
IDENTITY
Photos from the University of Texas Marine Science Institute's Fisheries and Mariculture Laboratory - Port Aransas, Texas, United States of America.
Biological features
Colour dark-brown dorsally, paler brown laterally and white ventrally; black lateral band as wide as eye extends from snout to base of caudal fin, bordered above and below by paler bands; below this is a narrower dark band. Black lateral band very pronounced in juvenile, but tends to be obscured in adult. Body elongate, sub cylindrical; head broad and depressed. Mouth large, terminal, with projecting lower jaw; villiform teeth in jaws and on roof of mouth and tongue. First dorsal fin with 7-9 (usually 8) short but strong isolated spines each depressed into a groove, not connected by a membrane, 28-33 rays. Second dorsal fin long, anterior rays somewhat elevated in adults. Pectoral fins pointed, becoming more falcate with age. Anal fin similar to dorsal, but shorter; 1-3 spines, 23-27 rays. Caudal fin lunate in adults, upper lobe longer than lower (caudal fin rounded in young, the central rays much prolonged). Scales small, embedded in thick skin; lateral line slightly wavy anteriorly.
Images gallery

15-20 kg broodstock cobia
|

Juvenile cobia in a raceway system
|

1 300 l larval rearing tanks submerged in a 30 000 l recirculating raceway system
|

Covered 20' recirculating broodstock tank for cobia
|
Photos from the University of Texas Marine Science Institute's Fisheries and Mariculture Laboratory - Port Aransas, Texas, United States of America.
PROFILE
Historical background
Aquaculture research with cobia was first reported in 1975 with the collection of wild caught cobia eggs off the coast of North Carolina. Larval development was described and, after the termination of the 131 day rearing trial, it was concluded that cobia had good aquaculture potential because of its rapid growth and good flesh quality. Additional research on cobia was conducted in the late 1980s and early 1990s in the USA and Taiwan Province of China, with the first reported captive spawning of the species occurring in the early 1990s in Taiwan Province of China Research continued, and by 1997 the technology to raise large quantities of cobia fry had been developed and Taiwan Province of China was producing juvenile fish for grow-out, mostly in nearshore cage systems.
In the USA, the first reported captive spawning of cobia occurred in 1996 at the University of Southern Mississippi's Gulf Coast Research Laboratory facility in Ocean Springs, Mississippi. Between 2000 and 2006, aquaculture facilities in Virginia, Texas, South Carolina and Florida also reported spawning cobia by either capturing gravid females, administering hormone injection or implants, or using photoperiod/water temperature manipulations to induce spawning.
As of 2006, two facilities in the USA (the University of Texas Marine Science Institute's Fisheries and Mariculture Laboratory in Port Aransas, Texas and the Aquaculture Center of the Florida Keys in Marathon, Florida) have reported consistent production of cobia eggs and juveniles since 2002. Grow-out production of cobia in cages is ongoing or has occurred in the Bahamas, Belize, Dominican Republic, Mexico, and Puerto Rico, with additional projects reportedly planned for the USA mainland, the Caribbean and Central America; however, there was not any large scale commercial production of cobia reported in 2006 and the industry in the western hemisphere could be characterized as 'developing'.
In the USA, the first reported captive spawning of cobia occurred in 1996 at the University of Southern Mississippi's Gulf Coast Research Laboratory facility in Ocean Springs, Mississippi. Between 2000 and 2006, aquaculture facilities in Virginia, Texas, South Carolina and Florida also reported spawning cobia by either capturing gravid females, administering hormone injection or implants, or using photoperiod/water temperature manipulations to induce spawning.
As of 2006, two facilities in the USA (the University of Texas Marine Science Institute's Fisheries and Mariculture Laboratory in Port Aransas, Texas and the Aquaculture Center of the Florida Keys in Marathon, Florida) have reported consistent production of cobia eggs and juveniles since 2002. Grow-out production of cobia in cages is ongoing or has occurred in the Bahamas, Belize, Dominican Republic, Mexico, and Puerto Rico, with additional projects reportedly planned for the USA mainland, the Caribbean and Central America; however, there was not any large scale commercial production of cobia reported in 2006 and the industry in the western hemisphere could be characterized as 'developing'.
Main producer countries
In addition to the countries indicated in the map, which is based on Member States statistical returns to FAO, work on cobia production activities have also been reported in the Bahamas, Belize, the Dominican Republic, Mexico, Philippines, Puerto Rico, United States of America and Viet Nam.
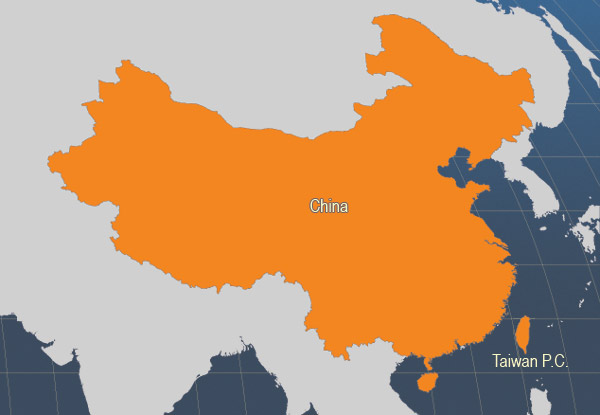

Main producer countries of Rachycentron canadum (FAO Fishery statistics, 2006)
Habitat and biology
Cobia are distributed worldwide in warm marine waters, except for the central and eastern Pacific, resulting in a very large potential area suitable for the production of native species. They can be found throughout the water column and are caught in both coastal and continental shelf waters, although they are typically considered to be an offshore species. Wild-caught cobia do not support a major commercial fishery and are uncommon throughout its range and generally considered incidental catch. They are often found associated with structures of various kinds, such as oil and gas platforms, weedlines, buoys, turtles, rays, and any type of flotsam.
