., 1996, 2000; Eckert and Sarti, 1997; Suarez, Dutton and Bakarbessy, 2000
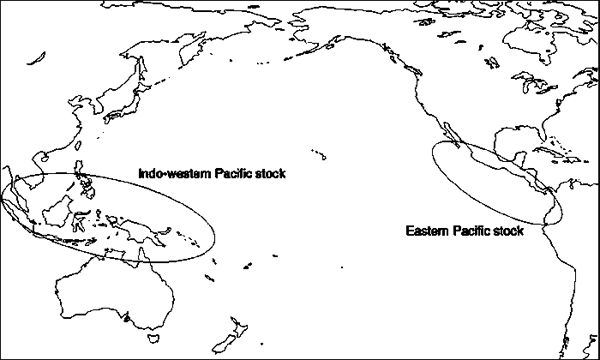
Figure 15. Location of the Pacific olive ridley turtle nesting stocks. Source: Bowen et al., 1998
Olive ridleys (Lepidochelys olivacea)
Background
The olive ridley turtle (Lepidochelys olivacea) has a circumtropical distribution and is probably the most abundant sea turtle species (Bowen et al., 1998). Olive ridleys resident in Pacific waters comprise two stocks (Figure 15) - an eastern Pacific stock that nests along the Pacific coast from Mexico to Colombia (Cliffton, Cornejo and Felger, 1982; Cornelius, 1982; Green and Ortiz-Crespo, 1982; Martinez and Paez, 2000) and a western Pacific stock that nests in coastal areas of southeastern Asia, New Guinea and northern Australia (Siow and Moll, 1982; Chantrapornsyl, 1992; Bowen et al., 1998; Putrawidjaja, 2000). Table 5 summarizes some of the nesting seasons for these two stocks.
Several major nesting populations of the eastern Pacific stock are increasing in abundance following protection from anthropogenic hazards (Figure 16) while some nesting populations in the eastern Pacific (Valverde, Cornelius and Mo, 1998) and Indo-western Pacific stocks are apparently in decline (Siow and Moll, 1982; Chantrapornsyl, 1992; Chantrapornsyl and Bhatia, 1993). The eastern Pacific stock was heavily exploited during the 1960s and 1970s for eggs, meat and skins (Green and Ortiz-Crespo, 1982; Trinidad and Wilson, 2000). Olive ridleys from both Pacific stocks are known to be caught incidentally in coastal fisheries (Cliffton, Cornejo and Felger, 1982; Robins, 1995; Poiner and Harris, 1996; Cheng and Chen, 1997; Slater et al., 1998), which can be a major source of mortality (Poiner and Harris, 1996). Olive ridleys are also exposed to incidental capture in some Pacific pelagic longline fisheries (McCraken, 2000; Polovina et al., 2004). A stochastic simulation model of the population dynamics of the eastern Pacific stock has been developed for NOAA Fisheries to help evaluate the impact of various competing risks on olive ridley population viability (Chaloupka, unpubl.).
Table 5. Summary of nesting seasons for Pacific olive ridley turtle stocks shown in Figure 15
|
Stock |
Nesting location |
Season (peak) |
Source |
|
Eastern Pacific |
Mexico |
Jun-Dec (Aug-Oct) |
Dash & Kar (1990) |
|
Costa Rica |
year round (Aug-Nov) |
Hughes & Richard (1974) |
|
|
Colombia |
Aug-Dec (Aug-Dec) |
Martinez & Paez (2000) |
|
|
Indo-western Pacific |
Northern Australia |
Jun-Aug (Jun-Jul) |
Limpus (2002 pers. comm.) |
|
Peninsula Malaysia |
Oct-Jan |
Siow & Moll (1982) |
|
|
West Thailand |
Oct-Feb (Dec-Jan) |
Chantrapornsyl (1992) |
|
|
Orissa |
Dec-Apr (Dec-Apr) |
Dash & Kar (1990) |
Hazards
The main hazards for olive ridleys are (see also NMFS and USFWS, 1998d):
egg harvesting;
commercial harvest of large turtles in the foraging grounds and on nesting beaches;
incidental capture in coastal and pelagic fisheries.
