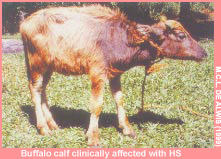
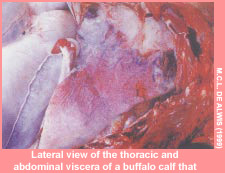
 Haemorrhagic septicaemia (HS) is an acute, fatal, septicaemic disease of cattle and buffaloes caused by specific serotypes of the bacterium Pasteurella multocida . Serotype classification of P. multocida is based on the presence of capsular and somatic antigens. To date, five capsular groups – A, B, D, E and F – have been identified by indirect haemagglutination test using heat-labile antigen preparations (Carter, 1967).
Haemorrhagic septicaemia (HS) is an acute, fatal, septicaemic disease of cattle and buffaloes caused by specific serotypes of the bacterium Pasteurella multocida . Serotype classification of P. multocida is based on the presence of capsular and somatic antigens. To date, five capsular groups – A, B, D, E and F – have been identified by indirect haemagglutination test using heat-labile antigen preparations (Carter, 1967).
Numerous serotypes of P. multocida are associated with a variety of animal disease syndromes affecting a wide range of domestic and feral species. In many instances, P. multocida plays a secondary role in the pathogenesis of these diseases or acts in combination with other agents. HS, on the other hand, is a primary pasteurollosis and is reproducible in susceptible host species using pure cultures of the causative organism alone.
Epidemiology
Although HS has never been reported in a few countries, such as Australia and the United Kingdom and some other European countries, it has a wide global distribution. In most African and Asian countries, the disease is endemic. Indeed most Asian countries rank HS as one of the most economically important diseases.
The acute nature of most cases of HS limits the efficacy of antimicrobial therapy of sick animals |
HS occurs commonly in cattle and buffaloes; buffaloes are more susceptible than cattle. In both species, young animals and young adults are more susceptible than older animals. The two common serotypes of P. multocida associated with the disease in these species are types B:2 (in Asia ) and E:2 (in Africa ). The Asian B:2 serotype has also been associated with sporadic septicaemic disease in pigs.
In Egypt and the Sudan , the presence of both E and B serotypes has been reported (Shigidi and Mustafa, 1979). The African form of HS occurs sporadically. Outbreaks are usually limited in extent and tend to be associated with stress conditions (Bastianello and Nesbit, 1994). The Asian form of HS occurs in countries with high seasonal rainfall and is usually endemic in marshy zones or along river deltas.
Because HS is primarily a bacterial disease, it should theoretically lend itself to effective antibiotic therapy. However, treatment is constrained by a number of factors. The acute nature of most cases of the disease limits the efficacy of antimicrobial therapy of sick animals. Vaccination, therefore, appears to be an alternative effective control option. A solid, long-lasting immunity is conferred on animals that recover from the natural disease and is more persistent than that induced by vaccination.
HS vaccines
Some Asian countries have achieved success in control of the disease by immunizing buffaloes and cattle with alum-precipitated or oil-adjuvant vaccines (Carter and De Alwis, 1989). Immunity is, however, of short duration – lasting 6 to 9 months in primary vaccination and 12 months after secondary vaccination. Large-scale vaccination of cattle against HS is not practised in many countries of Africa . An outbreak of HS in Zambia in 1979 was controlled by using a formalinized vaccine obtained from the Sudan (Francis, Schels and Carter, 1980).
 Despite the fact that the alum-precipitated vaccine is known to provide immunity of short duration, it is still the most common vaccine in use, as it is the easiest vaccine to inject. Oil-adjuvant vaccines, though known to be highly potent, are difficult to inject because of their high viscosity.
Despite the fact that the alum-precipitated vaccine is known to provide immunity of short duration, it is still the most common vaccine in use, as it is the easiest vaccine to inject. Oil-adjuvant vaccines, though known to be highly potent, are difficult to inject because of their high viscosity.