Cobia prefer warm water (>20 ºC) and typically have annual migratory patterns that are established and predictable. In the northwestern Gulf of Mexico, they arrive in the spring and can be caught into the early fall, spawning multiple times from April to September, with activity peaking in July. Sexual maturity is reported in males at 1-2 years and in females at 2-3 years, with females growing both larger and faster with maximum sizes up to 60 kg. Spawning occurs in both nearshore and offshore waters where females release several hundred thousand to several million eggs (1.4 mm diameter) which are then fertilized by the attending males. The viable eggs begin development, are heavily pigmented, buoyant, and hatch in approximately 24 hours. Cobia larvae grow rapidly and are large in comparison to most marine species at 3.5 mm TL at hatching. Juvenile fish are found in both nearshore and offshore waters, often among Sargassum patches or weedlines where they seek shelter from predators and can feed.Cobia are opportunistic feeders and examinations of stomach contents have revealed various fish, shrimp, squid, and, in particular, crabs.
Cobia prefer warm water (>20 ºC) and typically have annual migratory patterns that are established and predictable. In the northwestern Gulf of Mexico, they arrive in the spring and can be caught into the early fall, spawning multiple times from April to September, with activity peaking in July. Sexual maturity is reported in males at 1-2 years and in females at 2-3 years, with females growing both larger and faster with maximum sizes up to 60 kg. Spawning occurs in both nearshore and offshore waters where females release several hundred thousand to several million eggs (1.4 mm diameter) which are then fertilized by the attending males. The viable eggs begin development, are heavily pigmented, buoyant, and hatch in approximately 24 hours. Cobia larvae grow rapidly and are large in comparison to most marine species at 3.5 mm TL at hatching. Juvenile fish are found in both nearshore and offshore waters, often among Sargassum patches or weedlines where they seek shelter from predators and can feed.Cobia are opportunistic feeders and examinations of stomach contents have revealed various fish, shrimp, squid, and, in particular, crabs.
PRODUCTION
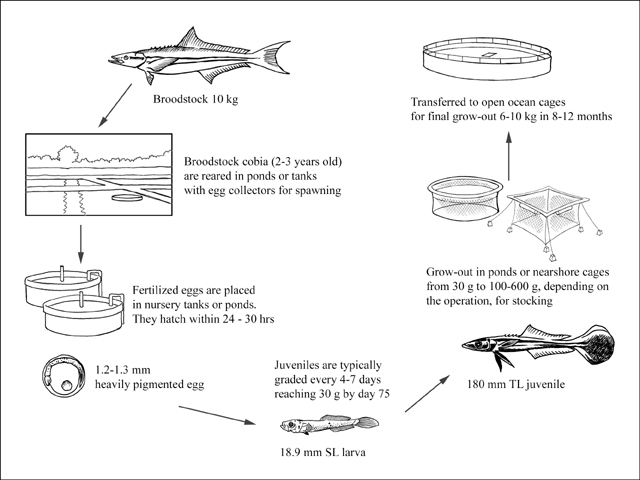
Although the majority of cobia aquaculture production currently comes from China, most of the detailed information about culture and grow-out methods is reported from Taiwan Province of China. Here, broodstock fish for spawning were initially caught from the wild; however, after the species became subsequently farmed, 1.5-2 year old cobia (approximately 10 kg) are now selected from the grow-out cages and transported to onshore ponds. These spawning ponds (400-600 m2 and 1.5 m deep) are stocked with 100 adult cobia at a sex ratio of 1:1. These spawn naturally year round, with peaks in the spring and fall when water temperatures are 23-27 ºC.
Spawning efforts in the USA have also been successful, utilizing round fibreglass tanks 5.5-6.0 m in diameter and 1.5-1.8 m deep to hold adult cobia. The tanks have an egg collector and are either operated as recirculating systems, flow-through, or a combination of both, depending on the biological filtration capacity of the system. Broodstock collection generally involves capturing and transporting juvenile or adult wild-caught cobia (often during their natural spawning season) into the tank systems, where 2-3 year old fish will spawn either naturally or after being induced with photoperiod and temperature manipulations. Research on maintaining and extending the cobia spawning season in the USA has resulted in the production of fertilized eggs during 10 months of the year thus far, with the goal of realizing year-round egg production in the future.
Cobia seed stock used for large scale commercial aquaculture production comes exclusively from hatcheries.
Cobia are spawned and fertilized eggs are collected, either from tanks or spawning ponds. After hatching and absorbing the yolk sac (usually by day 3), the larval cobia must initially be provided adequate amounts of the proper size food, such as enriched rotifers (Brachionus plicatilis) or copepod nauplii. In tank systems this food should be offered for at least the first four days, after which enriched, newly hatched enriched Artemia can be introduced, followed by weaning to dry feed at approximately 25-30 days post-hatch. The rearing density of cobia in tank systems during the early stages remains a challenging aspect of culture that will need to be improved upon for scaling to commercial viability. So far, a modest harvest of 1 fish/litre after weaning, regardless of the initial stocking rate is normal, although some promising research in the USA during 2005-2006 has resulted in the production of over 2 fish/litre and researchers are hoping to double that number in future trials.