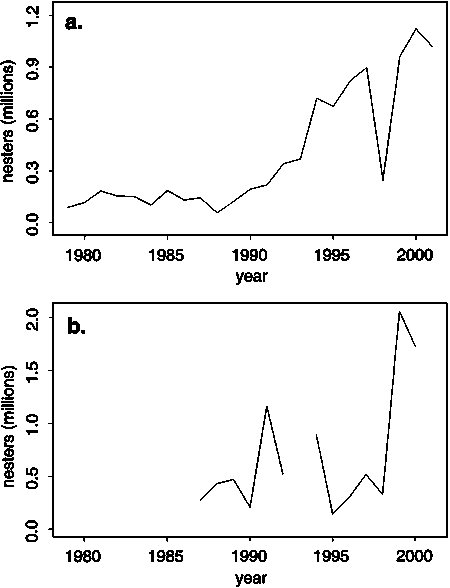
Figure 16. Trends in abundance of two major olive ridley nesting populations from the eastern Pacific stock. Panel (a) shows estimated nesting abundance at La Escobilla, Mexico. Panel (b) shows estimated nesting abundance at Ostional, Costa Rica - the nesting population at nearby Playa Nancite is apparently in decline (Valverde, Cornelius and Mo, 1998). Source: Chaves, 1998, 1999, 2002; Penaflores et al., 2000; F. Alberto Abreu, 2002, pers. comm.
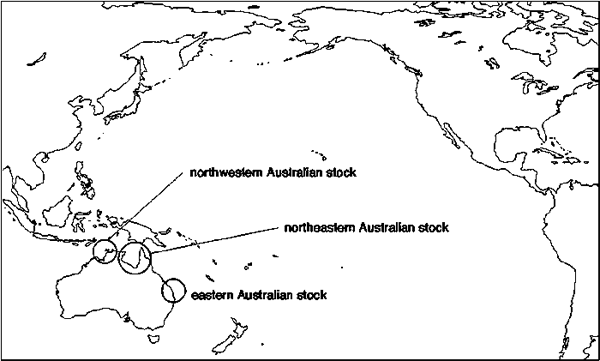
Figure 17. Location of the regional rookeries for the Pacific flatback turtle stocks. Source: Dutton, Broderick and Fitzsimmons, 2002; Limpus, 2002
Flatbacks (Natator depressus)
Background
The flatback is endemic to the northern Australian region with nesting restricted to Australia. Three management units are recognized in the Pacific (Dutton, Broderick and Fitzsimmons, 2002) - a northwestern Australian, a northeastern or Gulf of Carpentaria and an eastern Australian nesting stock (Figure 17). Crab Island in North Queensland (northeastern Australian stock) is the largest flatback rookery in the world (Limpus et al., 1983). Long-term monitoring of nesting abundance is only available for the eastern Australian stock that nests along central coastal Queensland (Mon Repos, Woongarra) and on offshore islands in the central Great Barrier Reef region such as Wild Duck (Limpus, 2002). All monitored nesting populations appear to be stable (see Figure 18) but there are no foraging ground abundance estimates for any population. There are only very limited demographic data available for the flatback turtle (Walker and Parmenter, 1990; Parmenter and Limpus, 1995; Limpus, 2002). There are no demographic models of flatback population dynamics available.
Table 6. Summary of nesting seasons for Pacific flatback turtle stocks shown in Figure 17
|
Stock |
Nesting location |
Season (peak) |
Source |
|
Northwestern Australian |
Fog Bay |
May-Jul |
Guinea (1994) |
|
Northeastern Australian |
Crab Island |
year round |
Limpus et al. (1983) |
|
Eastern Australian |
Wild Duck Island |
Oct-Jan |
Slater et al. (1998) |
Hazards
The eastern Australian stock might have been significantly reduced over the last century but there are no reliable data to confirm this view (Limpus, 2002). Extensive harvesting of eggs and turtles occurs in northern Australian waters (Limpus et al., 1983; Limpus, 2002). Nearly 60 percent of sea turtles caught in the Australian northern prawn fishery (Gulf of Carpentaria) are flatbacks (Poiner and Harris, 1996) while around 11 percent of the incidental capture of sea turtles in the Queensland east coast otter trawl fishery are flatbacks (Robins, 1995). The main hazards for flatbacks are:
pig and veranid predation of eggs at coastal rookeries;
egg harvesting;
incidental capture in coastal otter trawl fisheries;
indigenous harvest of large turtles in the foraging grounds and on nesting beaches.
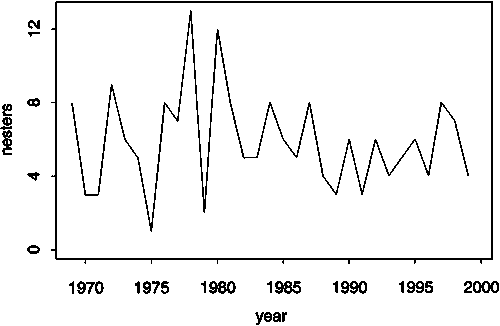
Figure 18. Trend in abundance of the Woongarra flatback nesting population from the eastern Australian stock. Source: Limpus, 2002
ACKNOWLEDGEMENTS
We are especially grateful to the following for providing data sets that were used in these analyses: Alberto Abreu, George Balazs, Kirsten Dobbs, Colin Limpus, Jeff Miller, Nicholas Pilcher and Hiroyuki Suganuma.