Vaccination appears to be an alternative effective control option |
A considerable amount of research aimed at producing oil-adjuvant vaccines of low viscosity has been done during the past decade in South Asia (De Alwis, unpublished). Indonesia and Sri Lanka have successfully used lower levels of lanoline, an emulsifying agent, to reduce viscosity. Malaysia has used the alum-precipitation technique to concentrate broth cultures in order to reduce the dose volume of the oil-adjuvant vaccine, which is believed to facilitate injection (De Alwis, unpublished). India achieved the same objective by using an agar-grown bacterin, but the production process was found to be too laborious to be feasible on a large-scale basis. Scientists in India , Pakistan and Thailand have used recently developed oil adjuvants and succeeded in reducing the viscosity considerably, but only in Thailand is a vaccine developed by this process used routinely. The double-emulsion vaccine has been used in India and Malaysia on an experimental basis.
 A live heterotypic vaccine made with P. multocida serotype B:3, 4 isolated from a fallow deer in the United Kingdom (Jones and Hussaini, 1982) protected cattle against a serotype B:2 challenge and conferred immunity against HS for one year in cattle vaccinated subcutaneously (Myint, Carter and Jones, 1987). FAO provided funds through its Technical Cooperation Programme to the Government of Myanmar to standardize this vaccine and subject it to further field tests. However, the overall adoption of the intranasal HS vaccine outside Myanmar , where it was developed, has been poor.
A live heterotypic vaccine made with P. multocida serotype B:3, 4 isolated from a fallow deer in the United Kingdom (Jones and Hussaini, 1982) protected cattle against a serotype B:2 challenge and conferred immunity against HS for one year in cattle vaccinated subcutaneously (Myint, Carter and Jones, 1987). FAO provided funds through its Technical Cooperation Programme to the Government of Myanmar to standardize this vaccine and subject it to further field tests. However, the overall adoption of the intranasal HS vaccine outside Myanmar , where it was developed, has been poor.
Recently, the safety, efficacy and cross-protectivity of a live intranasal HS vaccine containing P. multocida serotype B:3, 4 were tested in young cattle and buffaloes in Myanmar (Myint, Jones and Nyunt, 2005). In this study, the administration of 100 times the recommended dose to 50 cattle and 39 buffalo calves was innocuous. Seven months after vaccination, three out of three buffaloes were protected, and twelve months after vaccination, three of four buffaloes were protected, against a subcutaneous challenge with serotype B:2. The vaccinated cattle developed serum antibodies detectable by the passive mouse protection test. The serum of vaccinated cattle cross-protected mice against infection with P. multocida serotypes E:2, F:3, 4 and A:3, 4.
This finding could offer the possibility of further studies in parts of Africa , perhaps in Egypt and the Sudan , where both serotypes B:2 and E:2 have been isolated.
The use of a cross-protecting B:3, 4 deer strain of P. multocida in intranasal vaccination trials could be carried out to assess its efficacy on a pilot scale. The various etiologic types of HS strains could be mapped out in parts of East Africa where the disease is of particular significance.
Bastianello, S.S. & Nesbit, J.W. 1994. Haemorrhagic septicaemia. In J.A.W. Coetzer, G.R. Thomson & R.C. Tustin, eds. Infectious diseases of livestock, Vol. 2, pp. 1180 –1183. Oxford , UK , Oxford University Press.
Carter , G.R. 1967. Pasteurellosis: Pasteurella multocida and Pasteurella haemolytica. Adv. Vet. Sci. , 11: 321–379.
Carter, G.R. & De Alwis, M.C.L. 1989. Haemorrhagic septicaemia. In C. Adlam & J.M. Rutter, eds. Pasteurella and pasteurellosis, pp. 131–160 . London , Academic Press.
Francis, B.K.T., Schels, H.F. & Carter, O.R. 1980. Type E Pasteurella associated with haemorrhagic septicaemia in Zambia . Vet. Rec. , 107: 135.
Jones, T.O. & Hussaini, S.N. 1982. Outbreaks of Pasteurella multocida septicaemia in fallow deer ( Dama dama). Vet. Rec., 110: 451 –452.
Myint, A., Carter, G.R. & Jones, T.O. 1987. Prevention of experimental haemorrhagic septicaemia with a live vaccine. Vet. Rec., 120: 500 –501.
Myint, A., Jones, T.O. & Nyunt, H.H. 2005. Safety, efficacy and cross-protectivity of a live intranasal aerosol haemorrhagic septica emia vaccine. Vet. Rec., 256: 41 –45.
Shigidi, M.T.A. & Mustafa, A.A. 1979. Biochemical and serological studies on Pasteurella multocida isolated from cattle in the Sudan . Cornell Vet ., 69(1): 77–84.