In Taiwan Province of China, cobia are reported to be raised in a series of outdoor ponds until they reach a large enough size to be stocked into a nearshore or offshore grow-out cage system. During the larval rearing stage, 'greenwater' nursery ponds <5 000 m2 in area and 1-1.2 m deep with an adequate bloom of Chlorella, copepods, and rotifers are utilized. This method typically results in larval survival of 5-10 percent from hatch to day 20, after which time the fish are moved to two or three pond systems during the next 2 months, depending on the characteristics of the operation.
To reduce cannibalism and size variability cobia are graded weekly after day 45 post-hatch until they reach approximately 30 g (around day 75 post-hatch), which is considered the minimum size for stocking in cages. Cobia are fed 5-6 times a day to satiation at a rate of 5 percent body weight up to 30 g; after this the feeding rate is reduced to 2-3 percent body weight as the fish approaches 200 g. Some producers continue raising the juvenile fish from 30 g up to 600-1 000 g in outdoor ponds, while others use smaller (20-200 m3) nearshore cages. From this point onwards the overall goal, whether in ponds or cages, is to raise the young cobia to a point where they are large enough to be stocked into a grow-out cage system, yet small enough to be transported in large numbers with minimal mortality.
Early on in the cobia production cycle Taiwanese methods, which utilize outdoor ponds for broodstock and nursery phases, tend to be more extensive when compared to current efforts in the USA, which typically involve tank culture of broodstock and early juveniles. From that point on however, grow-out methods are similar in both locations, as they utilize net pens or cages of various sizes and types to rear the cobia to harvestable size.
Successful grow-out of cobia has been reported in nearshore and offshore cages, utilizing both surface and submerged systems during the longest and final stage of production. Taiwanese producers use 1 000-2 000 m3 cages, while some operations in the Caribbean have used 3000 m³ submersible systems successfully. In order to minimize grow-out time as well as disease issues, cobia produced in cages should be located in sites that provide warm (26 ºC and above) clean water and adequate flow rates through the cage system to provide high dissolved oxygen levels continuously. Harvest numbers vary depending on the stocking rates and water temperature, but the grow-out period for pellet fed cobia is generally about 1-1.5 years, with fish reaching a final weight of 6-10 kg at harvest densities of 10-15 kg/m3.
Cobia producers in Taiwan Province of China use both floating and sinking pellets (42-45 percent crude protein, and 15-16 percent lipid), typically fed 6 days a week at a rate of 0.5-0.7 percent BW/day towards the end of the grow-out phase. Cobia feed conversion in Taiwan Province of China is reported to be approximately 1.5:1. Operations in the Caribbean have used pellets manufactured in the USA that are typically higher in crude protein (50- 53 percent) with 10-15 percent lipid content.
It is reported that producers in Taiwan Province of China make selective or partial harvests of cobia from cages allowing them to meet current market demands, whether the fish are consumed domestically or destined for export. Little published information is available regarding cage harvest methods for cobia, although they would certainly be site specific and likely to employ techniques to crowd and net or pump fish and place them into a chilled container on a tending vessel, similar to those used in salmon farming.
Cobia in Taiwan Province of China are typically starved the day before harvest and 6 kg fish or larger are selected, killed, bled and chilled before whole fish or fillets are packed in ice. Cobia enters the market whole/gutted, headless, or filleted, depending on the final market destination.
Information on production costs for this species is minimal but was said to be approximately USD 2.20/kg in Taiwan Province of China in 2001, a figure which was noted to be very competitive with several other species of marine fish being cultured.
Production cycle

Production cycle of Rachycentron canadum
Production systems
Seed supply
Although the majority of cobia aquaculture production currently comes from China, most of the detailed information about culture and grow-out methods is reported from Taiwan Province of China. Here, broodstock fish for spawning were initially caught from the wild; however, after the species became subsequently farmed, 1.5-2 year old cobia (approximately 10 kg) are now selected from the grow-out cages and transported to onshore ponds. These spawning ponds (400-600 m2 and 1.5 m deep) are stocked with 100 adult cobia at a sex ratio of 1:1. These spawn naturally year round, with peaks in the spring and fall when water temperatures are 23-27 ºC.
Spawning efforts in the USA have also been successful, utilizing round fibreglass tanks 5.5-6.0 m in diameter and 1.5-1.8 m deep to hold adult cobia. The tanks have an egg collector and are either operated as recirculating systems, flow-through, or a combination of both, depending on the biological filtration capacity of the system. Broodstock collection generally involves capturing and transporting juvenile or adult wild-caught cobia (often during their natural spawning season) into the tank systems, where 2-3 year old fish will spawn either naturally or after being induced with photoperiod and temperature manipulations. Research on maintaining and extending the cobia spawning season in the USA has resulted in the production of fertilized eggs during 10 months of the year thus far, with the goal of realizing year-round egg production in the future.
Cobia seed stock used for large scale commercial aquaculture production comes exclusively from hatcheries.
Hatchery production
Cobia are spawned and fertilized eggs are collected, either from tanks or spawning ponds. After hatching and absorbing the yolk sac (usually by day 3), the larval cobia must initially be provided adequate amounts of the proper size food, such as enriched rotifers (Brachionus plicatilis) or copepod nauplii. In tank systems this food should be offered for at least the first four days, after which enriched, newly hatched enriched Artemia can be introduced, followed by weaning to dry feed at approximately 25-30 days post-hatch. The rearing density of cobia in tank systems during the early stages remains a challenging aspect of culture that will need to be improved upon for scaling to commercial viability. So far, a modest harvest of 1 fish/litre after weaning, regardless of the initial stocking rate is normal, although some promising research in the USA during 2005-2006 has resulted in the production of over 2 fish/litre and researchers are hoping to double that number in future trials.