Alfaro-Shigueto, J., Mangel, J., Vega, D. & Dutton, P.H. First confirmed occurrence of loggerhead turtles in Peru. Mar. Turtle Newsl. (In press)
Alvarado-Diaz, J., Arias-Coyotl, E. & Delgado-Trejo, C. 2003. Clutch frequency of the Michoacan green seaturtle. J Herpetol., 37: 183-185.
Balazs, G.H. & Chaloupka, M. 2004. Thirty-year recovery trend in the once depleted Hawaiian green sea turtle stock. Biol. Conserv., 117: 491-498.
Balazs, G.H. & Chaloupka, M. Spatial and temporal variability in somatic growth of green sea turtles resident in the Hawaiian Archipelago. Mar. Biol. (In press)
Basintal, P. 2002. Sea turtles conservation at the Sabah’s Turtle Islands Park, Malaysia. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, Western Pacific Regional Fishery Management Council, Honolulu, Hawaii, pp. 151-160.
Bowen, B.W., Abreu-Grobois, F.A., Balazs, G.H., Kamezaki, N., Limpus, C.J. & Ferl, R.J. 1995. Trans-Pacific migrations of the loggerhead turtle (Caretta caretta) demonstrated with mitochondrial DNA markers. Proc. Natl Acad. Sci. USA, 92: 3731-3734.
Bowen, B.W., Clark, A.M., Abreu-Grobois, F.A., Chaves, A., Reichart, H.A. & Ferl, R.J. 1998. Global phylogeography of the ridley sea turtles (Lepidochelys spp.) as inferred from mitochondrial DNA sequences. Genetica, 101: 179-189.
Bowen, B.W., Kamezaki, N., Limpus, C.J., Hughes, G.R., Meylan, A.B. & Avise, J.C. 1994. Global phylogeography of the loggerhead turtle (Caretta caretta) as indicated by mitochondrial DNA haplotypes. Evolution, 48: 1820-1828.
Bowen, B.W., Meylan, A.B., Ross, J.P., Limpus, C.J., Balazs, G.H. & Avise, J.C. 1992. Global population structure and natural history of the green turtle (Chelonia mydas) in terms of matriarchal phylogeny. Evolution, 46: 865-881.
Broderick, D., Moritz, C., Miller, J.D., Guinea, M., Prince, R.I.T. & Limpus, C.J. 1994. Genetic studies of the hawksbill turtle, Eretmochelys imbricata: evidence for multiple stocks in Australian waters. Pac. Conserv. Biol., 1: 123-131.
Chaloupka, M. 2001. Historical trends, seasonality and spatial synchrony in green turtle egg production. Biol. Conserv., 101: 263-279.
Chaloupka, M. 2002a. Stochastic simulation modelling of southern Great Barrier Reef green turtle population dynamics. Ecol. Modelling, 148: 79-109.
Chaloupka, M. 2002b. Phase 1 - Assessment of the suitability of Queensland Parks and Wildlife Service sea turtle data for use in models of the population dynamics of the southern Great Barrier Reef green turtle stock. Research Publication No. 74. Townsville, Queensland, Great Barrier Reef Marine Park Authority. 49 pp. (also available at www.gbrmpa.gov.au/corp site/info)
Chaloupka, M. 2003a. Phase 2 - Development of a population model for the southern Great Barrier Reef green turtle stock. Research Publication No. 81. Townsville, Queensland, Great Barrier Reef Marine Park Authority. 99 pp. (also available at www.gbrmpa.gov.au/corpsite/info)
Chaloupka, M. 2003b. Stochastic simulation modelling of loggerhead sea turtle population dynamics given exposure to competing mortality risks in the western South Pacific. In A.B. Bolten & B.E. Witherington, eds. Loggerhead sea turtles, pp. 274-294. Washington, DC, Smithsonian Books.
Chaloupka, M. 2004. Exploring the metapopulation dynamics of the southern Great Barrier Reef green sea turtle stock and the possible consequences of sex-biased local harvesting. In H. Akçakaya, M. Burgman, O. Kindvall, C. Wood, P. Sjogren-Gulve, J. Hattfield & M. McCarthy, eds. Species conservation and management: case studies. New York, Oxford University Press.
Chaloupka, M. & Balazs, G.H. Modelling the effect of fibropapilloma disease on the somatic growth dynamics of Hawaiian green sea turtles. (In review)
Chaloupka, M.Y. & Limpus, C.J. 1997. Robust statistical modelling of hawksbill sea turtle growth rates (southern Great Barrier Reef). Mar. Ecol. Prog. Ser., 146: 1-8.
Chaloupka, M.Y. & Limpus, C.J. 2001. Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters. Biol. Conserv., 102: 235-249.