Nursery
In Taiwan Province of China, cobia are reported to be raised in a series of outdoor ponds until they reach a large enough size to be stocked into a nearshore or offshore grow-out cage system. During the larval rearing stage, 'greenwater' nursery ponds <5 000 m2 in area and 1-1.2 m deep with an adequate bloom of Chlorella, copepods, and rotifers are utilized. This method typically results in larval survival of 5-10 percent from hatch to day 20, after which time the fish are moved to two or three pond systems during the next 2 months, depending on the characteristics of the operation.
To reduce cannibalism and size variability cobia are graded weekly after day 45 post-hatch until they reach approximately 30 g (around day 75 post-hatch), which is considered the minimum size for stocking in cages. Cobia are fed 5-6 times a day to satiation at a rate of 5 percent body weight up to 30 g; after this the feeding rate is reduced to 2-3 percent body weight as the fish approaches 200 g. Some producers continue raising the juvenile fish from 30 g up to 600-1 000 g in outdoor ponds, while others use smaller (20-200 m3) nearshore cages. From this point onwards the overall goal, whether in ponds or cages, is to raise the young cobia to a point where they are large enough to be stocked into a grow-out cage system, yet small enough to be transported in large numbers with minimal mortality.
Ongrowing techniques
Early on in the cobia production cycle Taiwanese methods, which utilize outdoor ponds for broodstock and nursery phases, tend to be more extensive when compared to current efforts in the USA, which typically involve tank culture of broodstock and early juveniles. From that point on however, grow-out methods are similar in both locations, as they utilize net pens or cages of various sizes and types to rear the cobia to harvestable size.
Successful grow-out of cobia has been reported in nearshore and offshore cages, utilizing both surface and submerged systems during the longest and final stage of production. Taiwanese producers use 1 000-2 000 m3 cages, while some operations in the Caribbean have used 3000 m³ submersible systems successfully. In order to minimize grow-out time as well as disease issues, cobia produced in cages should be located in sites that provide warm (26 ºC and above) clean water and adequate flow rates through the cage system to provide high dissolved oxygen levels continuously. Harvest numbers vary depending on the stocking rates and water temperature, but the grow-out period for pellet fed cobia is generally about 1-1.5 years, with fish reaching a final weight of 6-10 kg at harvest densities of 10-15 kg/m3.
Feed supply
Cobia producers in Taiwan Province of China use both floating and sinking pellets (42-45 percent crude protein, and 15-16 percent lipid), typically fed 6 days a week at a rate of 0.5-0.7 percent BW/day towards the end of the grow-out phase. Cobia feed conversion in Taiwan Province of China is reported to be approximately 1.5:1. Operations in the Caribbean have used pellets manufactured in the USA that are typically higher in crude protein (50- 53 percent) with 10-15 percent lipid content.
Harvesting techniques
It is reported that producers in Taiwan Province of China make selective or partial harvests of cobia from cages allowing them to meet current market demands, whether the fish are consumed domestically or destined for export. Little published information is available regarding cage harvest methods for cobia, although they would certainly be site specific and likely to employ techniques to crowd and net or pump fish and place them into a chilled container on a tending vessel, similar to those used in salmon farming.
Handling and processing
Cobia in Taiwan Province of China are typically starved the day before harvest and 6 kg fish or larger are selected, killed, bled and chilled before whole fish or fillets are packed in ice. Cobia enters the market whole/gutted, headless, or filleted, depending on the final market destination.
Production costs
Information on production costs for this species is minimal but was said to be approximately USD 2.20/kg in Taiwan Province of China in 2001, a figure which was noted to be very competitive with several other species of marine fish being cultured.
Diseases and control measures
Cobia are susceptible to many viruses, bacteria, and parasites that commonly afflict other warm water marine species. Managing disease and parasite issues has been identified, particularly by the Taiwanese, to be one of the major challenges with regard to cobia culture so far. The chart below lists some of the pathogens reported on cobia. It is intended to be used as an informative document only and culturists should refer to published disease reference sources for actual treatment and diagnosis. In addition, producers need to check on specific local and federal regulations with regard to approved chemicals and drugs for use on any food fish.
In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply an FAO recommendation.
Suppliers of pathology expertise
Assistance can be obtained from the following sources:
In some cases antibiotics and other pharmaceuticals have been used in treatment but their inclusion in this table does not imply an FAO recommendation.