Chaloupka, M. & Limpus, C. 2002. Estimates of survival probabilities for the endangered loggerhead sea turtle resident in southern Great Barrier Reef waters. Mar. Biol., 140: 267-277.
Chaloupka, M.Y. & Musick, J.A. 1997. Age, growth and population dynamics. In P.J. Lutz & J.A. Musick, eds. The biology of sea turtles, pp. 233-276. CRC Marine Science Series. Boca Raton, CRC Press.
Chan, E.H. & Liew, H.C. 1996. Decline of the leatherback population in Terengganu, Malaysia, 1956-1995. Chelonian Conserv. Biol., 2: 196-203.
Chan, E.H., Liew, H.C. & Mazlan, A.G. 1988. The incidental capture of sea turtles in fishing gear in Terengganu, Malaysia. Biol. Conserv., 43: 1-7.
Chantrapornsyl, S. 1992. Biology and conservation olive ridley turtle (Lepidochelys olivacea, Eschscholtz) in the Andaman Sea, southern Thailand. Phuket Mar. Biol. Cent. Res. Bull., 57: 51-66.
Chantrapornsyl, S. & Bhatia, O. 1993. Marine turtle conservation in the Philippines. In A. Nacu, R. Trono, J.A. Palma, D. Torres & F. Agas, eds. Proc. First ASEAN Symposium-Workshop on Marine Turtle Conservation, Manila, Philippines, pp. 193-198.
Charuchinda, M., Monanunsap, S. & Chantrapornsyl, S. 2002. Status of sea turtle conservation in Thailand. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, Western Pacific Regional Fishery Management Council, Honolulu, Hawaii, pp. 179-184.
Chaves, G. 1998. Plan de manejo para la utilización racional, manejo y conservación de los huevos de la tortuga marina lora (Lepidochelys olivacea) en el refugio nacional de vida silvestre Ostional, Santa Cruz, Guanacaste, Costa Rica. Escuela de Biología-Universidad de Costa Rica. Informe No. 1998. 20 pp.
Chaves, G. 1999. Anidación de la tortuga lora (Lepidochelys olivacea) en el Refugio Nacional de Vida Silvestre Ostional, Guanacaste. Escuela de Biologia-Universidad de Costa Rica. Informe No. 1. 18 pp.
Chaves, G. 2002. Anidación de la tortuga lora (Lepidochelys olivacea) en el Refugio Nacional de Vida Silvestre Ostional, Guanacaste. Escuela de Biologia-Universidad de Costa Rica. Informe No. 4. 23 pp.
Cheng, I.J. 2002. Current sea turtle research and conservation in Taiwan. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, Western Pacific Regional Fishery Management Council, Honolulu, Hawaii, pp. 185-190.
Cheng, I.J. & Chen, T.H. 1997. The incidental capture of five species of sea turtles by coastal setnet fisheries in the eastern waters of Taiwan. Biol. Conserv., 82: 235-239.
Chua, T.H. 1988. Nesting population and frequency of visits in Dermochelys coriacea in Malaysia. J Herpetol., 22: 192-207.
Cliffton, K., Cornejo, D.O. & Felger, R.S. 1982. Sea turtles of the Pacific coast of Mexico. In K.A. Bjorndal, ed. Biology and conservation of sea turtles, pp. 199-209. Washington, DC, Smithsonian Institution Press.
Cornelius, S.E. 1982. Status of sea turtles along the Pacific coast of middle America. In K.A. Bjorndal, ed. Biology and conservation of sea turtles, pp. 211-219. Washington, DC, Smithsonian Institution Press.
Cummings, V. 2002. Sea turtle conservation in Guam. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, Western Pacific Regional Fishery Management Council, Honolulu, Hawaii, pp. 37-38.
Dash, M.C. & Kar, C.S. 1990. The turtle paradise garirmatha. New Delhi, Interprint.
Dobbs, K. & Limpus, C. Managing traditional hunting on the Great Barrier reef - modelling the southern Great Barrier Reef green turtle stock. In Proc. 23rd Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC. (In press)
Donoso, M. & Dutton, P.H. Numbers, distribution and stock origin of sea turtles caught incidentally in the Chilean longline fishery for swordfish, 2001-2002. In Proc. 24th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC. (In press)
Donoso, M., Dutton, P., Serra, R. & Brito-Montero, J.L. 2000. Sea turtles found in waters off Chile. In H.J. Kalb & T. Wibbels, compilers. Proc. 19th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-443, pp. 218-219.
Dutton, P.H. Life history and population status of sea turtles in the Pacific. In Proc. International Workshop on Sea Turtle Bycatch in Longline Fisheries. NOAA Tech. Report. (In press)
Dutton, P.H., Balazs, G.H. & Dizon, A.E. 1997. Genetic stock identification of sea turtles caught in the Hawaii-based pelagic longline fishery. In S.P. Epperly & J. Braun, compilers. Proc. 17th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-415, pp. 45-46.