| DISEASE | AGENT | TYPE | SYNDROME | MEASURES |
| Marine velvet disease; Amyloodiniosis | Amyloodinium ocellatum | Parasitic dinoflagellate | Coughing; flashing; operculum flared out; reluctance to feed; visible on gills & fins; appears as small dark spots on gill filaments under a stereoscope | Copper sulphate pentahydrate; decrease salinity in some cases (freshwater dip); flush system; formalin bath/treatment; mechanical filtration down to at least 40 microns |
| Cryptocaryonosis; marine white spot | Cryptocaryon irritans | External protozoan | White foci visible on skin; interconnected, larger masses of whitish spots | Prolonged copper immersion; freshwater dips; formalin treatment; decrease salinity to 15 ‰ or less for 2 weeks; decrease system temperature to <19 ºC |
| Sessile, colonial, ciliate infestation | Epistylis spp. | Stalked ciliate | Reported during larval stage; white or reddish masses on the skin/fins, gill arches, or in mouth; more common in polluted waters; often associated with gram-negative bacterial condition called red sore disease | Formalin treatment; freshwater bath/dip; antibiotics for severe bacterial infection |
| Trichodinosis | Trichodina sp. | Protozoan parasite | Reported during nursery stage; found on skin & gills; loss of appetite; lethargy; chronic low level mortality; often leads to secondary infections | Formalin treatment; freshwater bath; copper treatment; Praziquantel bath or prolonged immersion |
| Monogenean infestation | Neobenedenia sp. | Monogenean flatworm parasite | Reported during grow-out stage; skin damage & ulceration; eroded fins; eye lesions which can lead to blindness | Formalin treatment; freshwater bath; copper treatment; Praziquantel bath or prolonged immersion |
| Myxidiosis | Sphaerospora-like myxosporidean | Myxosporidian parasite | Poor appetite & ascites; enlarged kidney exhibiting patches or nodules; skin ulcers; spores in the digestive tract | No known treatment; disinfect system; quarantine affected fish |
| Coccidiosis | Coccidia spp. | Protozoan parasite | Abdominal swelling; exopthalamy; cysts in liver tissue; varies with organ affected | Treat fish with oral monensin; reduce stress |
| Lymphocystis | Iridovirus | Virus | Reported during nursery stage; skin, fins & gills with white, bumpy growths | No known treatment; disinfect system; quarantine fish |
| Pasteurellosis | Photobacterium damsela subsp. Piscicida | Bacterium | Whitish, granulomatous deposits on kidney, liver & spleen | None known but vaccine being developed |
| Vibriosis | Vibrio alginolyticus; V. vulnificus & V. parahaemolyticus | Bacteria | Swollen abdomen; skin ulcers; protruded eyes; lethargy; darkening of skin; ascites in peritoneal cavity | Administer antibiotics; remove diseased fish; disinfect system; reduce stress |
| Secondary bacterial infection (after Neobenedenia infestation) | Streptococcus sp. | Bacterium | Can cause blindness in cobia; protruded eyes; skin ulcers; skin darkening | Administer antibiotics; remove diseased fish; disinfect system; reduce stress |
Suppliers of pathology expertise
Assistance can be obtained from the following sources:
- Aquatic Veterinarian and Disease Diagnostic Laboratory Resources (http://www.aquavets.com/)
- Texas Veterinary Medical Diagnostic Laboratories (http://tvmdlweb.tamu.edu/)
STATISTICS
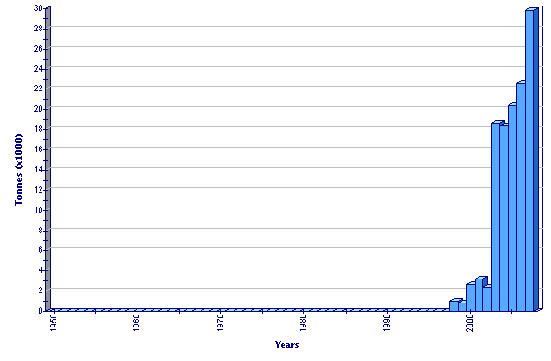
Production statistics
Global aquaculture production of Rachycentron canadum
(FAO Fishery statistics)
(FAO Fishery statistics)

Market and trade
China and Taiwan Province of China both find that the rapid growth rate and good flesh quality of cobia make it potentially one of the most important marine fish for future production. Since wild caught cobia does not represent a major fishery and the farming of cobia is in its infancy, details regarding the market and trade of this species are notably lacking. As a result of its current limited availability, many seafood consumers have probably never tasted cobia; increasing supplies from aquaculture, combined with effective marketing of this firm-textured, white-fleshed fish will be critically important for future market expansion.
Taiwan Province of China reports that this species enjoys a relatively high market value compared to other finfish. Larger cobia (8-10 kg) are sold whole there, while Japan is the primary destination for smaller (6-8 kg) fish sold both whole and headless (some for sashimi), with fillet product typically exported to other markets. The prices for cobia vary according to size; the market value in Taiwan Taiwan Province of China for whole fish that are 17 lb (7.7 kg) and larger was USD 5.50/kg in 2004 and reported to be less than that for smaller fish.
Taiwan Province of China reports that this species enjoys a relatively high market value compared to other finfish. Larger cobia (8-10 kg) are sold whole there, while Japan is the primary destination for smaller (6-8 kg) fish sold both whole and headless (some for sashimi), with fillet product typically exported to other markets. The prices for cobia vary according to size; the market value in Taiwan Taiwan Province of China for whole fish that are 17 lb (7.7 kg) and larger was USD 5.50/kg in 2004 and reported to be less than that for smaller fish.
STATUS AND TRENDS
The current status of cobia farming in Asia and the activities being demonstrated elsewhere suggest that the farming of this species has a bright future. Cobia has several desirable traits, most importantly a rapid growth rate and good flesh quality. Global production is likely to expand in the future. Researchers and commercial producers in China, Taiwan Province of China and the USA have established pond and tank spawning of cobia broodstock, and both larval and juvenile rearing protocols are currently being refined.
Improvements during the nursery phase of cobia production are critical if culturists are going to be able to supply the massive numbers of juveniles that would be required to stock large, commercial cage operations. Towards that goal, USA and Taiwanese researchers are working on the development of intensive and super-intensive recirculating systems for juvenile production, utilizing temperature control, UV sterilization, drum filters, protein skimmers and oxygenation units. So far, a Taiwanese high density juvenile production system has been quite successful, with optimum production and survival rates for cobia grown from 7 to 76 grams in four weeks at initial stocking densities of 370 fish/m³ and final harvest values reported to be 28 kg/m3.
Salinity tolerance of cobia is another interesting aspect of culture that warrants further research. Although typically taken from waters near oceanic salinity (30-35 ‰), juvenile cobia have been cultured successfully during 8 week trials in water as low as 5 ‰ with good FCRs and growth rates. The ability to grow cobia at various salinities could present aquaculturists with additional production opportunities with this species.