Dutton, P.H., Benson, S.R. & Eckert, S.A. Identifying origins of leatherback turtles from Pacific foraging grounds off central California, USA. Proc. 23rd Annual Symposium on Sea Turtle Biology and Conservation, Kuala Lumpur, Malaysia, 2003. NOAA Tech. Memo. (In press)
Dutton, P.H., Broderick, D. & Fitzsimmons, N. 2002. Defining management units: molecular genetics. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, Western Pacific Regional Fishery Management Council, Honolulu, Hawaii, pp. 93-101.
Dutton, P.H., Bowen, B.W., Owens, D.W., Barragan, A. & Davis, S.K. 1999. Global phylogeography of the leatherback turtle (Dermochelys coriacea). J. Zool. Lond., 248: 397-409.
Eckert, S.A. 1999. Global distribution of juvenile leatherback sea turtles. Hubbs Sea World Research Institute Technical Report 99-294. 13 pp.
Eckert, S.A. & Sarti, L.M. 1997. Distant fisheries implicated in the loss of the world’s largest leatherback nesting population. Mar. Turtle Newsl., 78: 2-7.
Ellis, D.M., Balazs, G.H., Gilmartin, W.G., Murakawa, S.K.K. & Katahira, L.K. 2000. Short-range reproductive migrations of hawksbill turtles in the Hawaiian islands as determined by satellite telemetry. In F.A. Abreu, R. Briseno, R. Marquez & L. Sarti, eds. Proc. 18th International Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-436, pp. 252-253.
Gardner, S.C. & Nichols, W.J. 2001. Assessment of sea turtle mortality rates in the Bahia Magdalena region, Baja California Sur, Mexico. Chelonian Conserv. Biol., 4: 197-199.
Green, D. & Ortiz-Crespo, F. 1982. Status of sea turtle populations in the central eastern Pacific. In K.A. Bjorndal, ed. Biology and conservation of sea turtles, pp. 221-233. Washington, DC, Smithsonian Institution Press.
Guinea, M.L. 1994. A possible model to explain winter nesting by the flatback turtle, Natator depressus, at Fog Bay, Northern Territory. In R. James, compiler. Proc. Marine Turtle Conservation Workshop, pp. 154-155. Canberra, Australian National Parks and Wildlife Service.
Hatase, H., Kinoshita, M., Bando, T., Kamezaki, N., Sato, K., Matsuzawa, Y., Goto, K., Omuta, K., Nakashima, Y., Takeshita, H. & Sakamoto, W. 2002. Population structure of loggerhead turtles, Caretta caretta, nesting in Japan: bottlenecks on the Pacific population. Mar. Biol., 141: 299-305.
Heithaus, M.R., Frid, A. & Dill, L.M. 2002. Shark-inflicted injury frequencies, escape ability, and habitat use of green and loggerhead turtles. Mar. Biol., 140: 229-236.
Hien, T.M. 2002. Status of sea turtle conservation in Viet Nam. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, pp. 191-194. Honolulu, Hawaii, Western Pacific Regional Fishery Management Council.
Hirth, H.F., Kasu, J. & Mala, T. 1993. Observations on a leatherback turtle Dermochelys coriacea nesting population near Piguwa, Papua New Guinea. Biol. Conserv., 65: 77-82.
Hitipeuw, C. & Maturbongs, J. 2002. Marine turtle conservation program: Jamursba-Medi nesting beach, north coast of the Bird’s Head Peninsula, Papua. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, pp. 161-175. Honolulu, Hawaii, Western Pacific Regional Fishery Management Council.
Horikoshi, K., Suganuma, H., Tachikawa, H., Sato, F. & Yamaguchi, M. 1994. Decline of Ogasawara green turtle population in Japan. In K.A. Bjorndal, A.B. Bolten, D.A. Johnson & P.J. Eliazar, eds. Proc. 14th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-351, pp. 235-237.
Hughes, D.A. & Richard, J.D. 1974. The nesting of the Pacific ridley turtle Lepidochelys olivacea on Playa Nancite, Costa Rica. Mar. Biol., 24: 97-107.
Julian, F. & Beeson, M. 1998. Estimates of marine mammal, turtle and seabird mortality for two California gillnet fisheries: 1990-1995. Fish. Bull., 96: 271-284.
Kamezaki, N. & Matsui, M. 1997. A review of biological studies on sea turtles in Japan. Japan J. Herpetol., 17: 16-32.