As the current world leader in the farming of cobia, China's future production is likely to be a major factor in the commercialization of this species. Thus far, the availability of trash fish to use as food for the expanding cobia industry in China has been identified as one of the main limiting factors for future production. Despite this feed shortage, producers have identified cobia as a species that has tremendous potential and cage systems are widely distributed in the southern coastal provinces (Guangdong and Hainan). The future global supply, market, and pricing of cobia will certainly be affected by the future production (or lack of) cobia in China and Taiwan Province of China, as well as in other countries in Southeast Asia.
Improvements during the nursery phase of cobia production are critical if culturists are going to be able to supply the massive numbers of juveniles that would be required to stock large, commercial cage operations. Towards that goal, USA and Taiwanese researchers are working on the development of intensive and super-intensive recirculating systems for juvenile production, utilizing temperature control, UV sterilization, drum filters, protein skimmers and oxygenation units. So far, a Taiwanese high density juvenile production system has been quite successful, with optimum production and survival rates for cobia grown from 7 to 76 grams in four weeks at initial stocking densities of 370 fish/m³ and final harvest values reported to be 28 kg/m3.
Salinity tolerance of cobia is another interesting aspect of culture that warrants further research. Although typically taken from waters near oceanic salinity (30-35 ‰), juvenile cobia have been cultured successfully during 8 week trials in water as low as 5 ‰ with good FCRs and growth rates. The ability to grow cobia at various salinities could present aquaculturists with additional production opportunities with this species.
As the current world leader in the farming of cobia, China's future production is likely to be a major factor in the commercialization of this species. Thus far, the availability of trash fish to use as food for the expanding cobia industry in China has been identified as one of the main limiting factors for future production. Despite this feed shortage, producers have identified cobia as a species that has tremendous potential and cage systems are widely distributed in the southern coastal provinces (Guangdong and Hainan). The future global supply, market, and pricing of cobia will certainly be affected by the future production (or lack of) cobia in China and Taiwan Province of China, as well as in other countries in Southeast Asia.
MAIN ISSUES
Depending on pond management, culture density, feed rate, and water exchange rates, the aquaculture production of cobia in ponds may result in water quality issues and excess nutrient loading in the effluent. Large-scale production of cobia at various life stages could potentially impact any coastal areas where such activities are located and production discharge would require monitoring. Cobia grow-out thus far has been reported in nearshore and offshore cage systems which, no matter where they are located, will also have some form of environmental impact. These operations carry with them some inherent risks including, but not limited to, escapees (genetic pollution), disease transmission, and nutrient loading in and around farm sites.
Since 1998, when significant aquaculture production of cobia was initially reported, it has been noted in Taiwan Province of China that while cobia production is successful and expanding there, diseases affecting the fish in culture are a major issue and best management practices must be implemented wherever possible. Because of the annual threat of typhoons, some farmers there have located their grow-out systems in more enclosed, lower energy areas, at the expense of higher water flow and flushing rates. This intensification of nearshore production has been reported to result in increased disease outbreaks and, in some cases, lower quality flesh from cobia raised in cage systems from more polluted areas.
Expansion of cobia cage culture in areas such as the Caribbean and Central America where cage sites may be close to sensitive coral reef would also require producers to monitor impacts and the nutrient loading of the nearby ecosystem. Cage culture environmental impacts are site-specific and, while water exchange rates and dilution factors in many areas are sufficient to prevent excess nutrient loading, every site has a carrying capacity that needs to be considered.
Another environmental consideration is that since cobia are higher trophic level carnivores, it is necessary to use feed containing fairly high crude protein levels (the specific nutritional requirements are currently being investigated) with a certain portion of this protein obtained from fish meal. For aquaculture to continue supplying the increasing demand for seafood worldwide, additional research into supplemental or alternative protein sources for use in feeds for species such as cobia is imperative. To that end, research on the specific nutritional requirements of this species in terms of protein and lipid levels and fish meal substitution in feed has been conducted and is ongoing.
As cobia aquaculture expands in the future, the efficient production of a high quality product with minimal environmental impact during culture should be the desired goal.
Since 1998, when significant aquaculture production of cobia was initially reported, it has been noted in Taiwan Province of China that while cobia production is successful and expanding there, diseases affecting the fish in culture are a major issue and best management practices must be implemented wherever possible. Because of the annual threat of typhoons, some farmers there have located their grow-out systems in more enclosed, lower energy areas, at the expense of higher water flow and flushing rates. This intensification of nearshore production has been reported to result in increased disease outbreaks and, in some cases, lower quality flesh from cobia raised in cage systems from more polluted areas.
Expansion of cobia cage culture in areas such as the Caribbean and Central America where cage sites may be close to sensitive coral reef would also require producers to monitor impacts and the nutrient loading of the nearby ecosystem. Cage culture environmental impacts are site-specific and, while water exchange rates and dilution factors in many areas are sufficient to prevent excess nutrient loading, every site has a carrying capacity that needs to be considered.
Another environmental consideration is that since cobia are higher trophic level carnivores, it is necessary to use feed containing fairly high crude protein levels (the specific nutritional requirements are currently being investigated) with a certain portion of this protein obtained from fish meal. For aquaculture to continue supplying the increasing demand for seafood worldwide, additional research into supplemental or alternative protein sources for use in feeds for species such as cobia is imperative. To that end, research on the specific nutritional requirements of this species in terms of protein and lipid levels and fish meal substitution in feed has been conducted and is ongoing.
As cobia aquaculture expands in the future, the efficient production of a high quality product with minimal environmental impact during culture should be the desired goal.
Responsible aquaculture practices
Best management practices should be implemented during the entire production process and include detailed monitoring of broodstock and production, the use of high quality and nutritionally complete diets, frequent sampling and observation of stock for diseases and/or parasites, and the timely removal of sick fish.
REFERENCES
Bibliography
| Arnold, C.R., Kaiser, J.B. & Holt, G.J. 2002. Spawning of cobia Rachycentron canadum in captivity. Journal of the World Aquaculture Society, 33:205-208. |
| Benetti, D.D., Alarcon, J.F., Stevens, O.M., O'Hanlon, B., Rivera, J.A., Banner-Stevens, G. and Rotman, F.J. 2003. Advances in hatchery and growout technology of marine finfish candidate species for offshore aquaculture in the Caribbean. Proceedings of the Gulf and Caribbean Fisheries Institute, 54:475-487. |
| Briggs, J.C. 1974. Cobia, Rachycentron canadum. In: A.J. McClane (ed.), McClane's new standard fishing encyclopedia and international angling guide, p. 219. Holt, Rinehart & Winston, New York, USA. |
| Chen, S.C., Kou, R.J., Wu, C.T., Wang, P.C. & Su, F.Z. 2001. Mass mortality associated with a Sphaerospora-like myxosporidean infestation in juvenile cobia, Rachycentron canadum (L.), marine cage cultured in Taiwan. Journal of Fish Diseases, 24:189-195. |
| Chou, R.L., Her, B.Y., Su, M.S., Hwang, G., Wu, Y.H. & Chen, H.Y. 2004. Substituting fish meal with soybean meal in diets of juvenile cobia Rachycentron canadum. Aquaculture, 229:325-333. |
| Chou, R.L., Su, M.S. & Chen, H.Y. 2001. Optimal dietary protein and lipid levels for juvenile cobia (Rachycentron canadum). Aquaculture, 193:81-89. |
| Collette, B.B. 1978. Rachycentridae. In: W. Fischer (ed.), FAO species identification sheets for fishery purposes, western and central Atlantic (Fishing area 31), Vol. 4. (unpaginated). FAO, Rome, Italy. |
| Craig, S.R., Schwarz, M.H. & McLean, E. 2006. Juvenile cobia (Rachycentron canadum) can utilize a wide range of protein and lipid levels without impacts on production characteristics. Aquaculture, 261:384-391. |
| Denson, M.R., Stuart, K.R., Smith, T.I.J., Weirich, C.R. & Segars, A. 2003. Effects of salinity on growth, survival, and selected hematological parameters of juvenile cobia Rachycentron canadum. Journal of the World Aquaculture Society, 34:496-504. |
| Ditty, J.G. & Shaw, R.F. 1992. Larval development, distribution, and ecology of cobia Rachycentron canadum (Family: Rachycentridae) in the northern Gulf of Mexico. Fishery Bulletin, 90:668-677. |
| Faulk, C.K. & Holt, G.J. 2003. Lipid nutrition and feeding of cobia Rachycentron canadum larvae. Journal of the World Aquaculture Society, 34:368-378. |
| Faulk, C.K. & Holt, G.J. 2005. Advances in rearing cobia Rachycentron canadum larvae in recirculating aquaculture systems: live prey enrichment and greenwater culture. Aquaculture, 249: 231-243. |
| Faulk, C.K. & Holt, G.J. 2006. Responses of cobia Rachycentron canadum larvae to abrupt or gradual changes in salinity. Aquaculture, 254:275-283. |
| Faulk, C.K., Benninghoff, A.D. & Holt, G.J. 2007. Ontogeny of the gastrointestinal tract and selected digestive enzymes in cobia Rachycentron canadum. Journal of Fish Biology, 70:1-17. |
| Fowler, H.W. 1936. The marine fishes of West Africa. Bulletin of the American Museum of Natural History, 70(2). 1493 pp. |
| Franks, J.S., Ogle, J.T., Lotz J.M., Nicholson, L.C., Barnes, D.N. & Larson, K.M. 2001. Spontaneous spawning of cobia, Rachycentron canadum, induced by human chorionic gonadotropin (HCG), with comments on fertilization, hatching, and larval development. Proceedings of the Gulf and Caribbean Fisheries Institute, 52:598-609. |
| Franks, J.S., Warren, J.R. & Buchanan, M.V. 1999. Age and growth of cobia, Rachycentron canadum, from the northeastern Gulf of Mexico. Fishery Bulletin 97:459-471. |
| Goode, G.B. 1884. The fisheries and fishery industries of the United States. Section 1: Natural history of useful aquatic animals. Text. U.S. Commission on Fisheries, Washington D.C., 895 pp. |
| Hardy, J.D, Jr. 1978. Development of fishes of the Mid-Atlantic Bight: an atlas of egg, larval, and juvenile stages. Vol. III. Aphredoderidae through Rachycentridae. U.S. Fish and Wildlife Service, Biological Services Program, Washington, D.C. FWS/OBS-78/12, 394 pp. |
| Hassler, W.W. & Rainville, R.P. 1975. Techniques for hatching and rearing cobia, Rachycentron canadum, through larval and juvenile stages. University of North Carolina Sea Grant College Program, UNC-SG-75-30, Raleigh, North Carolina, USA. 26 pp. |