Kamezaki, N., Matsuzawa, Y., Abe, O., Asakawa, H., Fujii, T. 2003. Loggerhead turtle nesting in Japan. In A.B. Bolten & B.E. Witherington, eds. Loggerhead sea turtles, pp. 210-217. Washington, DC, Smithsonian Books.
Liew, H.C. 2002. Status of marine turtle conservation and research in Malaysia. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, pp. 51-56. Honolulu, Hawaii, Western Pacific Regional Fishery Management Council.
Limpus, C.J. 2002. The status of the flatback turtle, Natator depressus, in eastern Australia. In A. Dossier, A. Foley & B. Brost, eds. Proc. 20th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-477, pp. 140-142.
Limpus, C.J. & Chaloupka, M. 1997. Nonparametric regression modelling of green sea turtle growth rates (southern Great Barrier Reef). Mar. Ecol. Prog. Ser., 149: 23-34.
Limpus, C.J. & Limpus, D.J. 2003. Loggerhead turtles in the equatorial and southern Pacific Ocean: a species in decline. In A.B. Bolten & B.E. Witherington, eds. The biology and conservation of loggerhead sea turtles, pp. 199-209. Washington, DC, Smithsonian Institution Press.
Limpus, C.J. & McLachlan, N. 1996. The conservation status of the leatherback turtle, Dermochelys coriacea, in Australia. In R. James, ed. Proc. Marine Turtle Conservation Workshop, pp. 68-72. Canberra, Australia, Australian National Parks and Wildlife Service.
Limpus, C.J., Couper, P.J. & Read, M.A. 1994. The green turtle, Chelonia mydas, in Queensland: population structure in a warm temperate feeding area. Mem. Queensland Mus., 35: 139-154.
Limpus, C.J., Parmenter, C.J., Baker, V. & Fleay, A. 1983. The Crab Island sea turtle rookery in the north-eastern Gulf of Carpentaria. Aust. Wildl. Res., 10: 173-184.
Limpus, C.J., Miller, J.D., Parmenter, C.J., Limpus, D.J. & Hamann, M. 2004. The green turtle population of Raine Island and the northern Great Barrier Reef: 1843-2001. Mem. Queensland Mus., 49: 349-440.
Limpus, C.J., Miller, J.D., Parmenter, C.J., Reimer, D., McLachlan, N. & Webb, R. 1992. Migration of green (Chelonia mydas) and loggerhead (Caretta caretta) turtles to and from eastern Australian rookeries. Wildl. Res., 19: 347-358.
Loop, K.A., Miller, J.D. & Limpus, C.J. 1995. Nesting by the hawksbill turtle (Eretmochelys imbricata) on Milman Island Great Barrier Reef. Aust. Wildl. Res., 22: 241-252.
Lu, H.J., Lee, K.T. & Liao, C.H. 1998. On the relationship between El Niño/Southern Oscillation and South Pacific albacore. Fisheries Res., 39: 1-7.
Mangel, J.C., Bernhard, H., Canja, S., Hau, S., Smith, K. & Williams, S. 2000. Summary of hawksbill turtles (Eretmochelys imbricata) nesting on Maui, Hawaii from 1991 to 1996. In H. Kalb & T. Wibbels, eds. Proc. 19th Annual Symposium on Sea Turtle Biology and Conservation, NOAA Tech. Memorandum NMFS-SEFSC-443, pp. 283-284.
Márquez-M., R. 1990. Sea turtles of the world: an annotated and illustrated catalogue of sea turtle species known to date. FAO Species Catalogue, Fisheries Synopsis No. 125, Vol. 11. Rome. 81 pp.
Martinez, L.M. & Paez, V.P. 2000. Nesting ecology of the olive ridley turtle (Lepidochelys olivacea) at La Cuevita, Chocoan Pacific Coast, Colombia, in 1998. Actualioades Biologicas Medellin, 22: 131-143.
Matsuzawa, Y., Sato, K., Sakamoto, W. & Bjorndal, K.A. 2002. Seasonal fluctuations in sand temperature: effects on the incubation period and mortality of loggerhead sea turtle (Caretta caretta) pre-emergent hatchlings in Minabe, Japan. Mar. Biol., 14: 639-646.
McCracken, M.L. 2000. Estimation of the sea turtle take and mortality in the Hawaiian longline fisheries. Administrative Report H-00-06, Honolulu Laboratory, Southwest Fisheries Science Center, National Marine Fisheries Service, NOAA, Honolulu, Hawaii, pp. 1-29.
Meylan, A.B. & Donnelly, M. 1999. Status justification for listing the hawksbill turtle (Eretmochelys imbricata) as critically endangered on the 1996 IUCN Red List of Threatened Animals. Chelonian Conserv. Biol., 3: 200-224.
Morreale, S.J., Standora, E.A., Spotila, J.R. & Paladino, F.V. 1996. Migration corridor for sea turtles. Nature, 384: 319-320.