| Hitzfelder, G.M., Holt, G.J., Fox, J.M. & McKee, D.A. 2006. The effect of rearing density on growth and survival of cobia, Rachycentron canadum, larvae in a closed recirculating aquaculture system. Journal of the World Aquaculture Society, 37:204-209. |
| Huang, T.S., Lin, K.J., Chen, C.C. & Tsai, W.S. 2002. Study on cobia, Rachycentron canadum, over-wintering using the indoor high-density recirculating system. Journal of Taiwan Fisheries Research,10:53-62 (in Chinese with English abstract). |
| Joseph, E.B., Norcross, J.J. & Massmann, W.H. 1964. Spawning of the cobia, Rachycentron canadum, in the Chesapeake Bay area, with observations of juvenile specimens. Chesapeake Scientist, 5(1-2):67-71. |
| Kaiser, J.B. & Holt, G.J. 2005a. Cobia aquaculture. In: A.M. Kelly & J.T. Silverstein (eds.), pp. 465-469, Aquaculture in the 21st Century. American Fisheries Society. Bethesda, Maryland, USA. |
| Kaiser, J.B. & Holt, G.J. 2005b. Species Profile: Cobia. SRAC Publication number 7202. Southern Regional Aquaculture Center, Mississippi, USA. 6 pp. |
| Kaiser, J.B. & Holt, G.J. 2004. Cobia: a new species for aquaculture in the US. World Aquaculture, 35:12-14. |
| Kilduff, P., DuPaul, W., Osterling, M., Olney, J, Jr. & Tellock, J. 2002. Induced tank spawning of cobia, Rachycentron canadum, and early larval husbandry. World Aquaculture, 33(2): 35-38. |
| Liao, I.C. 2003. Candidate species for open ocean aquaculture: the successful case of cobia Rachycentron canadum in Taiwan. In: C.J. Bridger & B.A. Costa-Pierce (eds.), pp. 205-213, Open Ocean Aquaculture: From Research to Commercial Reality. World Aquaculture Society, Baton Rouge, Louisiana, USA. |
| Liao, I.C., Huang, T.S., Tsai, W.S., Hsueh, C.M., Chang, S.L. & Leano, E.M. 2004. Cobia culture in Taiwan: current status and problems. Aquaculture, 237:155-165. |
| Liao, I-C. & Leaño, E.M. (eds.) 2007. Cobia Aquaculture: Research, Developments and Commercial Production. Asian Fisheries Society, Manila, Philippines, Fisheries Society of Taiwan, Keelung, Taiwan and World Aquaculture Society, Baton Rouge, USA. 178 pp. |
| Liu, P. C., Lin, J.Y., Hsiao, P.T. & Lee, K.K. 2004. Isolation and characterization of pathogenic Vibrio alginolyticus from diseased cobia Rachycentron canadum. Journal of Basic Microbiology, 44: 23-28. |
| Liu, P.C., Lin, J.Y. & Lee. K.K. 2003. Virulence of Photobacterium damselae subsp. piscicida in cultured cobia Rachycentron canadum. Journal of Basic Microbiology, 43:499-507. |
| Lopez, C., Rajan, P.R., Lin, J.H.Y., Kuo, T.Y. & Huey-Lang Yang. 2002. Disease outbreak in seafarmed cobia (Rachycentron canadum) associated with Vibrio spp., Photobacterium damselae ssp. piscicida, monogenean and myxosporean parasites. Bulletin of the European Association of Fish Pathologists, 22:206-211. |
| Resley, M.J., Webb, K.A. & Holt, G.J. Growth and survival of juvenile cobia Rachycentron canadum cultured at different salinities in recirculating aquaculture systems. Aquaculture, 253:398-407. |
| Shaffer, R.V. & Nakamura, E.L. 1989. Synopsis of biological data on the cobia Rachycentron canadum (Pisces: Rachycentridae). FAO Fisheries Synopsis 153 (National Marine Fisheries Service/S 153), U.S. Department of Commerce, NOAA Technical Report, National Marine Fisheries Service, 82. Washington, D.C., USA. |
| Su, M.S. & Liao, I.C. 2001. Present status and prospects of marine cage aquaculture in Taiwan. In: I.C. Liao, & J. Baker (eds.), pp. 193-201, Aquaculture and Fisheries Resource Management. TFRI Conference Proceedings, Vol. 4. Taiwan Fisheries Research Institute, Keelung, Taiwan. |
| Su, M.S., Chien, Y.H. & Liao, I.C. 2000. Potential of marine cage aquaculture in Taiwan: cobia culture. In: I.C. Liao & C.K. Lin (eds.), pp. 97-106, Cage Aquaculture in Asia. Asian Fisheries Society, Manila, Philippines & World Aquaculture Society - Southeast Asian Chapter, Bangkok, Thailand. |
| Sun, L., Chen, H., Huang, L, Wang, Z. & Yan, Y. 2006. Growth and energy budget of juvenile cobia (Rachycentron canadum) relative to ration. Aquaculture, 257:214-220. |
| Wang, J.T., Liu, Y.J., Tian, L.X., Mai, K.S., Du, Z.Y., Wang, Y. & Yang, H.J. 2005. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture, 249:439-447. |
| Webb, K.A., Hitzfelder, G.M., Faulk, C.K. & Holt, G.J. 2007. Growth of juvenile cobia, Rachycentron canadum, at three different densities in a recirculating aquaculture system. Aquaculture, 264:223-227. |
| Zhou, Q.C., Tan, B.P., Mai, K.S. & Liu, Y.J. 2004. Apparent digestibility of selected feed ingredients for juvenile cobia Rachycentron canadum. Aquaculture, 241:441-451. |
| Zhou, Q.C., Wu, Z.H., Tan, B.P., Chi, S.Y. & Yang, Q.H. 2006. Optimal dietary methionine requirement for juvenile cobia (Rachycentron canadum). Aquaculture 258:551-557. |