NMFS/USFWS. 1998a. Recovery plan for US Pacific populations of the loggerhead turtle (Caretta caretta). Silver Spring, Maryland, USA, National Marine Fisheries Service.
NMFS/USFWS. 1998b. Recovery plan for US Pacific populations of the leatherback turtle (Dermochelys coriacea). Silver Spring, Maryland, USA, National Marine Fisheries Service.
NMFS/USFWS. 1998c. Recovery plan for US Pacific populations of the hawksbill turtle (Eretmochelys imbricata). Silver Spring, Maryland, USA, National Marine Fisheries Service.
NMFS/USFWS. 1998d. Recovery plan for US Pacific populations of the olive ridley turtle (Lepidochelys olivacea). Silver Spring, Maryland, USA, National Marine Fisheries Service.
Parmenter, J.C. & Limpus, C.J. 1995. Female recruitment, reproductive longevity and inferred hatchling survivorship for the flatback turtle (Natator depressus) at a major eastern Australian rookery. Copeia 1995: 474-477.
Penaflores, C., Vasconcelos, J., Albavera, E. & Marquez, R. 2000. Twenty-five years nesting of olive ridley sea turtle Lepidochelys olivacea in Escobilla Beach, Oaxaca, Mexico. In F.A. Abreu-Grobois, R. Briseno, R. Marquez & L. Sarti, eds. Proc. 18th International Sea Turtle Symposium. NOAA Technical Memorandum NMFS-SEFSC-436, pp. 27-29.
Pilcher, N.J. & Ali, L. 1999. Reproductive biology of the hawksbill turtle Eretmochelys imbricata in Sabah, Malaysia. Chelonian Conserv. Biol., 3: 330-336.
Poiner, I.R. & Harris, A.N.M. 1996. The incidental capture, direct mortality and delayed mortality of turtles in Australia’s northern prawn fishery. Mar. Biol., 125: 813-825.
Polovina, J.J., Balazs, G.H., Howell, E.A., Parker, D.M., Seki, M.P. & Dutton, P.H. 2004. Forage and migration habitat of loggerhead (Caretta caretta) and olive ridley (Lepidochelys olivacea) sea turtles in the central North Pacific Ocean. Fish Oceanogr., 13: 36-51.
Puccia, C.J. & Levins, R. 1985. Qualitative modeling of complex systems: an introduction to loop analysis and time averaging. Cambridge, Massachusetts, Harvard University Press.
Putrawidjaja, M. 2000. Marine turtles in Irian Jaya, Indonesia. Mar. Turtle Newsl., 90: 8-10.
Ramohia, P.C., Pita, J. & da Wheya, N. 2001. Leatherback turtle (Dermochelys coriacea) tagging and nest monitoring survey, Sasakolo nesting beach, Isabel Province. Apia, Samoa, South Pacific Regional Environmental Programme (SPREP).
Robins, J.B. 1995. Estimated catch and mortality of sea turtles from the east coast otter trawl fishery of Queensland, Australia. Biol. Conserv., 74: 157-167.
Sarti, L.M., Roldán, R. & Dutton, P.H. 2002. Biologia y ecologia de las tortugas marinas en el Archipiélago de Revillagigedo: rutas migratorias y uso del habitat de las tortugas prietas en Isla Clarión. D.G.V.S. Semarnat, La Jolla Lab. NMFS. 47pp.
Sarti, L.M., Eckert, S.A., Garcia, N.T. & Barragan, A.R. 1996. Decline of the world’s largest nesting assemblage of leatherback turtles. Mar. Turtle Newsl., 74: 2-5.
Sarti-Marinez, L. 2002. Current population status of Dermochelys coriacea in the Mexican Pacific coast. In I. Kinan, ed. Proc. Western Pacific Sea Turtle Cooperative Research and Management Workshop, February 5-8, 2002, Honolulu, Hawaii, pp. 87-89. Western Pacific Regional Fishery Management Council. 300 pp.
Sato, K., Bando, T., Matsuzawa, Y., Tanaka, H., Sakamoto, W., Minamikawa, S. & Goto, K. 1997. Decline of the loggerhead turtle, Caretta caretta, nesting on Senri Beach in Minabe, Wakayama, Japan. Chelonian Conserv. Biol., 2: 600-603.
Seminoff, J. 2002. IUCN Red List Status assessment 2002: green turtle (Chelonia mydas). Marine Turtle Specialist Group review draft.
Seminoff, J., Nichols, W., Resendiz, A. & Brooks, L. 2003a. Occurrence of hawksbill turtles, Eretmochelys imbricata (Reptilia: Cheloniidae), near the Baja Peninsula, Mexico. Pac. Sci., 57: 1-9.
Seminoff, J.A., Resendiz, A., Nichols, W.J. & Jones, T.T. 2002. Growth rates of wild green turtles (Chelonia mydas) at a temperate foraging area in the Gulf of California, Mexico. Copeia (2002): 610-617.
Seminoff, J., Jones, T., Resendiz, A., Nichols, W. & Chaloupka, M. 2003b. Monitoring green turtles (Chelonia mydas) at a coastal foraging area in Baja California, Mexico: using multiple indices to describe population status. J. Mar. Biol. Assoc. UK, 83: 1355-1362.
Sharma, D.S.K. 2000. Impacts from development, nesting population trends and the future of marine turtles at Paku-Kertih, Terengganu. In H. Kalb & T. Wibbels, eds. Proc. 19th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-443, pp. 88-92.
Siow, K.T. & Moll, E.O. 1982. Status and conservation of estuarine and sea turtles in west Malaysia waters. In K.A. Bjorndal, ed. Biology and conservation of sea turtles, pp. 339-347. Washington, DC, Smithsonian Institution Press.
Slater, J., Limpus, C., Robins, J., Pantus, F. & Chaloupka, M. 1998. Risk assessment of sea turtle capture in the Queensland east coast otter trawl fishery. Report prepared for TRAWLMAC, Queensland Fish Management Authority on behalf of the Great Barrier Reef Marine Park Authority and the Queensland Departments of Environment and Primary Industries.
Spotila, J.R., Reina, R.D., Steyermark, A.C., Plotkin, P.T. & Paladino, F.V. 2000. Pacific leatherback turtles face extinction. Nature, 405: 529-530.
Spotila, J.R., Dunham, A.E., Leslie, A.J., Steyermark, A.C., Plotkin, P.T. & Paladino, F.V. 1996. Worldwide population decline of Dermochelys coriacea: are leatherback turtles going extinct? Chelonian Conserv. Biol., 2: 209-222.
Steyermark, A.C., Williams, K., Spotila, J.R., Paladino, F.V., Rostal, D.C., Morreale, S.J., Koberg, M.T. & Arauz, R. 1996. Nesting leatherback turtles at Las Baulas National Park, Costa Rica. Chelonian Conserv. Biol., 2: 173-183.
Suarez, A., Dutton, P. & Bakarbessy, J. 2000. Leatherback (Dermochelys coriacea) nesting on the north Vogelkop coast of Irian Jaya, Indonesia. In H. Kalb & T. Wibbels, eds. Proc. 19th Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-443, p. 260.
Suganuma, H. 1985. Green turtle research program in Ogasawara. Mar. Turtle Newsl., 33: 2-3.
Trinidad, H. & Wilson, J. 2000. The bioeconomics of sea turtle conservation and use in Mexico: history of exploitation and conservation policies for the olive ridley (Lepidochelys olivacea). In Microbehavior and Macroresults: Proc. Tenth Biennial Conference of the International Institute of Fisheries Economics and Trade.
Tuck, G.N., Polacheck, T. & Bulman, C.M. 2003. Spatio-temporal trends of longline fishing effort in the southern ocean and implications for seabird bycatch. Biol. Conserv., 114: 1-27.
Valverde, R.A., Cornelius, S.E. & Mo, C.L. 1998. Decline of the olive ridley sea turtle (Lepidochelys olivacea) nesting assemblage at Nancite Beach, Santa Rosa National Park, Costa Rica. Chelonian Conserv. Biol., 3: 58-63.
Walker, T.A. & Parmenter, J.C. 1990. Absence of a pelagic phase in the life cycle of the flatback turtle, Natator depressa (Garman). J. Biogeography, 17: 275-278.
Wetherall, J.A., Balazs, G.H., Tokunaga, R.A. & Yong, M.M. 1993. Bycatch of marine turtles in North Pacific high-seas driftnet fisheries and impacts on the stocks. North Pacific Fish Comm. Bull., 53: 519-538.
Zarate, P., Fernie, A. & Dutton, P.H. 2004. First results of the east Pacific green turtle Chelonia mydas, nesting population assessment. In Proc. 22nd Annual Symposium on Sea Turtle Biology and Conservation. NOAA Technical Memorandum NMFS-SEFSC-503, pp. 70-73.
| [2] Ecological Modelling Services
P/L PO Box 6150, University of Queensland, St Lucia, Queensland, 4067, Australia
[email protected] [3] National Marine Fisheries Service, NOAA Southwest Fisheries Science Center, 8604 La Jolla Shores Drive, La Jolla, CA 92038, USA [email protected] [4] Ecologically Related Species Section Pelagic Resources Division, National Research Institute of Far Seas Fisheries 5-7-1, Shimizu-orido, Shizuoka 424-8633, Japan [email protected] |